Abstract
The development of effective therapeutic vaccines to generate tumor-reactive cytotoxic T lymphocytes (CTLs) continues to be a top research priority. However, in spite of some promising results, there are no clear examples of vaccines that eradicate established tumors. Most vaccines are ineffective because they generate low numbers of CTLs and because numerous immunosuppressive factors abound in tumor-bearing hosts. We designed a peptide vaccine that produces large numbers of tumor-reactive CTLs in a mouse model of melanoma. Surprisingly, CTL tumor recognition and antitumor effects decreased in the presence of interferon γ (IFNγ), a cytokine that can provide therapeutic benefit. Tumors exposed to IFNγ evade CTLs by inducing large amounts of noncognate major histocompatibility complex class I molecules, which limit T-cell activation and effector function. Our results demonstrate that peptide vaccines can eradicate large, established tumors in circumstances under which the inhibitory activities of IFNγ are curtailed.
Introduction
Because cytotoxic T lymphocytes (CTLs) have the ability to recognize and kill tumor cells, considerable efforts are being devoted to the development of T-cell immunotherapies for cancer.1-3 CTLs express the CD8 coreceptor and recognize antigen on tumor cells as peptide/major histocompatibility class I (MHC-I) complexes. As a consequence of antigen recognition, CD8 CTLs exert antitumor function via the perforin-granzyme cytolytic pathway or through cytokines such as interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα), which exhibit cytostatic activity. The MHC-I–binding peptides recognized by tumor-reactive CD8 T lymphocytes are usually derived from genes preferentially expressed by transformed cells or from tissue-differentiation antigens. The identification of MHC-I–binding peptides that serve as tumor-rejection CD8 T-cell epitopes has opened the door to developing synthetic peptide cancer vaccines.4 The discovery of melanoma T-cell epitopes for humans and mice has led to studies assessing the utility of peptide vaccines for the treatment of established disease states. In many of these studies, promising, but ultimately not outstanding, therapeutic effects were attained, indicating that the use of synthetic peptides alone, with commonly used adjuvants such as incomplete Freund adjuvant, or in combination with cytokines constitute relatively weak and ineffective vaccines.5-8 Thus, several groups, including ours, have focused on optimizing peptide vaccines with the use of Toll-like receptor agonists and costimulatory antibodies as immunologic adjuvants.9-14
Our goal was to design a peptide immunization strategy that generates T-cell responses similar to those observed during an acute viral infection, in which 10% to 50% of all CD8 T cells are specific for the pathogen. We recently described a vaccine that we call TriVax (named for its 3 components: synthetic peptide, polyriboinosinic-polyribocytidylic acid [poly-IC], and anti-CD40 antibody), which achieved our stated goal.15,16 In addition to generating large CD8 T-cell responses to a melanosomal epitope (Trp2180), significant therapeutic effects (60% long-term survival) were observed against 3-day established B16 melanomas. The therapeutic effect of TriVax disappeared when CD8 T cells were depleted with antibodies or in perforin-deficient mice. Conversely, the elimination of CD4 T lymphocytes and natural killer cells had no significant effect.16 These results indicated that the major effector mechanism of TriVax is mediated by classic CD8 CTLs through perforin-mediated lysis of tumor cells. Nevertheless, the therapeutic effect of TriVax decreased if vaccination was administered in more advanced disease states, even though large numbers of functional CD8 T cells were detected in the tumor-bearing mice, suggesting that immune-suppressive activity at the tumor site was responsible for the tumor's evasion from the T cells. During these studies, we observed that the therapeutic effectiveness of TriVax was significantly higher in IFNγ-deficient mice compared with the immune-competent cohorts (100% vs 60% survival, respectively).16 These paradoxical results suggested that IFNγ, a lymphokine generally known for its therapeutic effects against infectious agents and tumors,17-19 may trigger immune-inhibitory activities, limiting the effectiveness of T cell–based therapies against cancer. We present data supporting this notion and describe a novel mechanism by which IFNγ renders tumor cells resistant to T-cell recognition and eradication. These findings should help to increase the effectiveness of cancer vaccines by helping to design strategies to reduce the negative effects of IFNγ.
Methods
Mice
Six- to 8-week-old female C57BL/6 (B6) mice were obtained from the National Cancer Institute/Charles River Program. IFNγ-deficient (IFNγ−/−) and Pmel-1 T-cell receptor (TCR)-transgenic20 mice were obtained from The Jackson Laboratory. All animal care and experiments were conducted according to our institutional animal care and use committee guidelines, and were approved by the H. Lee Moffitt Cancer Center institutional review board.
Cells
The murine melanoma cell line B16 (derived from B16F10, but passaged several times in mice21 ) was provided by Dr Alan Houghton (Memorial Sloan-Kettering Cancer Center, New York, NY). The chemically induced melanomas JB/MS and JB/RH were isolated by Berkelhammer et al,22 and were provided by V. Hearing (National Cancer Institute, National Institutes of Health). The JB/RH cells do not express H-2Kb and are referred to here as JB/RHKb−. The B16Kb− cell line is a variant of B16F10 selected in our laboratory. A subline of B16F1 that does not express H-2Kb (B16F1Kb−) was provided by R. Vile (Mayo Clinic, Rochester MN). The murine thymoma EL4 and fresh aliquots of B16F0, B16F1, and B16F10 were obtained from ATCC. An immortalized, nontumorigenic melanosomal cell line from B6 mice23 was provided by R. Halaban (Yale University, New Haven, CT). All of the cell lines were cultured in medium supplemented with 10% fetal bovine serum, as recommended by the providers. Transfected B16 cells were prepared with SuperFect reagent (QIAGEN) using various cDNA plasmids: a dominant-negative IFNγR124 (IFNγRDN) construct (W. Lee, University of Pennsylvania, Philadelphia, PA); a short hairpin RNA (shRNA) construct for the immunoproteasome PA28α subunit25 (H. Udono, RIKEN Yokohama Institute, Yokohama, Japan); a construct encoding a single-chain trimer H-2Kb-β2M-Ova257 (scKbOva)26 molecule (J. Connolly, Washington University, St. Louis, MO); and a plasmid encoding the heavy chain of H-2Kb (L. Pease, Mayo Clinic, Rochester, MN). After transfection, stable clones were isolated with a combination of drug selection, flow cytometric sorting, and cell cloning at limiting dilutions. Expression of the transfected products was assessed by either flow cytometry or Western blot analysis.
Peptides, antibodies, and tetramers
Synthetic peptides representing the CD8 T-cell epitopes, Trp2180 (SVYDFFVWL; H-2Kb–restricted),27 a heteroclitic analog Trp1455 (TAPDNLGYM, H-2Db–restricted),28 Hugp10025 (KVPRNQDWL; H-2Db–restricted),20 and Ova55 (KVVRFDKL; H-2Kb–restricted) were purchased from A&A Laboratories. The purity (> 95%) and identity of peptides were determined by high-performance liquid chromatography and mass spectrometry analysis. Rat anti–mouse CD40 monoclonal antibody (FGK45.5) was prepared from hybridoma culture supernatants. Anti–mouse programmed death ligand-1 (PD-L1; 10F.9G2)29 was purchased from BioXCell. H-2Kb/Trp2180 and H-2Db/Trp1455 tetramers were provided by the National Institute of Allergy and Infectious Diseases (NIAID) Tetramer Facility (Emory University Vaccine Center, Atlanta, GA). Fluorescent antibodies for flow cytometry were purchased from eBioscience.
Immunizations
For TriVax immunizations, mice were injected intravenously with a mixture of 200 μg of peptide, 100 μg of anti-CD40 monoclonal antibody (mAb), and 50 μg of a stabilized form of poly-IC (Poly-ICLC/Hiltonol; Oncovir). Approximately 2 weeks after the primary immunization, the mice were given an identical booster. For generating Pmel-1 TCR-transgenic CTLs, B6 mice received 1 × 106 splenocytes from Pmel-1 mice, and the next day they were vaccinated with Hugp10025TriVax. CTLs for all in vitro immunologic assays were obtained from TriVax-immunized mice 6 to 7 days after the second immunization.
Evaluation of cellular immune responses
Immunologic assays were performed as described previously.16 Briefly, for tetramer staining, cells were stained with fluorescein isothiocyanate-anti-MHC class II, PerCP Cy5.5-anti-CD8α, and PE-conjugated tetramers. Fluorescence was measured using a FACSCalibur flow cytometer (BD Biosciences), and analyzed using FlowJo software (TreeStar). Tetramer analyses were done by gating out the MHC-II–positive population and gating on the CD8+ population. Percent tetramer-positive cells refers to the percentage of the CD8+ population. Enzyme-linked immunosorbent spot (ELISPOT) and enzyme-linked immunosorbent assay (ELISA) (IFNγ and TNFα) were performed using purified CD8 T cells (Miltenyi Biotec) following the directions provided by the manufacturer (Mabtech). Spot counting was done with an ELISPOT reader system (Autoimmun Diagnostika). For cytotoxicity determinations, conventional 5-hour chromium-release assays were performed using freshly isolated CD8 T cells (effectors) against various target cells that were labeled with 51Cr in triplicate in 96-well V-bottom plates using various effector-to-target (E:T) ratios.
Antitumor effects
To study the therapeutic effects of vaccination, mice first received subcutaneous or intravenous B16 tumor inoculations (3 × 105 or 1 × 105 cells, respectively), and 7 days later were given their first immunization. Antitumor effects were evaluated by examination and measurements of tumor masses or by counting the number of lung tumor nodules (∼ 4 weeks after tumor injections, when the mice in the unvaccinated control group started to appear ill). Tumor growth was monitored every 2 to 4 days in individual tagged mice by measuring 2 opposing diameters with a set of calipers. Results are presented as the mean tumor size (area in square millimeters) ± SD for every treatment group at various time points until the termination of the experiment.
Statistical analyses
Statistical significance to assess the numbers of antigen-specific CD8 T cells (ELISPOT), cytokine levels (ELISA), and absolute number of lung tumor nodules were determined by unpaired Student t tests. Tumor sizes between 2 populations throughout time and cytotoxicity assays at various E:T ratios were analyzed for significance using 2-way analysis of variance (ANOVA). All analyses and graphics were done using Prism 5.01 software (GraphPad).
Results
IFNγ inhibits the therapeutic effects of peptide vaccination
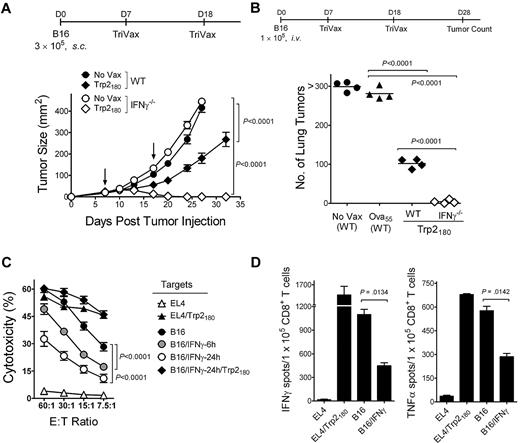
To evaluate the inhibitory effects of IFNγ in the therapeutic effectiveness of TriVax against established B16 tumors, responses of wild-type (WT) mice were compared with those obtained in IFNγ−/− mice in a 7-day subcutaneous tumor model using TriVax-containing Trp2180 peptide (H-2Kb restricted).27 All of the Trp2180TriVax-immunized IFNγ−/− mice rejected the tumors, while the WT mice did not, but WT mice were able to decrease the rate of tumor growth (Figure 1A). The inhibitory effects of IFNγ with Trp2180TriVax were also evident in a 7-day lung melanoma model (Figure 1B; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Similar results were obtained in the subcutaneous tumor model using TriVax containing a different T-cell epitope, Trp1455 (H-2Db–restricted28 ; supplemental Figure 1B).
IFNγ inhibits the therapeutic activity of peptide vaccination and decreases the capacity of CD8 T cells to recognize tumor cells. (A) Therapeutic effects induced by Trp2180TriVax against 7-day-established subcutaneous B16 tumors in WT and IFNγ−/− mice. Mice (4/group) were inoculated with tumor and vaccinated intravenously with TriVax (200 μg of Trp2180 peptide, 100 μg of anti-CD40 mAb, and 50 μg of poly-IC) on days 7 and 18 (arrows). Nonvaccinated mice (No Vax) were included as controls. Tumor sizes are presented as mean tumor areas in square millimeters. Points, mean for each group; bars, SD. (B) Mice (4/group) received B16 cells intravenously and were vaccinated with Trp2180TriVax or Ova55TriVax, as described. On day 28, the numbers of B16 pulmonary nodules were evaluated in individual mice. Horizontal lines, means of each group. (C) Freshly isolated purified CD8 T cells from Trp2180TriVax-immunized WT mice were evaluated for cytolytic activity against various targets: Trp2180 peptide-pulsed and unpulsed EL4 cells (triangles), nontreated B16, B16 incubated with 100 U/mL IFNγ for 6 or 24 hours (circles), and B16/IFNγ cells (24 hours) pulsed with Trp2180 peptide (diamonds). (D) Cytokine-release EliSpot assays using purified CD8 T cells from Trp2180TriVax-immunized WT mice were performed using several stimulator cells: Trp2180 peptide-pulsed and unpulsed EL4, IFNγ-treated (100 U/mL, 24 hours), and nontreated B16. P values were calculated with 2-way ANOVA (A,C) or unpaired Student t tests (B,D). Experiments were repeated 3 times with similar results.
IFNγ inhibits the therapeutic activity of peptide vaccination and decreases the capacity of CD8 T cells to recognize tumor cells. (A) Therapeutic effects induced by Trp2180TriVax against 7-day-established subcutaneous B16 tumors in WT and IFNγ−/− mice. Mice (4/group) were inoculated with tumor and vaccinated intravenously with TriVax (200 μg of Trp2180 peptide, 100 μg of anti-CD40 mAb, and 50 μg of poly-IC) on days 7 and 18 (arrows). Nonvaccinated mice (No Vax) were included as controls. Tumor sizes are presented as mean tumor areas in square millimeters. Points, mean for each group; bars, SD. (B) Mice (4/group) received B16 cells intravenously and were vaccinated with Trp2180TriVax or Ova55TriVax, as described. On day 28, the numbers of B16 pulmonary nodules were evaluated in individual mice. Horizontal lines, means of each group. (C) Freshly isolated purified CD8 T cells from Trp2180TriVax-immunized WT mice were evaluated for cytolytic activity against various targets: Trp2180 peptide-pulsed and unpulsed EL4 cells (triangles), nontreated B16, B16 incubated with 100 U/mL IFNγ for 6 or 24 hours (circles), and B16/IFNγ cells (24 hours) pulsed with Trp2180 peptide (diamonds). (D) Cytokine-release EliSpot assays using purified CD8 T cells from Trp2180TriVax-immunized WT mice were performed using several stimulator cells: Trp2180 peptide-pulsed and unpulsed EL4, IFNγ-treated (100 U/mL, 24 hours), and nontreated B16. P values were calculated with 2-way ANOVA (A,C) or unpaired Student t tests (B,D). Experiments were repeated 3 times with similar results.
IFNγ decreases the recognition of tumor cells by CTLs
These effects of IFNγ are confounding because this cytokine is associated with the production of antitumor effects.17,30 Moreover, it is known throughout the field that in vitro treatment of tumor cells with IFNγ increases their capacity to be recognized by CTLs. Notwithstanding this assumption, we observed the opposite effect, in which IFNγ-treated B16 decreased its susceptibility to lysis by freshly isolated CD8 T cells from Trp2180TriVax-immunized mice (Figure 1C). The inhibitory effects of IFNγ became evident as early as 6 hours after IFNγ treatment, but were more profound 24 hours after treatment. The IFNγ-treated B16 cells were effectively recognized by the CTLs if they were pulsed with Trp2180 peptide (Figure 1C), suggesting that IFNγ somehow decreases the antigenicity of the tumor cells. The decreased capacity of CD8 T cells from Trp2180TriVax-immunized mice to recognize the IFNγ-treated B16 was also evident in ELISPOT cytokine (IFNγ and TNFα) release assays (Figure 1D). The reduced antigenicity of the IFNγ-treated B16 was also observed using Trp1455TriVax-generated CD8 T cells (supplemental Figure 2A-B). The results obtained in the ELISPOT assays indicate that approximately 50% of tumor-reactive CD8 T cells were unable to recognize IFNγ-treated B16. Furthermore, visual examination of the ELISPOT plates clearly showed that the intensity of the spots, which is correlated with the amount of cytokine produced by each cell, was substantially lower in wells containing IFNγ-treated B16 compared with wells containing untreated B16 (supplemental Figure 2C). Notably, the decreased CD8 T-cell response observed with IFNγ-treated B16 was not evident when the tumor cells were treated with other cytokines such as IFNα, TNFα, or interleukin-10 (IL-10; supplemental Figure 3). It is possible that the unexpected observations on the negative effects of IFNγ could be limited to the particular B16 cell line that we were using (B16F10).21 However, 3 separate B16 cell lines (B16F0, B16F1, and B16F10, which were obtained directly from ATCC), a chemically induced melanoma cell line (JB/MS),22 and an immortalized, nontumorigenic melanocyte cell line23 all behaved in the same manner (supplemental Figure 4).
The inhibitory effect of IFNγ primarily targets the tumor
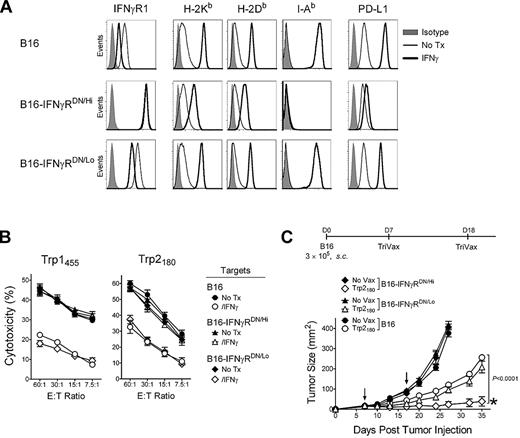
The above observations suggest that the inhibitory effects of IFNγ in the eradication of established tumors by TriVax could be due to effects that solely affect the tumor cells, such as the decrease of the antigenicity of B16 for MHC-I restricted CD8 T cells. However, it is also possible that IFNγ could exert additional inhibitory activities in other cells within the host, decreasing the effectiveness of TriVax. Thus, to evaluate whether the suppression by IFNγ was mostly mediated via its effects on the tumor, the B16 tumor was transduced with a plasmid a encoding a dominant-negative IFNγ receptor (IFNγRDN).24 Two B16 clones were isolated, one expressing high levels of IFNγRDN (B16-IFNγRDN/Hi) and another one expressing low IFNγRDN levels (B16-IFNγRDN/Lo). Treatment of the B16-IFNγRDN/Hi with IFNγ enhanced expression of MHC-I, MHC-II, or PD-L1 significantly less than B16 and B16-IFNγRDN/Lo (Figure 2A), and did not reduce the reactivity of these cells with the antigen-specific CD8 T cells compared with the nontransduced B16 cells or the B16-IFNγRDN/Lo cells (Figure 2B). More importantly, the therapeutic effectiveness of Trp2180TriVax was significantly higher against B16-IFNγRDN/Hi compared with B16-IFNγRDN/Lo or the parental B16 (Figure 2C). These results suggest that the inhibitory effects of IFNγ in limiting the effectiveness of TriVax are mediated mostly though its direct activity on the tumor.
Expression of IFNγRDN in tumor cells overcomes the inhibitory effects of IFNγ. (A) Expression levels of IFNγR1, MHC-I (H-2Kb and H-2Db), MHC-II (I-Ab), and PD-L1 on B16 and 2 stable B16 clones expressing high or low levels of a IFNγRDN receptor. B16, B16-IFNγRDN/Hi, and B16-IFNγRDN/Lo cells were incubated (IFNγ) or not (No Tx) with 100 U/mL of IFNγ for 40 hours, and stained with specific antibodies as indicated, followed by flow-cytometry analysis. (B) Freshly isolated and purified CD8 T cells from Trp1455TriVax or Trp2180TriVax-immunized WT mice (as indicated) were tested for cytolytic activity against several target cells treated (IFNγ) and nontreated (No Tx) with IFNγ (100 U/mL, 24 hours): Parental B16 and 2 stable B16 clones expressing high (B16-IFNγRDN/Hi) or low (B16-IFNγRDN/Lo) levels of IFNγRDN. (C) Therapeutic effects induced by Trp2180TriVax in WT mice against 7-day-established subcutaneous B16 tumors expressing or not IFNγRDN. P value (2-way ANOVA) compares Trp2180TriVax-immunized mice bearing B16-IFNγRDN/Hi with Trp2180TriVax-immunized mice bearing B16. *50% of the Trp2180TriVax-immunized mice bearing B16-IFNγRDN/Hi rejected their tumors. These experiments were repeated twice with similar results.
Expression of IFNγRDN in tumor cells overcomes the inhibitory effects of IFNγ. (A) Expression levels of IFNγR1, MHC-I (H-2Kb and H-2Db), MHC-II (I-Ab), and PD-L1 on B16 and 2 stable B16 clones expressing high or low levels of a IFNγRDN receptor. B16, B16-IFNγRDN/Hi, and B16-IFNγRDN/Lo cells were incubated (IFNγ) or not (No Tx) with 100 U/mL of IFNγ for 40 hours, and stained with specific antibodies as indicated, followed by flow-cytometry analysis. (B) Freshly isolated and purified CD8 T cells from Trp1455TriVax or Trp2180TriVax-immunized WT mice (as indicated) were tested for cytolytic activity against several target cells treated (IFNγ) and nontreated (No Tx) with IFNγ (100 U/mL, 24 hours): Parental B16 and 2 stable B16 clones expressing high (B16-IFNγRDN/Hi) or low (B16-IFNγRDN/Lo) levels of IFNγRDN. (C) Therapeutic effects induced by Trp2180TriVax in WT mice against 7-day-established subcutaneous B16 tumors expressing or not IFNγRDN. P value (2-way ANOVA) compares Trp2180TriVax-immunized mice bearing B16-IFNγRDN/Hi with Trp2180TriVax-immunized mice bearing B16. *50% of the Trp2180TriVax-immunized mice bearing B16-IFNγRDN/Hi rejected their tumors. These experiments were repeated twice with similar results.
Mechanisms involved in the inhibitory effects of IFNγ
A gene-chip analysis has revealed that IFNγ increases the expression (> 5-fold) of more than 1000 genes in B16 (data not shown), and many of these genes could be involved in decreasing tumor-cell recognition by CD8 T lymphocytes. One of the most likely explanations could be that the increased expression of the immunoproteasome by IFNγ could reduce the production of the Trp2180 and Trp1455 epitopes. There are reports that immunoproteasomes reduce the generation of some melanoma CTL epitopes.31 However, there is evidence that processing of the Trp2180 epitope is not compromised by the immunoproteasome.25,32 Nevertheless, interference RNA technology with the shRNA plasmid was used to inhibit the expression of the PA28α subunit,25 which is required for the immunoproteasome assembly and function. Although a significant reduction (> 90%) in the expression of PA28α in shPA28α-transduced B16 was achieved, these cells were not recognized by CTLs when treated with IFNγ (supplemental Figure 5). It has been reported that IFNγ can alter the extent of CD8 T-cell responses by inducing the expression of indoleamine 2,3-dioxygenase (IDO), which depletes tryptophan from the microenvironment.33 In addition, IFNγ can trigger autophagy, altering the cell's antigenic composition.34,35 Nevertheless, the addition of 1-methyl tryptophan (1MT, an IDO inhibitor),33 an excess of L-tryptophan, or inhibitors of autophagy (rapamycin and wortmannin) also fail to reverse the inhibitory effects of IFNγ on tumor-cell recognition by CD8 T cells (supplemental Figure 6A-B). Another possible way that IFNγ could reduce the capacity of CD8 T cells to interact with B16 cells could be through the increased expression of the inhibitory ligand PD-L1 (Figure 2A). However, adding blocking antibodies to PD-L1 or to PD1 to the in vitro immunologic assays did not reverse the effects of IFNγ (supplemental Figure 6C). Notwithstanding these findings, we observed that the implementation of in vivo PD1 blockade significantly increased the therapeutic effectiveness of Trp2180TriVax and Trp1455TriVax (Figure 3A-B). However, no tumor eradications like those observed in the IFNγ−/− mice were obtained, indicating that IFNγ inhibits the effectiveness of TriVax through more than one mechanism. Administration of the IDO inhibitor 1MT to tumor-bearing mice did not significantly increase the effectiveness of TriVax (supplemental Figure 7), suggesting that this inhibitory pathway may not be relevant in this model.
PD1 blockade increases the therapeutic efficacy of TriVax. WT mice (4/group) were inoculated subcutaneously with B16, and vaccinated with Trp2180TriVax (A) or Trp1455TriVax (B). Anti-PD-L1 mAb (10F.9G2) was administered intraperitoneally on days 2, 4, 6, and 8 after each TriVax administration at 200 μg/dose. Nonvaccinated mice (No Vax) and Ova55TriVax were included as controls. Arrows, days when TriVax administered; gray bars, time when anti–PD-L1 mAb was administered. Tumor sizes were determined in individual mice by measuring 2 opposing diameters and are presented as tumor areas in square millimeters. Points indicate means for each group of mice; and bars, SD. P values were calculated using 2-way ANOVA test comparing with the TriVax alone with TriVax plus anti–PD-L1 mAb. These experiments were repeated twice with similar results.
PD1 blockade increases the therapeutic efficacy of TriVax. WT mice (4/group) were inoculated subcutaneously with B16, and vaccinated with Trp2180TriVax (A) or Trp1455TriVax (B). Anti-PD-L1 mAb (10F.9G2) was administered intraperitoneally on days 2, 4, 6, and 8 after each TriVax administration at 200 μg/dose. Nonvaccinated mice (No Vax) and Ova55TriVax were included as controls. Arrows, days when TriVax administered; gray bars, time when anti–PD-L1 mAb was administered. Tumor sizes were determined in individual mice by measuring 2 opposing diameters and are presented as tumor areas in square millimeters. Points indicate means for each group of mice; and bars, SD. P values were calculated using 2-way ANOVA test comparing with the TriVax alone with TriVax plus anti–PD-L1 mAb. These experiments were repeated twice with similar results.
Noncognate MHC-I levels reduce the ability of CTL to recognize tumor cells
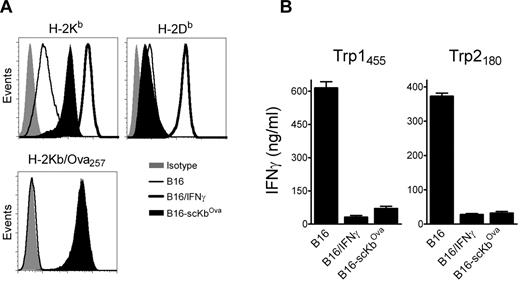
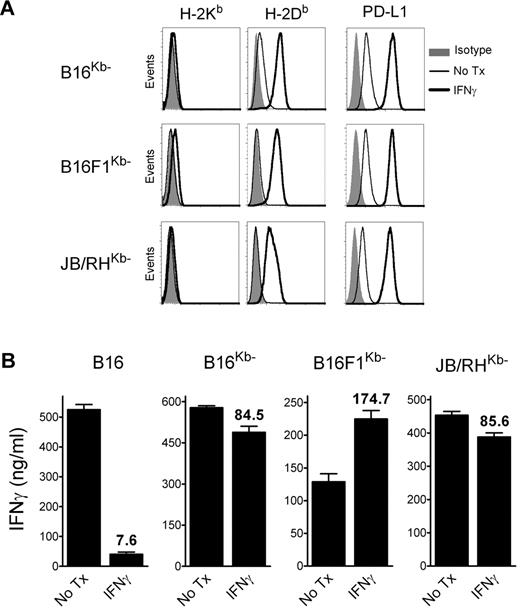
The above results suggested that, in addition to inhibiting CD8 T-cell function in vivo via the PD1 pathway, IFNγ decreases the overall antigenicity of melanoma cells in an immunoproteasome-independent manner, and that both mechanisms interfere with the therapeutic effectiveness of TriVax. We hypothesized that the CD8 T-cell-epitope density (ratio of cognate peptide/MHC-I complexes to total peptide/MHC-I complexes), and not necessarily the total amount of cognate peptide/MHC-I complexes per cell, could be critical for determining whether CD8 T lymphocytes recognize antigen-presenting cells (APCs). Thus, a large increase of noncognate peptide/MHC-I complexes induced by IFNγ would decrease the T-cell-epitope density if the cognate peptide/MHC-I complexes were not increased proportionally. To test this hypothesis, B16 cells expressing high levels of noncognate peptide/MHC-I complexes were produced by transfection using a plasmid encoding for a single-chain trimer H-2Kb-β2M-Ova257 (scKbOva) construct that allows the expression of surface peptide/MHC-I complexes in a transporter associated with antigen processing (TAP)- and proteasome-independent manner.26 Furthermore, the use of B16-scKbOva cells allowed an assessment of whether an increase of noncognate peptide/MHC-I in the absence of IFNγ treatment would decrease the tumor cell's antigenicity with CD8 T cells. The total levels of H-2Kb increased approximately 10-fold in the B16-scKbOva cells compared with the parental B16 cells, while IFNγ treatment increased by approximately 50-fold the expression of H-2Kb in the B16 cells; however, the levels of H-2Db in the B16-scKbOva cells were not significantly different (Figure 4A). In agreement with our hypothesis, the presence of noncognate Ova257/H-2Kb complexes in B16 cells in the absence of IFNγ treatment reduced the reactivity of the tumor cells with Trp1455- and Trp2180-reactive CD8 T cells (Figure 4B). The extent of the reduction of the T-cell response was similar to the one observed using IFNγ-treated B16. Another way to test the epitope-density hypothesis would be to lessen the large increase of noncognate peptide/MHC-I complexes induced by IFNγ. B16 tumors deficient in H-2Kb (H-2Kb-loss variants) would express lower levels of total surface MHC-I after IFNγ treatment compared with the parental B16 cells, and should be efficiently recognized by H-2Db-restricted CD8 T cells. Treatment of 3 different H-2Kb-loss variants, B16F1Kb−, B16Kb− (isolated by us), and JB/RHKb− (a chemically induced melanoma22 ), with IFNγ increased the expression of H-2Db and PD-L1, but not H-2Kb (Figure 5A). The effect of IFNγ on the immunogenicity of the H-2Kb− cells was assessed using freshly isolated Trp1455TriVax-derived CD8 T cells. As shown in Figure 5B, IFNγ treatment minimally decreased the antigenicity of B16Kb− and JB/RHKb−. Conversely, a substantial increase of antigenicity was observed in B16F1Kb−, while a substantial decrease was seen with the H-2Kb–expressing B16 cells. Similar findings were observed using Pmel-1 TCR-transgenic CD8 T cells specific for the H-2Db–restricted gp10025 epitope,20 except that IFNγ increased the antigenicity of all 3 H-2Kb-loss variants (supplemental Figure 8).
Expression levels of noncognate peptide/MHC-I complexes dictate the antigenicity of B16 cells. (A) Expression levels of MHC-I (H-2Kb and H-2Db, top panels) and H-2Kb/Ova257 complexes (bottom panel) on a stable B16 clone expressing single-chain H-2Kb/Ova257 (B16-scKbOva) compared with B16 and IFNγ-treated B16 (100 U/mL, 24 hours) cells measured by flow cytometry. Levels of H-2Kb/Ova257 complexes were measured using antibody 25-D1.16.53 (B) Antigenicity of B16-scKbOva was evaluated with freshly isolated CD8 T cells from Trp1455TriVax- and Trp2180TriVax-immunized WT mice using cytokine release ELISA. IFNγ-treated (100 U/mL, 24 hours) and nontreated parental B16 cells were included for comparison. Cultures consisted of 3 × 105 CD8 T cells coincubated with 1 × 105 stimulator cells for 40 hours before removing culture supernatants for cytokine measurements. Results represent the average values of IFNγ (columns) and SD (error bars) from triplicate wells. These experiments were repeated twice with similar results.
Expression levels of noncognate peptide/MHC-I complexes dictate the antigenicity of B16 cells. (A) Expression levels of MHC-I (H-2Kb and H-2Db, top panels) and H-2Kb/Ova257 complexes (bottom panel) on a stable B16 clone expressing single-chain H-2Kb/Ova257 (B16-scKbOva) compared with B16 and IFNγ-treated B16 (100 U/mL, 24 hours) cells measured by flow cytometry. Levels of H-2Kb/Ova257 complexes were measured using antibody 25-D1.16.53 (B) Antigenicity of B16-scKbOva was evaluated with freshly isolated CD8 T cells from Trp1455TriVax- and Trp2180TriVax-immunized WT mice using cytokine release ELISA. IFNγ-treated (100 U/mL, 24 hours) and nontreated parental B16 cells were included for comparison. Cultures consisted of 3 × 105 CD8 T cells coincubated with 1 × 105 stimulator cells for 40 hours before removing culture supernatants for cytokine measurements. Results represent the average values of IFNγ (columns) and SD (error bars) from triplicate wells. These experiments were repeated twice with similar results.
IFNγ-treated H-2Kb-loss tumor variants are recognized by H-2Db–restricted CD8 T cells. (A) Expression levels of MHC-I and PD-L1 on H-2Kb–loss variants (B16Kb−, B16F1Kb−, and JB/RHKb−). The cells were incubated (IFNγ) or not (No Tx) with 100 U/mL of IFNγ for 24 hours, and stained with antibodies as indicated, followed by flow cytometric analysis. (B) CD8 T cells, which were freshly isolated from mice vaccinated with Trp1455TriVax (H-2Db–restricted), were evaluated for their capacity to recognize B16, 2 B16 H-2Kb-loss variants (B16Kb− and B16F1Kb−), and an H-2Kb− chemically induced melanoma (JB/RHKb−) using a cytokine-release ELISA assay. The cells were either incubated with IFNγ (100 U/mL, 24 hours) or not (No Tx). Cultures consisted of 3 × 105 CD8 T cells coincubated with stimulator cells (3:1 ratio) for 40 hours before removing culture supernatants for cytokine measurements. Results represent the average values of IFNγ (columns) and SD (error bars) from triplicate wells. Numbers above columns represent the percentage response of the IFNγ-treated cells compared with that of the nontreated group.
IFNγ-treated H-2Kb-loss tumor variants are recognized by H-2Db–restricted CD8 T cells. (A) Expression levels of MHC-I and PD-L1 on H-2Kb–loss variants (B16Kb−, B16F1Kb−, and JB/RHKb−). The cells were incubated (IFNγ) or not (No Tx) with 100 U/mL of IFNγ for 24 hours, and stained with antibodies as indicated, followed by flow cytometric analysis. (B) CD8 T cells, which were freshly isolated from mice vaccinated with Trp1455TriVax (H-2Db–restricted), were evaluated for their capacity to recognize B16, 2 B16 H-2Kb-loss variants (B16Kb− and B16F1Kb−), and an H-2Kb− chemically induced melanoma (JB/RHKb−) using a cytokine-release ELISA assay. The cells were either incubated with IFNγ (100 U/mL, 24 hours) or not (No Tx). Cultures consisted of 3 × 105 CD8 T cells coincubated with stimulator cells (3:1 ratio) for 40 hours before removing culture supernatants for cytokine measurements. Results represent the average values of IFNγ (columns) and SD (error bars) from triplicate wells. Numbers above columns represent the percentage response of the IFNγ-treated cells compared with that of the nontreated group.
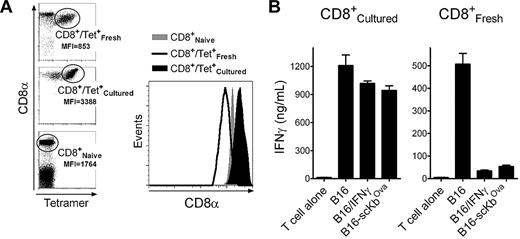
Increasing CD8 coreceptor levels overcomes the inhibition of IFNγ
The above results indicate that the reduction in immunogenicity observed in the IFNγ-treated melanoma cells was due to a large increase in noncognate peptide/MHC-I complexes. It is possible that such increase could hinder the ability of TCR to encounter the respective cognate peptide/MHC-I complexes. Alternatively, the possibility exists that the large amount of noncognate peptide/MHC-I sequesters CD8 coreceptor/Lck complexes away from the cognate peptide/MHC molecules in the immune synapse, decreasing Lck function and interfering with proper T-cell activation. To investigate the second possibility, we took advantage of the observation that placing CD8 T cells from TriVax-immunized mice in tissue culture for 1 week resulted in enhanced expression levels (∼ 4-fold) of surface CD8 molecules compared with the freshly isolated T cells (Figure 6A). By increasing the levels of CD8 on the T cells, it should be possible to offset the inhibition caused by an excess of noncognate peptide/MHC-I. Indeed, tissue-cultured Trp1455-reactive CD8 T cells were significantly more effective in recognizing the IFNγ-treated B16 and the B16-scKbOva cells compared with freshly isolated Trp1455-reactive T cells (Figure 6B). Similar results were obtained using Pmel-1 T cells (supplemental Figure 9) and TriVax-generated Trp2180 reactive CD8 T cells (data not presented). Although these results suggest that the enhancement of CD8 coreceptor expression in the cultured T lymphocytes could be responsible for overcoming the negative effects of IFNγ on tumor cell recognition, it is possible that other changes on the T cells when they were placed in tissue culture may have helped to increase their function.
Up-regulation of CD8 coreceptors after in vitro culture restores the capacity of T cells to recognize B16 cells expressing high levels of noncognate MHC-I. Tissue-culture CD8 T cells were produced by placing the purified CD8 T cells from Trp1455TriVax-immunized mice in medium containing 50 U/mL IL-2 and 20 ng/mL IL-7 for 7 days. (A) Comparison of the levels of CD8α expression between freshly isolated and cultured CD8 T cells from Trp1455TriVax-immunized mice compared with naive CD8 T cells from nonvaccinated mice. MFI, mean fluorescence intensity of CD8α. Right panel shows histograms gating on the Trp1455 tetramer-positive populations. (B) Antigen-induced IFNγ production of cultured and freshly isolated CD8 T cells from Trp1455TriVax-immunized mice evaluated by ELISA. CD8 T cells were evaluated for their capacity to recognize B16 treated or not with IFNγ (100 U/mL, 24 hours) and B16-scKbOva. CD8 T cells (3 × 105) were incubated with tumor cells (1 × 105) for 40 hours, and supernatants were removed for cytokine measurements. Supernatants from T cells without tumor cells (T cell alone) were included as controls. Results represent the average amounts of IFNγ and SD (error bars) from triplicate cultures. These experiments were repeated twice with similar results.
Up-regulation of CD8 coreceptors after in vitro culture restores the capacity of T cells to recognize B16 cells expressing high levels of noncognate MHC-I. Tissue-culture CD8 T cells were produced by placing the purified CD8 T cells from Trp1455TriVax-immunized mice in medium containing 50 U/mL IL-2 and 20 ng/mL IL-7 for 7 days. (A) Comparison of the levels of CD8α expression between freshly isolated and cultured CD8 T cells from Trp1455TriVax-immunized mice compared with naive CD8 T cells from nonvaccinated mice. MFI, mean fluorescence intensity of CD8α. Right panel shows histograms gating on the Trp1455 tetramer-positive populations. (B) Antigen-induced IFNγ production of cultured and freshly isolated CD8 T cells from Trp1455TriVax-immunized mice evaluated by ELISA. CD8 T cells were evaluated for their capacity to recognize B16 treated or not with IFNγ (100 U/mL, 24 hours) and B16-scKbOva. CD8 T cells (3 × 105) were incubated with tumor cells (1 × 105) for 40 hours, and supernatants were removed for cytokine measurements. Supernatants from T cells without tumor cells (T cell alone) were included as controls. Results represent the average amounts of IFNγ and SD (error bars) from triplicate cultures. These experiments were repeated twice with similar results.
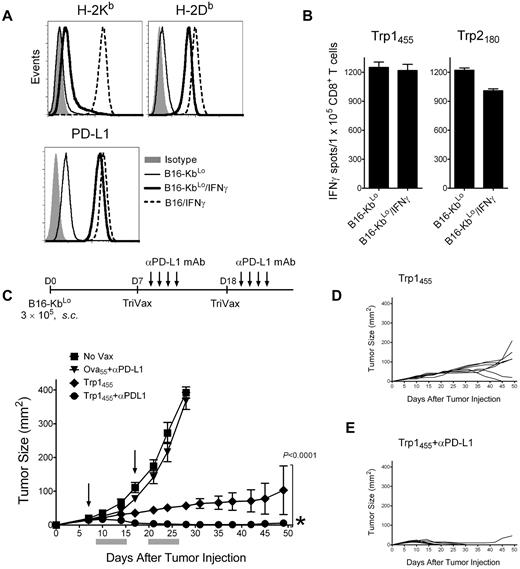
Overcoming the negative effects of IFNγ results in tumor eradication by TriVax
In view of the above results, TriVax should be highly effective in controlling tumors that do not substantially increase the levels of noncognate MHC-I as the result of IFNγ. Thus, a B16 tumor line expressing low levels of H-2Kb after IFNγ treatment was produced by transfecting B16Kb− cells with a plasmid encoding the heavy chain of H-2Kb. The resulting B16-KbLo cell line up-regulated to the same extent the levels of H-2Db (and PD-L1) compared with the parental B16 in response to IFNγ (Figure 7A). In contrast, the levels of H-2Kb in the IFNγ-treated B16-KbLo cells were approximately 10-fold lower compared with the IFNγ-treated parental B16 cells. As predicted, IFNγ treatment of B16-KbLo did not substantially reduce their reactivity with Trp1455 or Trp2180 CD8 T cells (Figure 7B). Next, we assessed the therapeutic effectiveness of Trp1455TriVax against 7-day-established, subcutaneous B16-KbLo tumors. Because the B16-KbLo expresses high PD-L1 levels when exposed to IFNγ (Figure 7A), one group of mice received PD1 blockade therapy in addition to TriVax. The results presented in Figure 7C-E show that TriVax immunization was effective in completely eradicating large, established tumors in the majority of the mice when administered in combination with anti–PD-L1 antibodies, with tumors that do not overly express noncognate MHC-I as a consequence of exposure to IFNγ. Furthermore, even in the absence of PD1 blockade, the therapeutic effects of TriVax were quite dramatic. Similar remarkable results were obtained in a more advanced disease stage (12-day-established B16-KbLo subcutaneous tumors; supplemental Figure 10).
Decreasing noncognate MHC-I levels together with PD1 blockade allows TriVax to eliminate advanced tumors in WT mice. (A) Expression levels of H-2Kb, H-2Db, and PD-L1 in B16-KbLo cells treated or not with IFNγ (100 U/mL, 24 hours). Results with IFNγ-treated parental B16 cells are included for comparison. (B) Responses (ELISPOT) of freshly isolated CD8 T cells from Trp1455TriVax- and Trp2180TriVax-immunized WT mice against IFNγ treated and nontreated B16-KbLo cells. Results represent the average number of spots from triplicate wells with SD (error bars) of the means. (C) Therapeutic effects induced by Trp1455TriVax against 7-day-established B16-KbLo tumors in WT mice. Mice (8/group) were inoculated with B16-KbLo cells and immunized with Trp1455TriVax or Ova55TriVax as indicated. Anti-PD-L1 mAb was administered intraperitoneally on days 2, 4, 6, and 8 after TriVax administration. Nonvaccinated mice (No Vax) were also included as controls. Arrows, days when the vaccines were administered; gray bars, period of anti–PD-L1 mAb treatment. (D-E). Tumor-growth curves are shown for individual mice from the Trp1455TriVax and Trp1455TriVax plus anti–PD-L1 groups. Tumor sizes were determined in individual mice by measurements of 2 opposing diameters and are presented as tumor areas in square millimeters. Points, mean for each group of mice; bars, SD. P < .0001, between the Trp1455TriVax and the Trp1455TriVax + anti–PD-L1 mAb group (obtained using a 2-way ANOVA analysis). *Seven of 8 mice in Trp1455TriVax + anti–PD-L1 mAb rejected their tumors; **1 mouse from Trp1455TriVax rejected its tumor. These experiments were repeated twice with similar results.
Decreasing noncognate MHC-I levels together with PD1 blockade allows TriVax to eliminate advanced tumors in WT mice. (A) Expression levels of H-2Kb, H-2Db, and PD-L1 in B16-KbLo cells treated or not with IFNγ (100 U/mL, 24 hours). Results with IFNγ-treated parental B16 cells are included for comparison. (B) Responses (ELISPOT) of freshly isolated CD8 T cells from Trp1455TriVax- and Trp2180TriVax-immunized WT mice against IFNγ treated and nontreated B16-KbLo cells. Results represent the average number of spots from triplicate wells with SD (error bars) of the means. (C) Therapeutic effects induced by Trp1455TriVax against 7-day-established B16-KbLo tumors in WT mice. Mice (8/group) were inoculated with B16-KbLo cells and immunized with Trp1455TriVax or Ova55TriVax as indicated. Anti-PD-L1 mAb was administered intraperitoneally on days 2, 4, 6, and 8 after TriVax administration. Nonvaccinated mice (No Vax) were also included as controls. Arrows, days when the vaccines were administered; gray bars, period of anti–PD-L1 mAb treatment. (D-E). Tumor-growth curves are shown for individual mice from the Trp1455TriVax and Trp1455TriVax plus anti–PD-L1 groups. Tumor sizes were determined in individual mice by measurements of 2 opposing diameters and are presented as tumor areas in square millimeters. Points, mean for each group of mice; bars, SD. P < .0001, between the Trp1455TriVax and the Trp1455TriVax + anti–PD-L1 mAb group (obtained using a 2-way ANOVA analysis). *Seven of 8 mice in Trp1455TriVax + anti–PD-L1 mAb rejected their tumors; **1 mouse from Trp1455TriVax rejected its tumor. These experiments were repeated twice with similar results.
Discussion
We have demonstrated that IFNγ interferes with the effectiveness of antigen-specific CD8 T cells to eliminate tumors with a therapeutic peptide vaccine in a mouse melanoma model. At the present time, we do not know if these observations will extend to other types of vaccines, other tumor types, or human tumors. However, it is likely that in any circumstance in which tumors are induced to express high levels of noncognate MHC-I and PD-L1, effective recognition by CD8 T cells may be compromised. The present findings are perplexing because IFNγ has long been considered to provide antitumor benefits through its antiproliferative activity and its ability to enhance antigen processing for both MHC-I and MHC-II pathways.30 However, our results indicate that by blocking the function of IFNγ directly on the tumor cells, vaccine-generated CD8 T lymphocytes were able to eradicate large, established tumors. IFNγ generates tumor resistance to MHC-I–restricted CD8 T lymphocytes through 2 main mechanisms: (1) induction of high levels of PD-L1 (B7-H1), which inhibits the survival and function of T lymphocytes,36-39 and (2) as described here, by reducing the capacity of the tumor cells to be recognized by CD8 T cells. It is known that exposure to IFNγ enhances the expression of PD-L1 in numerous cell types, including tumors.39 Because signaling of PD1 reduces the ability of T lymphocytes to become fully activated, proliferate, and exert effector function, PD1 blockade using anti-PD1 or anti PD-L1 antibodies has been used to improve the therapeutic effectiveness of T cells against chronic viral infections37 and tumors.40 Indeed, in combination with TriVax, PD1 blockade significantly increased the antitumor effects against B16 melanoma, but no complete cures were achieved. It was not so well known—and was actually counterintuitive—that IFNγ would reduce the capacity of tumor cells to stimulate CD8 T lymphocytes, since this cytokine is known to enhance the expression and function of various antigen-processing components.30 We considered the possibility that immunoproteasomes induced by IFNγ reduced the generation of the T-cell epitopes studied here in a manner similar to that described in other systems.31 However, reports in the literature for Trp218025,32 and our results with Trp1455 using shRNA to inhibit the immunoproteasome do not support this possibility. Conversely, a substantial increase of specific peptide/MHC-I complexes induced by IFNγ could result in the induction of high-antigen-dose tolerance (or anergy), inhibiting T-cell effector function.41,42 However, the results indicate that this was not the case in our model system, because the addition of exogenous peptide to the IFNγ-treated B16 tumor cells resulted in enhanced T-cell recognition of the tumor cells (Figure 1C).
In addition to the effects that IFNγ exerts directly on the tumor cells, which decrease the efficacy of TriVax, it is possible that this cytokine could also affect quantitatively and qualitatively the CD8 T-cell response to vaccination. Indeed, we observed that the cytolytic activity of TriVax-generated CTLs from IFNγ-deficient mice was slightly higher (∼ 20%) compared with CTLs from WT mice.16 In contrast, we found no significant differences in the percentages of tetramer-positive CD8 T cells (or the total numbers of tumor-reactive T cells) between IFNγ and WT mice in the same studies. It has been reported in some cancer models that the administration of IL-12 induces effective antitumor responses through the expression of the IFNγ-inducible chemokines interferon γ–induced protein 10 kDa (IP-10) and monokine induced by IFN-γ (MIG).43,44 Our studies in IFNγ-deficient mice suggest that efficient infiltration of TriVax-generated CD8 T cells occurs in the absence of IFNγ. It will be important to study the type of signals that attract the TriVax-generated CD8 T cells into the tumor site and other effects that this cytokine may exert on the tumor-bearing host that could positively or negatively affect the outcome of therapeutic vaccination.
The results presented herein point to the likelihood that high levels of noncognate MHC-I induced by IFNγ on tumor cells could be detrimental to their recognition by CTLs. This possibility is supported by the findings that the expression of high levels of noncognate MHC-I in the tumor cells without IFNγ treatment using the scKbOva construct reproduced the results using IFNγ-treated B16. Moreover, the results using H-2Kb− cells that generate lower levels of total noncognate MHC-I compared with the parental cells after IFNγ treatment provide additional support to this novel concept. Although we do not know exactly how an excess of noncognate MHC-I on APCs could decrease the ability of CD8 T lymphocytes to become activated when recognizing antigen, we considered the possibility that the noncognate MHC-I could be competing with cognate MHC-I for access to CD8 molecules on the T cells. Our results showing that augmenting the expression of CD8 molecules using tissue-cultured T cells reduced the inhibitory effects of IFNγ agree with this presumption. The CD8 coreceptor plays an important role in T-cell activation by delivering the Src-family tyrosine kinase Lck into the proximity of the CD3 subunits in the TCR complex.45-47 Lck sequestration from the TCR/CD3 complex plays a role in T cell–positive selection in the thymus,48 and it has been proposed to regulate ongoing immune responses and to help maintain peripheral tolerance and MHC restriction.49-51 The present findings indicate that coreceptor tuning for MHC-I–restricted T lymphocytes is not only regulated by levels of CD8 on the T cells, but also by the overall levels of MHC-I on the APCs. While low levels of noncognate MHC-I may enhance APC/T-cell interactions by facilitating cell-to-cell adhesion,52 high levels of noncognate MHC-I decrease T-cell activation.
We believe that the reduction of tumor-cell reactivity with MHC-I–restricted T lymphocytes induced by IFNγ has not been previously demonstrated because most researchers perform in vitro cultures (to expand and activate the effector T cells) before performing immunologic tests such as cytotoxicity assays and, as shown here, the increase of CD8 coreceptor levels obscures this phenomenon. In addition, in many cases, immune responses using cytokine-release assays (ELISPOT and intracellular cytokine staining) are only measured using peptide-pulsed APCs and not tumor cells. Because TriVax generates such extensive T-cell responses, we were able to perform cytotoxicity assays using freshly isolated CD8 T cells, which express lower levels of CD8 coreceptor molecules compared with cultured T cells. Thus, the use of tumor cells in ELISPOT/ELISA assays and the use of freshly isolated CD8 T cells in cytotoxicity assays allowed us to recognize this novel mechanism of immune evasion evoked by tumors as a consequence of exposure to IFNγ. It is tempting to speculate that one of the functions of IFNγ during infections is to protect noninfected cells from cross-reactive T cells by increasing noncognate MHC-I (and PD-L1) levels, and that tumor cells simply maintain this benefit to avoid immune destruction. Nevertheless, our results suggest that blocking the action of IFNγ in cancer patients undergoing T cell–based immunotherapy could improve their therapeutic outcome.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank A. Salazar for providing Poly-ICLC (Hiltonol) and the NIH Tetramer Core Facility for providing peptide/MHC tetramers.
This work was supported by NIH grants R01CA103921 and R01CA136828 and by funds provided by the Donald A. Adam Comprehensive Melanoma Research Center of the Moffitt Cancer Center and the Bankhead Coley Pre-Specialized Programs of Research Excellence at the Moffitt Cancer Center.
National Institutes of Health
Authorship
Contribution: E.C. and H.C. designed and conceptualized the research, analyzed the data, and wrote the manuscript; and H.C. and Y.L. performed all of the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Esteban Celis, Moffitt Cancer Center, 12902 Magnolia Dr, SRB2, Tampa, FL 33612; e-mail: ecelis@moffitt.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal