Gr1+CD11b+ cells are characterized as myeloid-derived suppressor cells potentially involved in angiogenesis. We demonstrate that Gr1+CD11b+ cells isolated from ischemic muscle in a hind-limb ischemic C57BL/6 mouse model play a role in vessel formation after ischemic injury. Gr1dimCD11b+ cells, a subpopulation of Gr1+CD11b+ cells, within skeletal muscle were increased in context of ischemia. Strikingly, astrocyte-plexus formed from muscle-derived Gr1dimCD11b+ cells in Matrigel culture, followed by formation of isolectin and von Willebrand Factor–expressing cells, similar to that reported for angiogenesis in retina. When isolated muscle-derived Gr1dimCD11b+ cells were injected into ischemic muscles, recovery of blood flow was significantly enhanced and these cells were incorporated into vessel walls. This suggests that Gr1dimCD11b+ cells are recruited into ischemic regions after ischemia and may be involved in angiogenesis by their capacity to generate vascular cells.
Introduction
Tumor angiogenesis and vasculogenesis are modulated by monocytes and myeloid progenitors.1,2 Myeloid macrophage lineage cells with a myeloid derived suppressor cell (MDSC) phenotype of Gr1+CD11b+ are significantly increased in spleen and bone marrow (BM) of animals bearing tumors.3,,–6 MDSCs regulate tumorigenesis through induction of angiogenesis7 and suppress T cell–mediated immune responses.8,9 However, populations of Gr1+CD11b+ cells are morphologically heterogenous and contain neutrophils, immature dendritic cells, monocytes and early myeloid progenitors. In our study, we found that the number of tissue-residing Gr1+CD11b+ cells was markedly increased in ischemic muscle after femoral artery dissection. Between the 2 cell populations that comprise Gr1+CD11b+ cells, Gr1highCD11b+ and Gr1dimCD11b+, we primarily focused on Gr1dimCD11b+ cells because Gr1highCD11b+ cells, mostly neutrophils, increase in number with inflammatory response after surgery. To determine whether increases in the numbers of Gr1dimCD11b+ cells in ischemic muscle might be related to neovascularization, we evaluated whether muscle-derived Gr1dimCD11b+ cells could differentiate into endothelial cells in vitro and if direct injection of muscle-derived Gr1dimCD11b+ cells enhanced recovery of blood perfusion in ischemic hind limbs of C57BL/6 mice.
Methods
Mouse hind-limb ischemia model and isolation of Gr1dimCD11b+ cells from ischemic muscle
Ischemic injury was induced in female C57BL/6 mice (The Jackson Laboratory; 10-12 weeks old) by femoral artery and vein dissection,10,11 with Indiana University School of Medicine Institutional Animal Care and Use Committee approval. Single cell suspension was made from ischemic muscle using 0.2% collagenase type 2 (210 U/mg; Worthington) and 0.2% dispase (0.95 U/mg; Invitrogen) digestion.12,13 Isolated cell suspensions were incubated with fluorescein isothiocyanate–conjugated anti–mouse CD11b and allophycocyanin–conjugated anti–mouse Gr-1 antibody (BD Biosciences). Gr1dimCD11b+ cells and Gr1−CD11b− cells were sorted by MoFlo XDP Cell Sorter (Beckman Coulter).
FACS analysis
Isolated Gr1dimCD11b+ cells were analyzed for expression of PE-CD115, F4/80 (eBiosciences), CD45 (BD Bioscience), and Ly6C (Miltenyi Biotec) antibodies using FACS Vantage (Becton Dickinson).
Real time quantitative PCR
Real-time quantitative polymerase chain reaction (q-PCR) analysis was performed using following primers: MCP-1 forward (F): GGCTCAGCCAGATGCAGTTAA, reverse (R): CTACTCATTGGGATCATCTTGCT), SDF-1 (F:CAGCCGTGCAACAATCTGAAG, R:CTGCATCAGTGACGGTAAACC), MIP-1α (F:TCTTCTCAGCGCCATATGGA, R:CGTGGAATCTTCCGGCTGTA) and VEGF-A (F:ACCATGAACTTTCTGCTCTCTTG, R: GAACTTGATCACTTCATGGGACT). Real time q-PCR was performed by MyiQ Real Time PCR Detection Systems (Bio-Rad) and relative quantification analysis was generated with Bio-Rad IQ5 software.
Intramuscular injection of muscle derived Gr1dimCD11b+ cells and evaluation of blood flow
Isolated muscle-derived Gr1dimCD11b+ cells or Gr1−CD11b− cells (5 × 105 cells/mouse) were injected into ischemic adductor muscles 2 days after femoral vessel dissection. Blood perfusions in both ischemic and nonischemic limbs of the same mouse were evaluated using laser Doppler perfusion scanner (Moore Instruments).10,14
In vitro differentiation and in vivo localization of injected Gr1dimCD11b+ cells
For in vitro analysis, sorted muscle-derived Gr1dimCD11b+ cells and Gr1−CD11b− cells were separately seeded at 0.5 × 105/cm2 onto Matrigel (BD Bioscience)–coated chamber Slide (Lab-Tek) and cultured in EGM-2 medium (Lonza). Cultured cells were stained with anti–rabbit Glial Fibrillary Acidic Protein (GFAP; Abcam), anti–rabbit von Willebrand Factor (VWF; Santa Cruz V Biotechnology), and anti-isolectin (Sigma-Aldrich) antibodies. Binding of primary antibodies was detected with Alexa 566 anti–rabbit (Molecular Probes) antibody with nuclei stained with 4′,6-diamidino-2-phenylindole. To detect localization of injected cells and whether they incorporated into vessels, we isolated muscle-derived Gr1dimCD11b+ cells from C57BL/6 (CD45.2+) mice and injected them into ischemic muscle of congenic CD45.1 mice (B6.SJL-PtpreaPep3b/BoyJ; Jackson Immunoresearch Laboratories). Frozen tissues were stained with anti–mouse SMA (Abcam) antibody and biotin-conjugated anti–mouse CD45.2 (eBioscience). Binding of primary antibodies was detected with Alexa 488 anti–rabbit and Alexa 555–conjugated streptavidin (Molecular Probes). Tissue sections and cultured cells stained for different antigens were photographed under a Zeiss LSM 510 Meta Microscope with AxioVision software (Carl Zeiss).
Statistical analysis
Data are given as mean plus or minus SEM. Comparisons of laser Doppler imaging index between 2 groups (Gr1dimCD11b+ cells-injected group vs Gr1−CD11b− cells-injected group), considering the time effect, used Bonferroni correction. A P value of less than .05 was considered statistically significant.
Results and discussion
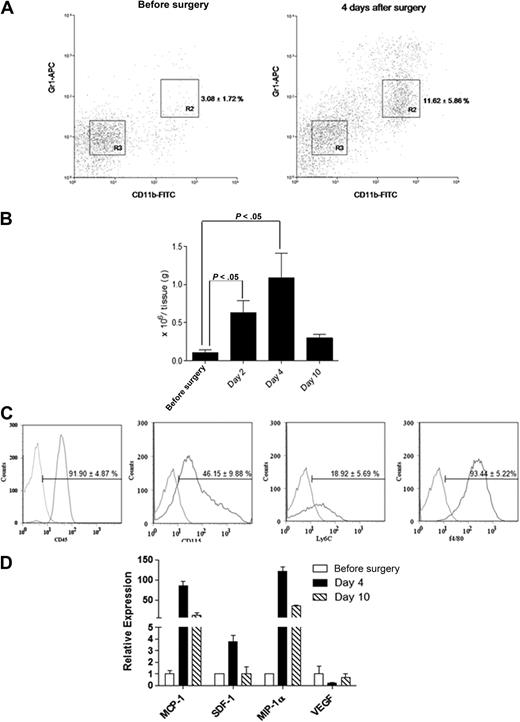
Femoral artery dissection is a common model used for studying non-tumor angiogenesis.10,15 We assessed cells with a MDSC phenotype in a C57BL/6 hind-limb ischemic model. Infiltrated Gr1dimCD11b+ cells within the ischemic hind limb were analyzed by flow cytometry (Figure 1). The percentage of infiltrated Gr1dimCD11b+ cells (R2 fraction; Figure 1A) was markedly increased after surgery (n = 5 each; 3.08% ± 1.72% before surgery vs 11.62% ± 5.86% in ischemic muscle at day 4; P < .05). Isolated Gr1dimCD11b+ cells increased in injured muscles at day 4 (n = 5 each; 0.10 ± 0.06 × 106/g tissue presurgery vs 1.13 ± 0.32 ×106/g tissue at day 4; P < .05), and dropped to noninjured muscle levels by day 10 (0.42 ± 0.33 × 106/g tissue; Figure 1B). We reasoned that Gr1dimCD11b+ cells might play a role in healing after ischemic injury. Infiltrated Gr1dimCD11b+ cells were positive for myelocyte/monocyte (CD45, CD115 and Ly6C) and macrophage (F4/80) markers (Figure 1C). Expression of candidate chemokines implicated in directing monocyte migration were analyzed.16 MCP-1 and MIP-1α mRNA expression was increased 85 and 121-fold in ischemic muscles 4 days after surgery with a lesser increase in SDF-1 (Figure 1D), suggesting that MCP-1 and MIP-1α, and perhaps SDF-1 may be involved in ischemic tissue recruitment of Gr1dimCD11b+ cells.
Ischemic muscles recruit Gr1dimCD11b+ cells after femoral artery dissection. Cell suspensions from nonsurgically treated muscles and ischemic muscles of femoral artery dissected C57BL/6 mice were stained with anti-CD11b and anti-Gr1 monoclonal antibodies. (A) Representative dot plots from nonsurgically treated muscles of C57BL/6 mice and ischemic muscles of femoral artery dissected C57BL/6. Percentages of cells are shown as the mean ± SEM (B) Total number of Gr1dimCD11b+/g tissue (n = 5 each). (C) Flow cytometric analysis of Gr1dimCD11b+ cells. Curves to right in each panel indicate staining with specific antibody, and curves to left represent staining with isolated control antibodies. (D) Real-time q-PCR expression profile of MCP-1, SDF-1, MIP-1α, and VEGF in ischemic muscle before surgery, and 4 and 10 days after surgery.
Ischemic muscles recruit Gr1dimCD11b+ cells after femoral artery dissection. Cell suspensions from nonsurgically treated muscles and ischemic muscles of femoral artery dissected C57BL/6 mice were stained with anti-CD11b and anti-Gr1 monoclonal antibodies. (A) Representative dot plots from nonsurgically treated muscles of C57BL/6 mice and ischemic muscles of femoral artery dissected C57BL/6. Percentages of cells are shown as the mean ± SEM (B) Total number of Gr1dimCD11b+/g tissue (n = 5 each). (C) Flow cytometric analysis of Gr1dimCD11b+ cells. Curves to right in each panel indicate staining with specific antibody, and curves to left represent staining with isolated control antibodies. (D) Real-time q-PCR expression profile of MCP-1, SDF-1, MIP-1α, and VEGF in ischemic muscle before surgery, and 4 and 10 days after surgery.
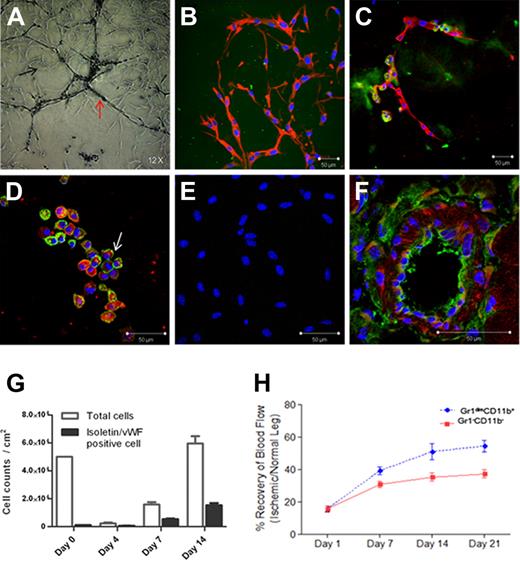
When new vessels are generated in retina, astrocyte-plexus is formed and endothelial cells proliferate.17,,–20 Four days after Gr1dimCD11b+ cell culture in Matrigel, astrocyte-plexus formed (black arrow; Figure 2A), followed by isolectin-positive endothelial cell formation (red arrow; Figure 2A, green; Figure 2C) as reported in retina. Astrocyte-plexus was positive for GFAP (red; Figure 2B), and NG-2 (data not shown), markers for astrocytes/pericytes. Isolectin-positive cells (green; Figure 2D) also expressed VWF (arrow; red; Figure 2D) in their cytoplasm. Isolectin and VWF expression was not detected in cultures of control Gr1−CD11b− cells (Figure 2E) isolated from the R3 fraction (Figure 1A). Most Gr1dimCD11b+ cells died 4 days after culture in Matrigel and the remaining cells increased 30-fold 2 weeks after culturing (Figure 2G). Less than 3% of Gr1dimCD11b+ cells were isolectin/VWF double positive cells on day 0. Isolectin/VWF double positive cells were slightly decreased by day 4, but increased after further culturing. It is possible that remaining cells after 4 days of culture might be endothelial precursor cells. As GFAP positive astrocyte-plexus, and isolectin/VWF-positive endothelial cells arose from Gr1dimCD11b+ cells, we conclude that infiltrated Gr1dimCD11b+ cells in ischemic muscles are still a heterogeneous cell population, with at least 1 subpopulation generating endothelial marker positive cells.
Cell culture and in vivo transplantation of muscle-derived Gr1dimCD11b+ and Gr1−CD11b− cells. (A) Images of cultured Gr1dimCD11b+ cells 4 days after in Matrigel. (B-C) Immunofluorescence of cultured Gr1dimCD11b+ cells with anti–rabbit GFAP antibody (red) and isolectin (green) 4 days after in Matrigel. (D) Immunofluorescence of cultured Gr1dimCD11b+ cells with anti–rabbit von Willebrand Factor (VWF) antibody (arrow; red) and isolectin (green) 4 days after in Matrigel. (E) No isolectin and VWF expressions were observed in cultured Gr1−CD11b− cells. (F) Red fluorescent signals indicate localization of transplanted C57BL/6 derived Gr1dimCD11b+ cells (CD45.2). Ten days after cell injection, CD45.2 positive cells were present in the vascular wall of the vessels of Boy/J mice. (G) Growth of Gr1dimCD11b+ cells in Matrigel culture. Total cells and isolectin/VWF double positive cells were counted at the indicated times. Quantitative analysis of hind-limb perfusion; (H) LDP index was significantly higher in the Gr1dimCD11b+ cells (5 × 105 cells /mouse) injected group compared with the control group injected with Gr1− CD11b− cells (5 × 105 cells /mouse) 3 weeks after cell injection. Data are mean ± SEM; P = .003.
Cell culture and in vivo transplantation of muscle-derived Gr1dimCD11b+ and Gr1−CD11b− cells. (A) Images of cultured Gr1dimCD11b+ cells 4 days after in Matrigel. (B-C) Immunofluorescence of cultured Gr1dimCD11b+ cells with anti–rabbit GFAP antibody (red) and isolectin (green) 4 days after in Matrigel. (D) Immunofluorescence of cultured Gr1dimCD11b+ cells with anti–rabbit von Willebrand Factor (VWF) antibody (arrow; red) and isolectin (green) 4 days after in Matrigel. (E) No isolectin and VWF expressions were observed in cultured Gr1−CD11b− cells. (F) Red fluorescent signals indicate localization of transplanted C57BL/6 derived Gr1dimCD11b+ cells (CD45.2). Ten days after cell injection, CD45.2 positive cells were present in the vascular wall of the vessels of Boy/J mice. (G) Growth of Gr1dimCD11b+ cells in Matrigel culture. Total cells and isolectin/VWF double positive cells were counted at the indicated times. Quantitative analysis of hind-limb perfusion; (H) LDP index was significantly higher in the Gr1dimCD11b+ cells (5 × 105 cells /mouse) injected group compared with the control group injected with Gr1− CD11b− cells (5 × 105 cells /mouse) 3 weeks after cell injection. Data are mean ± SEM; P = .003.
To evaluate whether muscle-derived Gr1dimCD11b+ cells contribute to neovascularization, we injected ischemic muscle-derived Gr1dimCD11b+ or control Gr1−CD11b− cells into adductor muscles of C57BL/6 mice after femoral artery dissection. Laser Doppler perfusion (LDP) index of the Gr1dimCD11b+ cell-injected group (54.5% ± 3.6% on day 21) was significantly higher than that of the Gr1−CD11b− group (37.4% ± 2.5% on day 21; p = .003; n = 9 each; Figure 2H), demonstrating improved blood perfusion in the Gr1dimCD11b+ cell-injected group. Without injection of cells, results were similar to the Gr1−CD11b− cell group (data not shown).
To explore whether injected muscle-derived Gr1dimCD11b+ cells directly incorporate into the vessel wall, C57BL/6 derived Gr1dimCD11b+ cells (CD45.2) were injected into ischemic muscle of CD45.1 positive Boy/J mice after surgery. At day 10, CD45.2 positive cells were clearly present in the CD45.1 positive Boy/J vascular wall of ischemic tissues (Figure 2F). The percentage of chimeric vessels formed by injected CD45.2 Gr1dimCD11b+ cells was 16.0% (± 4.2%), suggesting that Gr1dimCD11b+ cells may directly participate in vessel formation. However, our results do not exclude a paracrine effect of injected Gr1dimCD11b+ cells.
The results suggest that muscle-derived Gr1dimCD11b+ cells play a positive role in ischemia-induced neovascularization, events with possible clinical implications.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
These studies were supported in part by US Public Health Service grants NIH R01 HL056416, NIH R01 HL067384, and a project in NIH P01 HL053586 from the National Institutes of Health to H.E.B.
National Institutes of Health
Authorship
Contribution: J.A.K., K.M., and H.E.B. designed experiments, analyzed results, and wrote and revised drafts of the manuscript; and H.-D.C., B.J., S.-J.P., T.C., and S.M.-C. performed experiments and offered important advice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hal E. Broxmeyer, PhD, Indiana University School of Medicine, Department of Microbiology and Immunology, 950 West Walnut St, R2-302, Indianapolis, IN 46202; e-mail: hbroxmey@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal