The serum ferritin concentration is a clinical parameter measured widely for the differential diagnosis of anemia. Its levels increase with elevations of tissue iron stores and with inflammation, but studies on cellular sources of serum ferritin as well as its subunit composition, degree of iron loading and glycosylation have given rise to conflicting results. To gain further understanding of serum ferritin, we have used traditional and modern methodologies to characterize mouse serum ferritin. We find that both splenic macrophages and proximal tubule cells of the kidney are possible cellular sources for serum ferritin and that serum ferritin is secreted by cells rather than being the product of a cytosolic leak from damaged cells. Mouse serum ferritin is composed mostly of L-subunits, whereas it contains few H-subunits and iron content is low. L-subunits of serum ferritin are frequently truncated at the C-terminus, giving rise to a characteristic 17-kD band that has been previously observed in lysosomal ferritin. Taken together with the fact that mouse serum ferritin is not detectably glycosylated, we propose that mouse serum ferritin is secreted through the nonclassical lysosomal secretory pathway.
Introduction
Ferritin in mammals is a mostly intracellular, cytosolic iron storage and detoxification protein. It consists of H- and L-subunits that assemble into a 24-subunit hollow sphere in which iron is sequestered.1 However, ferritin can also be detected extracellularly, in cerebrospinal fluid and sinovial fluid. In serum it is an extracellular ferritin that has been used extensively in diagnostic tests. In the clinical setting, serum ferritin evaluation is most commonly used to estimate body iron stores as low serum ferritin correlates with iron depletion, whereas high serum ferritin correlates with elevated body iron stores or with inflammation in patients with normal body iron stores.2,3
Characterization of serum ferritin has produced many controversial results regarding subunit composition, iron content and other features. It has been compared in some studies to the “natural apoferritin” fraction found in many tissues, which is essentially devoid of iron4 while other studies have claimed that serum ferritin contains considerable amounts of iron.5
In insects, ferritin is a secreted protein composed of 2 types of subunits and the heteropolymer functions most likely as an iron transporter in the hemolymph. Each of the subunits has a classical secretion signal and ferritin is secreted from the insect fat body.6 In contrast, mammalian H- and L-ferritin subunits lack signals mediating endoplasmic reticulum (ER) targeting, but ferritin can traffic to the lysosomal compartment through autophagy.7 Binding of human serum ferritin to the lectin Concanavalin A (ConA) has previously suggested that ferritin is glycosylated8,9 and actively secreted through the ER-Golgi pathway.10 Indeed, it was shown in hepatocyte cell-models that inhibition of ER-Golgi trafficking abolished secretion of ferritin.11,12 However, it was never shown that serum ferritin was N-glycosylated, and ferritin did not enter the ER even when translated on rough ER.13,14 Thus, the mechanism of ferritin secretion remains unclear. The question whether serum ferritin is actively secreted or leaked from damaged cells, the mechanism by which ferritin may be secreted and the cellular sources of serum ferritin remain to be elucidated. Both hepatocytes and macrophages have been suggested as cellular sources4 and it was demonstrated that ferritin secreted from macrophages can function as an iron donor to developing erythroid cells.15,16 Serum ferritin levels are high in mice with total or macrophage-specific ablation of the iron regulatory protein 2 (IRP2), whereas serum levels are normal in mice with hepatocyte or intestinal epithelial cell-specific ablations of IRP2.17 These data strongly support that macrophages contribute significantly to serum ferritin.
The function of serum ferritin is not known, but many studies are based on the notion that serum ferritin performs a physiologic role, such as delivering iron from one cell type to another, recruiting free iron from interstitial spaces, promoting angiogenesis or possibly contributing to immunologic signaling events.18,,,–22 Evidence for the presence of a ferritin-receptor has been derived initially from cellular ferritin-binding and -uptake assays and binding of ferritin in tissue sections where binding was specific mostly for H-subunit rich ferritin.19,23,–25 Three distinct candidates for ferritin-receptors have been characterized and cloned recently. TIM-226 is a murine ferritin-receptor that specifically binds H-subunit and is primarily expressed on splenic B cells, epithelial cells of the bile duct, and kidney distal tubular cells. A TIM-2 analog was not detected in human tissues, where transferrin receptor was recently reported to bind and internalize H-subunit containing ferritin.27 SCARA5 specifically recognizes ferritin L-subunits21,26 and was identified in embryonic kidney and adult testis epithelial cells.28
Early characterizations of serum ferritin were performed mainly on human serum ferritin from iron-overloaded patients.4,29 To broaden the knowledge of serum ferritin to other species and conditions, we characterized mouse serum ferritin in control mice and chemically iron-loaded animals. We used mass spectrometry (MS), transmission electron microscopy (TEM), alternating current (AC) magnetic susceptometry for ferritin–iron core analysis,30 Western blotting, and glycosylation analysis, along with additional sensitive assays for iron determination, to characterize serum ferritin and to gain further insight into mechanisms of secretion and the possible role of serum ferritin in reallocation of body iron stores. We concluded that murine serum ferritin is mainly secreted by macrophages through a nonclassical secretion process involving secretory lysosomes and not the ER.
Methods
Mice
All mice were on a C57bl/6J background. All mouse experiments were performed under an animal protocol approved by the Israel Institute of Technology.
Iron overload in mice and serum collection
Male mice weighing between 18 and 24 g were injected intraperitoneally with a total of 100 mg of Fe in the form of Fe-dextran (Sigma-Aldrich). Iron-dextran (110 μL) in saline (91 mg of iron per mL) was injected 5 days a week for 2 weeks. Blood was collected 3 days after last injection. Blood from 5 mice was pooled, serum was separated, and stored at −20°C. Four different pools were analyzed. Sera of non–iron-overloaded mice were collected similarly but not pooled.
Purification of ferritin from tissues
Ferritin from livers and hearts from Fe-dextran–loaded mice was isolated as described,31 with slight modifications: ammonium sulfate precipitation was performed at 55% saturation and molecular sieve fractionation was done using Sephacryl S-300.
Size fractionation by Gel filtration
Total serum and ferritin purified from liver and heart were size-fractionated by fast-protein liquid chromatography (FPLC), using gel filtration on a Sephacryl S-300 26/60 column. Fractions were evaluated for absorbance at 280 nm for proteins, 420 nm for the ferritin–iron core, and 260 nm for nucleic acid contamination.
Ferritin quantification by ELISA
Ferritin concentration was determined as described.32 Pools of 5 fractions were initially screened by enzyme-linked immunosorbent assay (ELISA) to cover the whole eluate of the Sephacryl column. Individual fractions were analyzed from ferritin positive pools.
AC magnetic susceptometry
Characterization of ferritin was carried out in a Quantum Design MPMS-5S SQUID magnetometer with an AC susceptibility option. The measurements were performed with an AC amplitude of 0.45 mT at a frequency of 1 Hz in the temperature range 1.8 to 300 K. For this characterization both the isolated mouse liver ferritin and the mouse serum were freeze-dried for 6 hours in a Heto PowerDry PL3000 drier and the resulting solid was placed into gelatin capsules.
Glycosylation analysis
Immunoprecipitated ferritin was deglycosylated using Peptide: N-Glycosidase F (PNGase-F; NEB) or N-Glycanase (Prozyme), following manufacturer's instructions. In short, beads were diluted in reaction buffer with addition of sodium dodecyl sulfate (SDS)/β-mercaptoethanol and denatured for 5 minutes at 100°C. NP-40 and N-glycanase were added to reaction mixture and incubated for 2 hours at 37°C. Alternatively, serum was incubated with 100μL of ConA-sepharose (Amersham) bead slurry overnight and washed 3 times with 1% triton buffer and once with phosphate-buffered saline. ConA-bound proteins and supernate were analyzed by immunoblot.
Mass spectrometry, TEM, immunoprecipitation, immunoblot, immunofluorescence (IF), iron assays, and macrophage-related experiments including splenectomy are described in detail in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Serum ferritin is fully assembled into 24-mer polymers and no individual subunits are detected
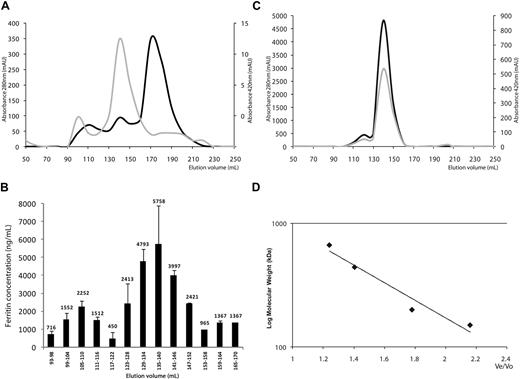
To determine whether mouse serum ferritin consisted of several biophysically distinct species, we separated the serum proteins from highly iron-overloaded mice using gel filtration. Eluted fractions were evaluated for absorbance at 280 nm and 420 nm for protein and iron, respectively (Figure 1A). Ferritin concentrations in the eluate fractions were estimated by ELISA in pooled fractions to detect possible traces of ferritin in the whole eluate and to cover a large range of molecular weights. Immune-reactive ferritin was detected only in high-molecular-weight ranges, whereas no ferritin was detected in fractions of low molecular weight, indicating that no individual subunits or subunit dimers were present in serum. In the high-molecular-weight range, ferritin concentrations were determined in individual fractions (Figure 1B). A serum ferritin peak was detected at a molecular weight of 480 kD. This peak overlapped with that of intact polymeric liver ferritin, purified from livers of iron dextran loaded mice (Figure 1C). This overlap implied that most serum ferritin consists of 24-subunit polymers. In some sera an additional peak elutes at a much higher molecular weight, which is outside of the marker range and may represent a dimer or a multimeric aggregate.
Serum ferritin is a fully assembled 24-subunit polymer. (A) Gel filtration of mouse serum on a Sephacryl S-300 26/60 column using an Akta basic fast-protein liquid chromatography (FPLC) machine (Pharmacia). Absorbance was measured at 280 nm for protein (light grey curve) and 420 nm suggestive for iron (dark grey curve). Note the different scales for 280 nm and 420nm. Absorbance maxima at 420 nm were found at 105 to 110 and 135 to 140 mL, suggesting the presence of ferritin in these fractions. A large peak with absorbance at 280 nm at 170 to 175mL corresponds to non–iron-containing irrelevant serum proteins (1 representative experiment is shown of 3). (B) Ferritin quantification by enzyme-linked immunosorbent assay (ELISA). (C) Purified mouse liver ferritin was loaded onto the same column and eluted as a single peak at 135 to 140 mL. Absorbance was measured at 280 nm for protein (light grey curve), 260 nm for nucleic acid contamination (not shown), and 420 nm suggestive for iron (dark grey curve). (D) Calibration curve obtained with molecular weight markers for gel filtration chromatography (Sigma-Aldrich) run on the same column.
Serum ferritin is a fully assembled 24-subunit polymer. (A) Gel filtration of mouse serum on a Sephacryl S-300 26/60 column using an Akta basic fast-protein liquid chromatography (FPLC) machine (Pharmacia). Absorbance was measured at 280 nm for protein (light grey curve) and 420 nm suggestive for iron (dark grey curve). Note the different scales for 280 nm and 420nm. Absorbance maxima at 420 nm were found at 105 to 110 and 135 to 140 mL, suggesting the presence of ferritin in these fractions. A large peak with absorbance at 280 nm at 170 to 175mL corresponds to non–iron-containing irrelevant serum proteins (1 representative experiment is shown of 3). (B) Ferritin quantification by enzyme-linked immunosorbent assay (ELISA). (C) Purified mouse liver ferritin was loaded onto the same column and eluted as a single peak at 135 to 140 mL. Absorbance was measured at 280 nm for protein (light grey curve), 260 nm for nucleic acid contamination (not shown), and 420 nm suggestive for iron (dark grey curve). (D) Calibration curve obtained with molecular weight markers for gel filtration chromatography (Sigma-Aldrich) run on the same column.
Serum ferritin consists mainly of L-subunits, but some H-subunits are detectable
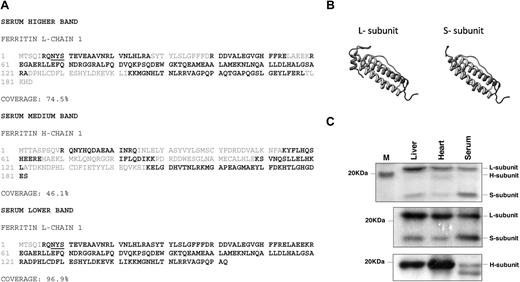
Because the presence of at least 1 H-subunit in a 24-mer cage is mandatory for iron loading,33 we further analyzed the subunit ratio and the amino acid sequence of serum ferritin. The presence of both H- and L-ferritin subunits was confirmed by MS of the main ferritin peak from Figure 1 and of serum ferritin immunoprecipitated with an L-subunit–specific antibody and separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; Figure 2A,C). The amino acid sequences of the serum ferritin H- and L-chains were found to be identical with those of intracellular ferritin subunits (Figure 2A). Red letters indicate sequences detected by MS after a trypsin digest. Interestingly, a prominent 17-kD band was detected by protein staining of immunoprecipitated ferritin from serum of iron-overloaded mice (Figure 2C). Mass spectrometry (MS) analysis of peptide fragments after digestion with 3 different proteases identified this band as a truncated L-subunit from which 21 C-terminal amino acids were missing (Figure 2A-B).
Full-length L- and H-subunits and a truncated L-subunit are detected in serum ferritin. (A) The main ferritin peak from Figure 1B was concentrated and separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Alternatively, serum ferritin was immunoprecipitated and separated on SDS-PAGE. Relevant protein bands were analyzed by LC-MS/MS mass spectrometry. Black-labeled amino acid sequences were detected by MS, showing the presence of L-, H- and a truncated L-subunit. L-subunit contains 1 potential site for N-glycosylation at the N-terminus of the protein (underlined) that faces the exterior surface of the assembled protein (determined by comparison to the human 24 subunit assembled structure; http://www.ncbi.nlm.nih.gov/Structure/CN3D/cn3d.shtml). (B) The structure of the L-subunit sequences was predicted using the Swiss Model Web site (http://www.ebi.ac.uk/swissprot). The truncated L- ferritin lacks the entire fifth short helix at the C-terminal end of the protein. (C) Top panel: Coomassie staining of immunoprecipitated liver, heart, and serum ferritin from iron-overloaded mice detects L-subunit very clearly while H-subunit in serum is hardly detectable. A 17-kD band (marked) S-subunit is prominent in serum (1 representative experiment is shown of 5). Middle and bottom panels: Immunoprecipitation of 5 μg of liver, heart, and serum ferritin with an anti–mouse liver ferritin antibody followed by Western blot with the same anti–liver antibody (middle panel) or with an anti–H-subunit antibody (bottom panel) shows that serum ferritin contains many fewer H-subunits than liver and heart ferritin (1 representative experiment is shown of 3 for L ferritin Western and of 6 for H-ferritin Western). It is important to note that in contrast to human ferritin, mouse L-ferritin subunits migrate at around 21 kD and H-ferritin subunits migrate slightly faster.
Full-length L- and H-subunits and a truncated L-subunit are detected in serum ferritin. (A) The main ferritin peak from Figure 1B was concentrated and separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Alternatively, serum ferritin was immunoprecipitated and separated on SDS-PAGE. Relevant protein bands were analyzed by LC-MS/MS mass spectrometry. Black-labeled amino acid sequences were detected by MS, showing the presence of L-, H- and a truncated L-subunit. L-subunit contains 1 potential site for N-glycosylation at the N-terminus of the protein (underlined) that faces the exterior surface of the assembled protein (determined by comparison to the human 24 subunit assembled structure; http://www.ncbi.nlm.nih.gov/Structure/CN3D/cn3d.shtml). (B) The structure of the L-subunit sequences was predicted using the Swiss Model Web site (http://www.ebi.ac.uk/swissprot). The truncated L- ferritin lacks the entire fifth short helix at the C-terminal end of the protein. (C) Top panel: Coomassie staining of immunoprecipitated liver, heart, and serum ferritin from iron-overloaded mice detects L-subunit very clearly while H-subunit in serum is hardly detectable. A 17-kD band (marked) S-subunit is prominent in serum (1 representative experiment is shown of 5). Middle and bottom panels: Immunoprecipitation of 5 μg of liver, heart, and serum ferritin with an anti–mouse liver ferritin antibody followed by Western blot with the same anti–liver antibody (middle panel) or with an anti–H-subunit antibody (bottom panel) shows that serum ferritin contains many fewer H-subunits than liver and heart ferritin (1 representative experiment is shown of 3 for L ferritin Western and of 6 for H-ferritin Western). It is important to note that in contrast to human ferritin, mouse L-ferritin subunits migrate at around 21 kD and H-ferritin subunits migrate slightly faster.
The presence of H-subunit in the 24-subunit polymer was further demonstrated by immunoprecipitation of the ferritin polymer with an L-subunit–specific antibody, followed by Western blot with an H-subunit–specific antibody. Clearly, serum ferritin contained much less H-subunit than liver ferritin (Figure 2C). Protein staining and quantification of ferritin on reducing SDS-PAGE revealed the extremely low H-subunit content of serum ferritin (Figure 2C and Table 1). Liver ferritin contains a significant fraction of natural apoferritin that is composed of L-subunit homopolymers.4 The very low amount of H-subunit found in serum ferritin implied that serum ferritin contained even more L-subunit homopolymer apoferritin than liver.4 But the finding that serum ferritin was exclusively assembled into 24-mers together with the detection of H-subunit in L-subunit immunoprecipitations, suggested that there are some H- and L-subunit heteropolymers in serum that can contain iron.
Distribution of L-, H-, and S-subunits in liver, heart, and serum ferritin
| . | Liver (%) . | Heart (%) . | Serum (%) . |
|---|---|---|---|
| Coomassie | |||
| L-subunit | 74.2 ± 2.2 | 65.8 ± 9.4 | 41.2 ± 6.2 |
| H-subunit | 10 ± 3.3 | 18.8 ± 7.1 | 5.3 ± 2.9 |
| S-subunit | 15.8 ± 2.9 | 15.4 ± 3.7 | 53.5 ± 7.7 |
| Western-blot | |||
| L-subunit | 68.61 ± 0.34 | 65.60 ± 2.05 | 47.03 ± 2.05 |
| H-subunit | 10* | 18.16 ± 0.95 | 3.26 ± 1.94 |
| S-subunit | 21.39 ± 0.34 | 16.24 ± 1.44 | 49.71 ± 2.09 |
| . | Liver (%) . | Heart (%) . | Serum (%) . |
|---|---|---|---|
| Coomassie | |||
| L-subunit | 74.2 ± 2.2 | 65.8 ± 9.4 | 41.2 ± 6.2 |
| H-subunit | 10 ± 3.3 | 18.8 ± 7.1 | 5.3 ± 2.9 |
| S-subunit | 15.8 ± 2.9 | 15.4 ± 3.7 | 53.5 ± 7.7 |
| Western-blot | |||
| L-subunit | 68.61 ± 0.34 | 65.60 ± 2.05 | 47.03 ± 2.05 |
| H-subunit | 10* | 18.16 ± 0.95 | 3.26 ± 1.94 |
| S-subunit | 21.39 ± 0.34 | 16.24 ± 1.44 | 49.71 ± 2.09 |
Intensity of bands was evaluated from coomassie stained gels (n = 4) using Adobe Photoshop as described in supplemental Methods.
As the H-subunit in serum is hardly visible by Coomassie and therefore quantifications are not very accurate, quantification of Western blots was done using 10% H-subunit of liver ferritin as reference point (n = 3).
Iron content and core analysis of serum ferritin
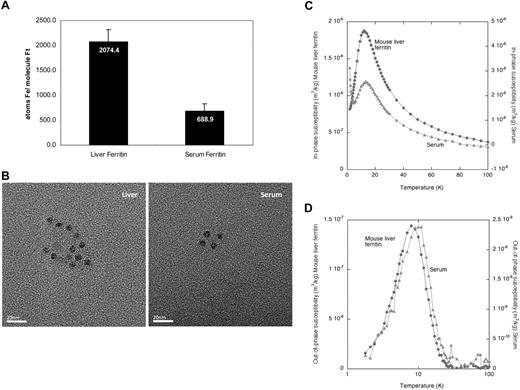
To evaluate the iron content of serum ferritin, we measured the average number of iron atoms in the ferritin protein molecule. Serum ferritin contained significantly less iron than liver ferritin (Figure 3A), estimated by measuring the iron in immunoprecipitated ferritin by the ferrozine assay and the ferritin protein concentration by ELISA.
Serum ferritin contains minimal iron and its iron core resembles the liver ferritin core structure. (A) Equal amounts of liver and serum ferritin were immunoprecipitated and iron content was determined by the ferrozine assay. Ferritin protein concentration was determined by ELISA before precipitation and loss of ferritin during precipitation was calculated by quantitative Western blot of immunoprecipitated and nonimmunoprecipitated ferritin. The average iron atoms/ferritin molecule ratio was calculated. An average of 2 measurements is shown. (B) Visualization of ferritin–iron core from serum ferritin by transmission electron microscopy (TEM) reveals similar core size as in liver ferritin. TEM analysis of liver ferritin and serum was performed at room temperature on a Jeol 2000 FXII microscope using a 120 000× magnification. Images were captured using a Gatan Model 694 CCD camera with Gatan Digital Micrograph software. Images were processed with Adobe Photoshop. Size bar indicates 20 nm. (C) Temperature dependence of the in-phase susceptibility of the mouse serum and isolated mouse liver ferritin. Paramagnetic tail in serum is visible at the left end of curve. (D) Temperature dependence of the out-of-phase susceptibility of the mouse serum and isolated mouse liver ferritin.
Serum ferritin contains minimal iron and its iron core resembles the liver ferritin core structure. (A) Equal amounts of liver and serum ferritin were immunoprecipitated and iron content was determined by the ferrozine assay. Ferritin protein concentration was determined by ELISA before precipitation and loss of ferritin during precipitation was calculated by quantitative Western blot of immunoprecipitated and nonimmunoprecipitated ferritin. The average iron atoms/ferritin molecule ratio was calculated. An average of 2 measurements is shown. (B) Visualization of ferritin–iron core from serum ferritin by transmission electron microscopy (TEM) reveals similar core size as in liver ferritin. TEM analysis of liver ferritin and serum was performed at room temperature on a Jeol 2000 FXII microscope using a 120 000× magnification. Images were captured using a Gatan Model 694 CCD camera with Gatan Digital Micrograph software. Images were processed with Adobe Photoshop. Size bar indicates 20 nm. (C) Temperature dependence of the in-phase susceptibility of the mouse serum and isolated mouse liver ferritin. Paramagnetic tail in serum is visible at the left end of curve. (D) Temperature dependence of the out-of-phase susceptibility of the mouse serum and isolated mouse liver ferritin.
To further characterize the bio-mineralization properties of the iron core, TEM and AC magnetic susceptibility measurements were performed on serum and isolated liver ferritin (Figure 3B-D). TEM observations of isolated mouse liver ferritin and mouse serum revealed the presence of electron dense particles with an average diameter of 6.2 nm (± 0.8 nm, n = 25) and 6.1 nm (± 0.6 nm, n = 27), respectively (Figure 3B). In unstained samples, like those presented in the figure, the protein shells of the ferritin and apoferritin molecules were not visible. However, evidence for presence of a proteinaceous shell came from the spherical shape and the confined size of the electron-dense cores, which appeared to be mutually separated by the non–electron-dense protein shells.
The elemental iron content determined from Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) of freeze-dried purified mouse liver ferritin and serum samples demonstrated that the presence of other magnetogenic elements in both samples was negligible, which enabled us to interpret AC susceptometry measurements particularly in respect to iron. The paramagnetic tail that was observed in the in-phase component of the serum ferritin at low temperatures (Figure 3C left end of light curve), indicated that a paramagnetic iron-containing species was present in that sample, which may correspond to serum transferrin.
Analysis of the temperature dependence of the AC susceptibility per mass of sample in freeze-dried serum and liver ferritin showed a maximum at 12 K for both samples in the in-phase susceptibility (χ′) indicating the presence of mineral particles in both samples (Figure 3C). Additional evidence for such particles comes from the single bell-shaped maxima located at 8 K for the mouse liver ferritin and 10 K for the serum sample in the nonzero out-of-phase susceptibility (χ″) measurement (Figure 3D). Both the shape and the temperature location of these 2 χ″(T) maxima suggested that the crystalline structure and the particle size distribution of the mineral particles in serum and liver ferritin were very similar (Figure 3D). As liver ferritin stores iron mainly in the form of ferrihydrite, these results suggested that the iron of serum ferritin is also stored in a ferrihydrite core. However, it is unclear where iron is loaded onto extracellular ferritin and by which route it is secreted. To further understand the intracellular pathway of ferritin secretion, we analyzed the glycosylation pattern of serum ferritin.
Evidence for a nonclassical secretion pathway for ferritin
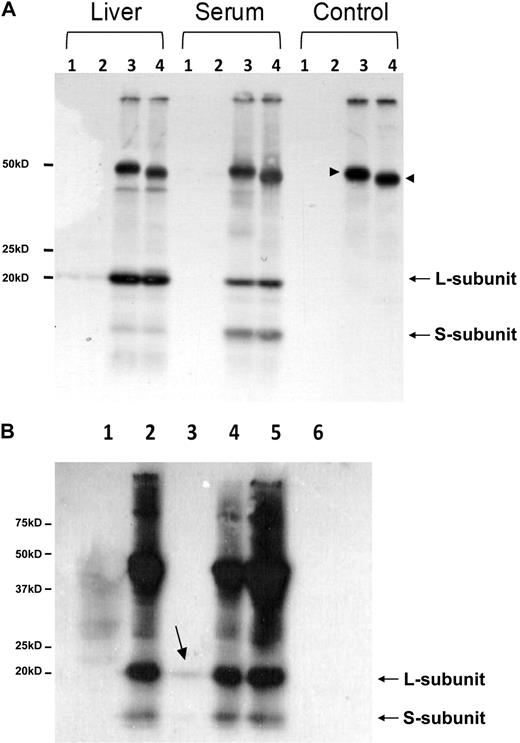
Although neither H- nor L-subunit of ferritin have a classical signal sequence for ER targeting (http://www.cbs.dtu.dk/services/SignalP/), ConA binding of human serum ferritin is indicative of the presence of mannose- or glucose-like molecules on the protein surface. This motivated us to further analyze the glycosylation pattern of mouse serum ferritin as N-glycosylation is well accepted to be a marker for ER-Golgi processing. A glycosylation consensus site is found in the N-terminus of the L-subunit (Figure 2A underlined), an area that faces the cytosol in a fully assembled 24-mer, and would therefore be easily accessible to deglycosylation enzymes (determined by comparison to the human ferritin structure MMDB ID:76115 at http://www.ncbi.nlm.nih.gov/sites/entrez?db = structure). But surprisingly, evaluation of the N-glycosylation of mouse serum ferritin by immunoprecipitation followed by PNGase-F digest did not reveal a noteworthy molecular weight change associated with deglycosylation (Figure 4A) even though the IgG heavy chain was visibly deglycosylated (arrowheads). Furthermore the molecular weight mass detected by MS of the peptide containing the glycosylation consensus site matched the predicted mass, indicating that no peptide modifications had occurred. Binding of serum to ConA and analysis of the eluate detected a very small fraction of ferritin that bound to ConA. This fraction had the same molecular weight as nonglycosylated ferritin and therefore may have represented ferritin bound nonspecifically to ConA (Figure 4B). Commonly, serum ferritin glycosylation is quantified by subtracting the ferritin detected by ELISA in eluates of a Sepharose-ConA column from the levels of ferritin detected in eluate of a control Sepharose column. Sera from control mice showed less than 2% ferritin binding to ConA (data not shown). These results ruled out the possibility that the lack of ConA binding was specifically associated with iron overload. Taken together, these results substantially undermined the common assumption that serum ferritin is highly glycosylated, and did not support ferritin secretion through the classical ER-Golgi route.
Mouse serum ferritin is not detectably glycosylated. (A) Deglycosylation of immunoprecipitated liver and serum ferritin from iron-overloaded mice with Peptide: N-Glycosidase F (PNGase-F) followed by separation on SDS-PAGE and Western blot with an anti–mouse liver ferritin antibody. Lanes 1 and 2 are pre-clears before and after deglycosylation, respectively. Lanes 3 and 4 are immunoprecipitated samples before and after deglycosylation, respectively. No change in molecular weight was detected in L- ferritin subunit after PNGase F digestion. Note the significant amount of the S-subunit detected in serum, compared with liver ferritin. An internal positive control is marked with arrowheads and shows that IgG heavy chain was successfully deglycosylated (1 representative experiment is shown of 5). (B) ConA binding of mouse serum ferritin. Serum was incubated with protein A sepharose and with ConA beads (lanes 1 and 3, respectively) and their supernatants were immunoprecipitated with anti–liver ferritin antibody (lanes 2 and 4). Serum was directly immunoprecipitated with anti–liver ferritin antibody (lane 5) and ConA beads were loaded in lane 6. All samples were analyzed on SDS-PAGE followed by Western blot analysis (1 representative experiment is shown of 5).
Mouse serum ferritin is not detectably glycosylated. (A) Deglycosylation of immunoprecipitated liver and serum ferritin from iron-overloaded mice with Peptide: N-Glycosidase F (PNGase-F) followed by separation on SDS-PAGE and Western blot with an anti–mouse liver ferritin antibody. Lanes 1 and 2 are pre-clears before and after deglycosylation, respectively. Lanes 3 and 4 are immunoprecipitated samples before and after deglycosylation, respectively. No change in molecular weight was detected in L- ferritin subunit after PNGase F digestion. Note the significant amount of the S-subunit detected in serum, compared with liver ferritin. An internal positive control is marked with arrowheads and shows that IgG heavy chain was successfully deglycosylated (1 representative experiment is shown of 5). (B) ConA binding of mouse serum ferritin. Serum was incubated with protein A sepharose and with ConA beads (lanes 1 and 3, respectively) and their supernatants were immunoprecipitated with anti–liver ferritin antibody (lanes 2 and 4). Serum was directly immunoprecipitated with anti–liver ferritin antibody (lane 5) and ConA beads were loaded in lane 6. All samples were analyzed on SDS-PAGE followed by Western blot analysis (1 representative experiment is shown of 5).
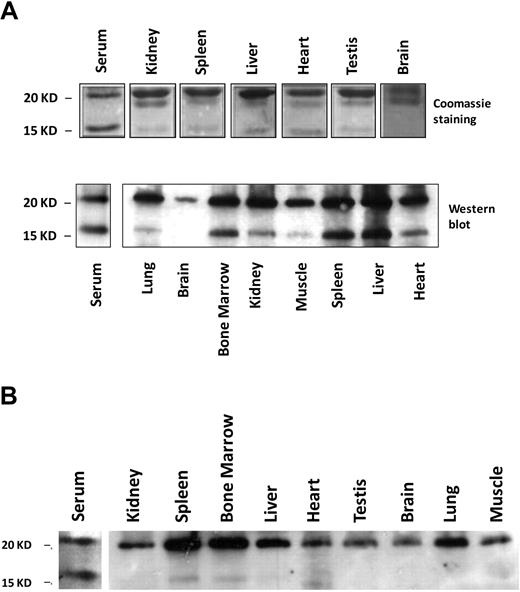
The 17-kD band mentioned in Figure 2 in mouse serum ferritin resembles the truncated L-subunit of lysosomal ferritin from iron-loaded rats.34 The high levels of the smaller 17-kD L-subunit in serum led us to name it the “S-subunit.“ We hypothesize that the S-subunit may be the product of lysosomal processing and that serum ferritin may be secreted through the nonclassical lysosomal secretion pathway.35 The new C-terminus is located in the turn between the D- and E-helices and faces the exterior of the shell in a fully assembled ferritin molecule, where it may be accessible to lysosomal proteases without disassembly of the 24-mer (determined by comparison to the bullfrog and human ferritin crystal structures MMDB-ID 50282 and 76116, respectively). Iron loaded mice express the S-subunit in all peripheral tissues, but the S- to L-subunit ratio is significantly higher in serum than in any of the analyzed tissues (Table 1 and Figure 5A). In contrast, most tissues of non–iron-overloaded mice (Figure 5B) did not express the S-subunit, whereas it was highly represented in sera. Spleen and bone marrow, both tissues rich in macrophages, were the only tissues in which low levels of the S-subunit were detected, which made us wonder whether macrophages are a source of serum ferritin.
Serum ferritin is characterized by a high representation of the 17-kD truncated subunit of the L-chain. (A) Serum or tissue-lysates from iron-dextran overloaded mice were subjected to IP, using an anti–liver ferritin antibody (Serum, 20 μg ferritin/IP; liver lysate, 200 μg protein/IP; and all other tissue lysates, 1 mg protein/IP). IPs were separated on SDS-PAGE and stained with Coomassie (top panel; 1 representative experiment is shown of 4). Western blot with anti–liver ferritin antibody from tissue-lysates (40 μg protein/IP) and immunoprecipitated serum from iron-dextran overloaded mice (bottom panel; 1 representative experiment is shown of 3). (B) Western blot as in panel A from tissue lysates (40 μg protein/lane) and serum (200 ng of ferritin/IP) from untreated mice (1 representative experiment is shown of 3).
Serum ferritin is characterized by a high representation of the 17-kD truncated subunit of the L-chain. (A) Serum or tissue-lysates from iron-dextran overloaded mice were subjected to IP, using an anti–liver ferritin antibody (Serum, 20 μg ferritin/IP; liver lysate, 200 μg protein/IP; and all other tissue lysates, 1 mg protein/IP). IPs were separated on SDS-PAGE and stained with Coomassie (top panel; 1 representative experiment is shown of 4). Western blot with anti–liver ferritin antibody from tissue-lysates (40 μg protein/IP) and immunoprecipitated serum from iron-dextran overloaded mice (bottom panel; 1 representative experiment is shown of 3). (B) Western blot as in panel A from tissue lysates (40 μg protein/lane) and serum (200 ng of ferritin/IP) from untreated mice (1 representative experiment is shown of 3).
Macrophages are a major cellular source of serum ferritin
Serum ferritin levels are elevated in mice that lack IRP2,17,36 and more recently, it was observed that serum ferritin levels were elevated specifically when IRP2 was absent in macrophages whereas absence of IRP2 in hepatocytes did not affect serum ferritin levels.17 Red-pulp macrophages of the spleen play a central role in iron recycling and therefore the effect of splenectomy on serum ferritin levels was evaluated. A significant decrease in serum ferritin was detected after splenectomy compared with sham operated mice (209 ± 10 ng/mL, n = 13 and 510 ± 77 ng/mL, n = 6, respectively), suggesting that either splenic lymphocytes or macrophages were a source of serum ferritin.
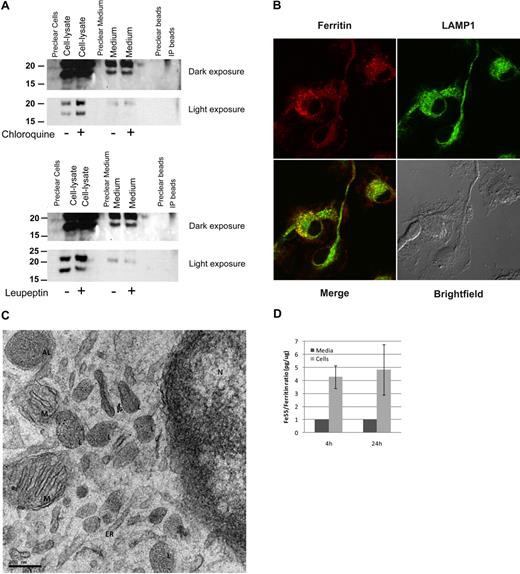
We demonstrated by immunoprecipitation (IP)–Western (Figure 6A) and by metabolic labeling and Ferritin-IP (not shown) that primary cultures of bone marrow–derived macrophages (BMDMs) secrete ferritin into their culture medium and the S-subunit is clearly detectable in the medium. The presence of ferritin in the medium was not a result of a cellular leak, as the L-/S-subunit ratio was not equal in the cellular lysates compared with the ferritin in the medium. Moreover, cytosolic IRP1 was detected only in cell-lysates and not in the culture medium (not shown). The lysosomotropic agent chloroquine inhibited ferritin processing, causing an accumulation of intracellular ferritin that was not reflected in the amount of ferritin secreted. The lysosomal protease-inhibitor leupeptin had only a minor effect on ferritin in BMDMs. Immunofluorescence of ferritin and the lysosomal marker LAMP1 showed cytosolic ferritin that did not colocalize with LAMP1 and vesicular ferritin of which much but not all colocalized with LAMP1, indicating that ferritin is distributed throughout the cytosol in BMDMs and is present in the lysosomes as well (Figure 6B). Analysis of BMDMs by TEM clearly showed localization of ferritin–iron cores (visible as small black dots) to lysosomal and autophagosomal compartments, whereas neither the ER nor the nuclear envelope, where synthesis of secretory proteins is high, showed ferritin molecules (Figure 6C). It is important to note that only holoferritin and not apoferritin is detected by this method. Further evidence that ferritin is secreted actively came from detection of a difference in the iron content of intracellular ferritin compared with ferritin in the culture medium. Comparison of ferritin-protein/ferritin-Fe55 ratio of intracellular and secreted ferritin from murine BMDMs that were loaded with Fe55-transferrin before the experiment showed that the radioactive iron content of the secreted ferritin was significantly lower than that of the intracellular ferritin (Figure 6D).
Macrophages actively secrete ferritin. (A) BMDMs were incubated with 15μM chloroquine or 80 μg/mL leupeptin for 18 hours. Ferritin was immunoprecipitated from cell lysates and medium, separated on SDS-PAGE and detected by Western blot with the anti–L-subunit antibody. For each experiment, 1 representative gel is shown of 4 similar experiments. (B) BMDM immunofluorescence of ferritin and LAMP1. Image visualization was made on a Zeiss LSM 510 META laser scanning confocal microscope with a Plan-Apochromat 63×/1.4 oil DIC lens and scan zoom 2.0, resulting in a final magnification of ×126. Alexa Fluor 488 and 568 were used as fluorochromes. Images were captured using LSM5 software program and further processed with Adobe Photoshop. (C) Electron microscopy (EM) analysis of BMDMs. L indicates lysosome; AL, autophagolysosome; M, mitochondria; ER, endoplasmic reticulum; and N, nucleus. Sections were examined with a T12 transmission electron microscope at 120keV (FEI). Image acquisition was done using a 2k CCD ultrascan CCD camera (Gatan). (D) Comparison of ratio of ferritin-Fe55/ferritin-protein showed that the radioactive iron content of the secreted ferritin was significantly lower than that of the intracellular ferritin. BMDMs were incubated overnight with transferrin-Fe55. Ferritin protein and Fe55 content was determined in lysates and medium by immunoprecipitation followed either by Western blot with a purified ferritin standard curve for protein quantification or scintillation counting for Fe55 determination.
Macrophages actively secrete ferritin. (A) BMDMs were incubated with 15μM chloroquine or 80 μg/mL leupeptin for 18 hours. Ferritin was immunoprecipitated from cell lysates and medium, separated on SDS-PAGE and detected by Western blot with the anti–L-subunit antibody. For each experiment, 1 representative gel is shown of 4 similar experiments. (B) BMDM immunofluorescence of ferritin and LAMP1. Image visualization was made on a Zeiss LSM 510 META laser scanning confocal microscope with a Plan-Apochromat 63×/1.4 oil DIC lens and scan zoom 2.0, resulting in a final magnification of ×126. Alexa Fluor 488 and 568 were used as fluorochromes. Images were captured using LSM5 software program and further processed with Adobe Photoshop. (C) Electron microscopy (EM) analysis of BMDMs. L indicates lysosome; AL, autophagolysosome; M, mitochondria; ER, endoplasmic reticulum; and N, nucleus. Sections were examined with a T12 transmission electron microscope at 120keV (FEI). Image acquisition was done using a 2k CCD ultrascan CCD camera (Gatan). (D) Comparison of ratio of ferritin-Fe55/ferritin-protein showed that the radioactive iron content of the secreted ferritin was significantly lower than that of the intracellular ferritin. BMDMs were incubated overnight with transferrin-Fe55. Ferritin protein and Fe55 content was determined in lysates and medium by immunoprecipitation followed either by Western blot with a purified ferritin standard curve for protein quantification or scintillation counting for Fe55 determination.
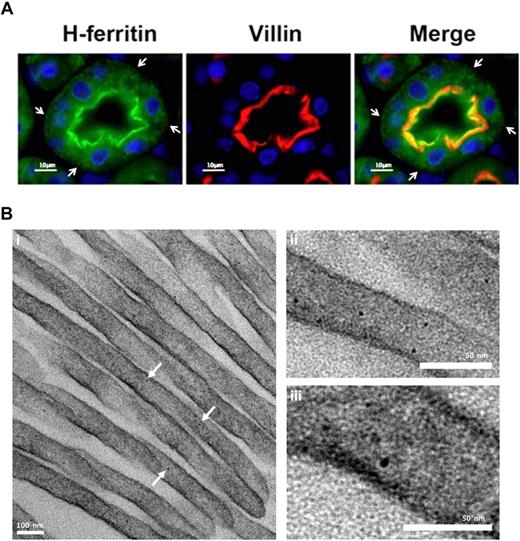
It is interesting to note that serum ferritin in mice with complete IRP2 deletion in all tissues is more than 3 times higher than in the mice with macrophage specific deletion of IRP2 (217 ± 4 ng/mL, n = 17 and 61 ± 2 ng/mL, n = 14, respectively,17 ) which implies that additional tissues likely also contribute to serum ferritin. The kidney may play an important role in systemic iron homeostasis.37,38 The fact that large amounts of iron seem to be moving through lysosomal compartments of the kidney,37 and that the 17-kD L-subunit band was highly expressed in kidney of iron-overloaded mice (Figure 5A) has led us to hypothesize that the kidney may be an additional source of serum ferritin. By IF and TEM we detected ferritin in 2 locations of the proximal tubule cells. A high concentration was visible near the apical brushborder of the kidney proximal tubule cells (Figure 7A) and some ferritin was also detected in a punctate distribution throughout the cell and clearly at the basolateral membrane (Figure 7A arrows). Interestingly, ferritin was found in the apical microvilli, suggesting that there is a need for sequestration of reabsorbed iron at this location (Figure 7B).
Structured subcellular localization of ferritin in proximal tubule brush border cells. (A) Immunofluorescence of kidney cortex sections using antibodies against H ferritin (green) and villin (red). Nuclei were stained with DAPI (blue). H ferritin and villin showed colocalization near the brush border region of proximal convoluted tubule cells. Ferritin concentration decreased toward the basolateral membrane of the cell and distribution showed a punctate pattern. Vesicle-like structures are visible at the basolateral membrane (arrowheads). Villin is an actin-binding protein found exclusively in the brush border of renal and intestinal epithelial cells. Experiments with an L-subunit–specific antibody gave similar results. Image visualization was made on a Nikon Eclipse E600 using a 63× magnification. CY3 and FITC were used as fluorochromes. Images were captured using a Nikon Digital Camera DXM1200F with Nikon ACT-1 Version 2.62 software. Images were processed with Adobe Photoshop. (B) Kidney cortex EM: Electron-dense particles of 5 to 7 nm in diameter that have clear characteristics of ferritin–iron cores are abundant in the proximal tubule brush border. Kidney cortex specimens were fixed with 2.5% glutaraldehyde and reduced osmium, followed by dehydration and embedding in Epon resin. Finally, microtome sections were mounted on copper grids and stained with bismuth subnitrate before visualization on a Technai T12 TEM at 120keV (FEI). Image acquisition was done using a 2k CCD ultrascan CCD camera (Gatan). Arrows denote electron dense ferritin particles.
Structured subcellular localization of ferritin in proximal tubule brush border cells. (A) Immunofluorescence of kidney cortex sections using antibodies against H ferritin (green) and villin (red). Nuclei were stained with DAPI (blue). H ferritin and villin showed colocalization near the brush border region of proximal convoluted tubule cells. Ferritin concentration decreased toward the basolateral membrane of the cell and distribution showed a punctate pattern. Vesicle-like structures are visible at the basolateral membrane (arrowheads). Villin is an actin-binding protein found exclusively in the brush border of renal and intestinal epithelial cells. Experiments with an L-subunit–specific antibody gave similar results. Image visualization was made on a Nikon Eclipse E600 using a 63× magnification. CY3 and FITC were used as fluorochromes. Images were captured using a Nikon Digital Camera DXM1200F with Nikon ACT-1 Version 2.62 software. Images were processed with Adobe Photoshop. (B) Kidney cortex EM: Electron-dense particles of 5 to 7 nm in diameter that have clear characteristics of ferritin–iron cores are abundant in the proximal tubule brush border. Kidney cortex specimens were fixed with 2.5% glutaraldehyde and reduced osmium, followed by dehydration and embedding in Epon resin. Finally, microtome sections were mounted on copper grids and stained with bismuth subnitrate before visualization on a Technai T12 TEM at 120keV (FEI). Image acquisition was done using a 2k CCD ultrascan CCD camera (Gatan). Arrows denote electron dense ferritin particles.
Discussion
To approach open questions on the biophysical form, iron content, mechanism of secretion, cellular source, and most importantly, function of secreted ferritin in mammals, we characterized mouse serum ferritin, a representative for extracellular ferritin.
Biophysical characteristics of serum ferritin imply active secretion through the lysosomal secretory pathway
Mouse serum ferritin is assembled into a 24-subunit polymer that, in agreement with earlier work,39 consists mainly of L-subunit and was considered to represent natural apoferritin.4 Here we showed by MS, immunoblot and iron analysis that mouse serum ferritin contains low amounts of H-subunits and iron. MS analysis demonstrated that amino-acid sequences of both subunits were identical to the cytosolic ferritin subunits, indicating that serum ferritin originates from the same genes as intracellular ferritin, in contrast to an earlier report.40 High serum ferritin correlating with overexpression of the L-subunit in hyperferritinemia cataract syndrome and high macrophage ferritin in ferroportin disease further support this notion.41
Sera from control or iron-dextran loaded mice contain ferritin that is highly enriched with a C-terminally truncated 17-kD L-subunit that we named S-subunit (Figure 2, 5). The S-subunit seems to correspond to the truncated subunit detected in lysosomal ferritin in siderosomes,34 and is either the product of lysosomal processing or a splice variant targeted to the lysosome. As the S-subunit was exclusively found in lysosomal fractions,34 the high ratio of S- to L-subunit found in serum ferritin implies that serum ferritin has passed through a lysosomal compartment. Processing by specific acidic proteases within the lysosome could give rise to the 17-kD band. After lysosomal processing, ferritin may be further degraded and its iron stored as hemosiderin, or lysosomal ferritin may be secreted by cells that have a lysosomal secretory pathway. While high representation of the S-subunit was characteristic for serum of control mice, in tissues the S-subunit was mainly detected in iron-overloaded animals and at much lower levels than the full-length L-subunit. Thus, the unique ratio of the 2 types of L-subunits found in sera suggests an active secretion pathway rather than a leak of cellular content of ruptured cells to the blood stream. Also in cultured BMDMs, the analysis of iron content of intracellular and secreted ferritin showed significantly lower Fe55 content in the secreted ferritin compared with intracellular ferritin (Figure 6D). The fact that the Fe55/ferritin-protein ratio was different in cultured cells compared with their medium was further evidence that ferritin was indeed secreted from macrophages.
Possible mechanisms of ferritin secretion
The signal sequence for ER targeting found in insect ferritin6 is lacking in mammalian ferritin and, indeed, mammalian ferritin is mostly cytosolic. The large body of work demonstrating ConA binding of human ferritin, has generally led to the conclusion, that serum ferritin is N-glycosylated and therefore secreted through the ER-Golgi pathway,8,–10 but this conclusion may be erroneous. Most quantifications of ConA-bound human serum ferritin were done by subtracting ferritin concentration from eluates of ConA-Sepharose columns from total ferritin, without further analysis of the ConA bound fraction.42 It is therefore possible that this ConA fraction was nonspecifically bound (as demonstrated in Figure 4B) or that serum ferritin noncovalently binds glucose- or mannose-like molecules in some physiologic or pathologic conditions. A glycosylated 23-kD G-subunit was detected in the pre-MS era,8 but it may represent a protein that copurified with serum ferritin (supplemental Figure 1). Further evidence for the ER-Golgi route for ferritin secretion was reported from hepatocyte cell-lines.11,12 However, hepatocytes may not be the main source of serum ferritin (see the next section).
We found that mouse serum ferritin does not bind to ConA, matching reports on canine and bovine serum ferritin43,44 and it also is not enzymatically deglycosylated (Figure 4A). While the presence of N-glycosylation is evidence for secretion through the ER-Golgi secretory pathway, the absence of N-glycosylation does not exclude this pathway. For example, albumin does not carry any N-glycosylation, but is classically secreted through the ER-Golgi route,45 though albumin has no glycosylation consensus site. In contrast, ferritin L-subunit has a glycosylation consensus site that in our hands showed no evidence for N-glycosylation. This may indeed indicate that serum ferritin was not secreted through the ER-Golgi pathway. As mentioned in the previous paragraph, the enrichment of the 17-kD fragment in serum ferritin strongly suggests that mouse serum ferritin has passed through a lysosomal compartment. Ferritin entry to the lysosomal compartment is mediated by autophagy7 and secretion of proteins from the lysosomal compartment is well documented. Interestingly, secretory lysosomes have been specifically described for 2 cell types: the hematopoietic lineage, including macrophages, and renal tubular cells.35 Further evidence for a lysosomal pathway of ferritin secretion comes from our finding that ferritin is clearly visible in BMDM lysosomes and autophagolysosomes (Figure 6B-C). Indications that secretion of ferritin may be regulated are further supported by the finding that an unidentified serum factor inhibits ferritin secretion,12 but further investigation is required to elucidate this regulatory mechanism. The inhibition of lysosomal processing of ferritin by Chloroquine, which impairs its secretion (Figure 6A), further supports the lysosomal secretory pathway suggested for serum ferritin.
Cellular source of serum ferritin
Genetic evidence for the contribution of macrophages to serum ferritin levels arises from cell type–specific ablations of IRP2, which diminishes translational repression of ferritin, resulting in ferritin over-expression. Mice with macrophage specific deletion of the IRP2 gene have elevated serum ferritin levels, while neither mice with intestinal epithelial nor hepatocyte specific ablation of IRP2 show elevated serum ferritin levels.17 Positive evidence that hepatocytes are not a source of serum ferritin could not be obtained. These genetic findings, and the fact that splenectomy caused decreased serum ferritin concentrations in mice, compared with sham operated control-mice support the notion that macrophages are an important cellular source for serum ferritin. In addition, the 17-kD L-subunit was detected in control sera and in spleen and bone marrow, both tissues with significant macrophage representation, but not in other tissues (Figure 5B). Although macrophages appear to be a significant source of serum ferritin, they most likely are not the only source. In mice with macrophage specific ablation of IRP2, serum ferritin levels are much less elevated than in mice with total ablation of IRP2.
In the kidney, glomerular filtration of transferrin and subsequent reabsorption of transferrin and iron, have been demonstrated.37 The divalent metal transporter (DMT1) which is highly expressed in endosomes and lysosomes of proximal tubule cells may import the iron to the cytosol hence it can be exported to the blood stream by ferroportin. Alternatively, ferritin could export iron from these cells after loading near the apical brushborder of the proximal tubule cells, where a high accumulation of ferritin colocalizes with villin, an actin-binding protein involved in formation of microvilli in the intestine and the kidney (Figure 7A). Subsequently, ferritin is also found in a punctate distribution near the basolateral membrane, a distribution that may represent vesicular localization, indicating that ferritin may have entered the lysosomal compartment, where it may facilitate intracellular transport across these polarized cells and secretion of iron from these cells.
Iron in serum ferritin
Liver- and brain–ferritin–iron cores are mainly composed of ferrihydrite under physiologic conditions.46 Mouse serum ferritin contains a measurable but low amount of iron. This low iron content and the extreme similarity of the serum and liver ferritin cores, assessed by TEM and AC susceptometry,30 suggest that the few serum ferritin cages that contain an H-subunit accumulate iron as ferrihydrite as well. The fact that the average iron content of liver ferritin is much higher than the average iron found in serum ferritin, despite the fact that the core sizes are similar, supports the notion that most ferritin in serum is apoferritin.
The presence of iron in extracellular ferritin suggests that cells that possess a dedicated ferritin uptake pathway could benefit from this iron. But the amount of iron transported in serum ferritin is low compared with Tf-Fe (supplemental data). We therefore suggest that serum ferritin is not a major pathway of systemic iron transport, but locally secreted ferritins may play such a role in selected tissues.16,47,48 Both kidney and macrophage ferritins are rich in H-subunits (Figure 5A and not shown), and ferritin secreted from macrophages contains iron (Figure 6D). Thus, H-subunit containing heteropolymers could be selectively and locally removed by H-subunit–specific receptors for ferritin, such as TIM-2, and the residual ferritin, containing mostly L-subunits, few H-subunits and little iron would remain in the blood stream.
This leads to the question of the source of serum ferritin iron. Lysosomal “fast” ferritin was reported to contain less iron than cytosolic ferritin34 and modification at the C-terminus of the L-subunit in neuroferritinopathy enlarges the tight pores around the 4-fold axis,49 which may suggest that lysosomal ferritin can release some of the iron that it originally acquired in the cytosol. Alternatively, poly(rC)-binding protein 1 (PCBP1) has been identified as an iron chaperone that facilitates cytosolic iron loading of ferritin. Thus, yeast cells that over-express human ferritin showed poor but detectable iron loading in the absence of PCBP150 and the low iron content of serum ferritin may indicate that iron-loading of lysosomal apoferritin takes place in a compartment where PCBP1 is not present. To further characterize the location of secreted ferritin iron-loading, it will be interesting to compare iron-content, -core size and magnetic properties of secreted ferritin, which is assembled in the presence or absence of PCBP1.
In conclusion, mouse serum ferritin is an extracellular ferritin with unique characteristics: it consists of fully assembled 24-mers that contain a high fraction of 17-kD C-terminally truncated L-subunits, very few H-subunits, low iron content, and lack of glycosylation. Macrophages and kidney proximal tubule cells are likely the major cellular sources for serum ferritin. We propose that serum ferritin represents a subpopulation of intracellular ferritin that has translocated to the lysosomal compartment where it may protect this compartment and its membranes from reactive iron. From the lysosome, ferritin can be secreted through the secretory-lysosomal pathway. Secreted H-subunit containing molecules, which contain iron, may be selectively removed by H-subunit–specific ferritin-receptors, leaving behind a serum ferritin population rich in L-subunits, that contains little iron and bears signs of lysosomal processing. Serum ferritin generally reflects body iron status, but our results support the notion that serum ferritin more specifically reflects macrophage iron status. If macrophages are indeed the main source of serum ferritin, it may explain the fact that serum ferritin is elevated in inflammation, when increased hepcidin levels inhibit iron recycling from macrophages, causing macrophage iron retention, systemic iron deficiency and anemia. Our results further imply that serum ferritin may represent the end product of a pathway that begins with autophagy of cytosol, and is coupled to lysosomal processing and secretion of ferritin. This ferritin secretion pathway may represent an important and underappreciated mechanism by which cells can regulate and secrete iron.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof Abraham M. Konijn and Fanis Missirlis for support and critical reading of the manuscript, Lev Feitelberg and MsYelena Ring for technical assistance, the Prof Yuval Shoham laboratory and especially Dan Goldman for use of FPLC and hospitality, and Dr Wing-Hang Tong for being devil's advocate. Blood policy limits to 50 references and we apologize to all researchers whose original work had to be cited through reviews.
This work was funded by the US-Israel Binational Science Foundation, Grant no. 2007466 (E.M.-H. and T.R.) and the Instituto de Salud Carlos III (Spain), project PI060549 (F.J.L.)
Authorship
Contribution: L.A.C. coordinated the experiments and did most of the experimental work; L.G. and F.J.L. did the iron core analysis by TEM and magnetic susceptometry; A.W. did some of the SDS-PAGE and Western blots; Y.L.-B. did the immunofluorescence of the macrophages; D.-l.Z. and D.R.C. did the kidney work; R.S. did macrophage and kidney TEMs; A.M. did the mouse work; B.G. and M.W.H. did splenectomy and contributed to the discussion; T.A.R. contributed to discussion and experimental design, analyzed data, supervised D.-l.Z. and D.C., and edited the manuscript; and E.G.M.-H. designed the research, analyzed data, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation of L.G. is Departamento de Biomateriales y Materiales Bioinspirados, Instituto de Ciencia de Materiales de Madrid, Madrid, Spain. The current affiliation of R.S. is Imaging and Characterization Laboratory, King Abdullah University of Science and Technology, Thuwal, Kingdom of Saudi Arabia.
Correspondence: Esther G. Meyron-Holtz, Faculty of Biotechnology and Food Engineering, Israel Institute of Technology, Technion City, 32000 Haifa, Israel; meyron@tx.technion.ac.il

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal