Aberrant JAK-STAT activation characterizes human myeloproliferative neoplasms. The study by Oh and colleagues in this issue of Blood identifies STAT activation through loss of negative feedback by novel mutations of the adapter protein Lnk.1
Human myeloproliferative neoplasms (MPNs) result from dysregulated cytokine signaling. Further understanding of the key signaling nodes and relevant driver mutations in these disorders is biologically and clinically important. The best example of this targeted approach has been the treatment of chronic myelogenous leukemia (CML). The Philadelphia chromosome translocation t(9;22) product BCR-ABL was identified in the 1980s in CML cells. The aberrant protein phosphorylation due to the activated kinase activity of the BCR-ABL fusion protein led to remarkably successful new drugs targeting the kinase domain. The patients lacking BCR-ABL were essentially without a genetic explanation for many years but this is starting to change quickly. In these disorders, one common feature has been cytokine-independent proliferation due to constitutive activation of the Janus kinase (JAK)–signal transducer and activator of transcription (STAT) signaling pathway (see figure). Significant advances in genetic analysis beyond metaphase cytogenetics such as single nucleotide polymorphism arrays and high-throughput sequencing have led to rapid identification of additional genetic lesions for human MPNs. Identification of the V617F mutation in the pseudokinase domain of JAK2 in 20052-4 was rapidly followed by identification of mutation W515L in the myeloproliferative leukemia (MPL) receptor in 2006,5 and JAK2 exon 12 mutations in 2007.6 These activating mutations were found in humans, validated in cell lines, and shown to recapitulate the disease and pathophysiology in mouse retroviral and transgenic models, thus setting the standard for characterization of newly identified disease-associated mutations.
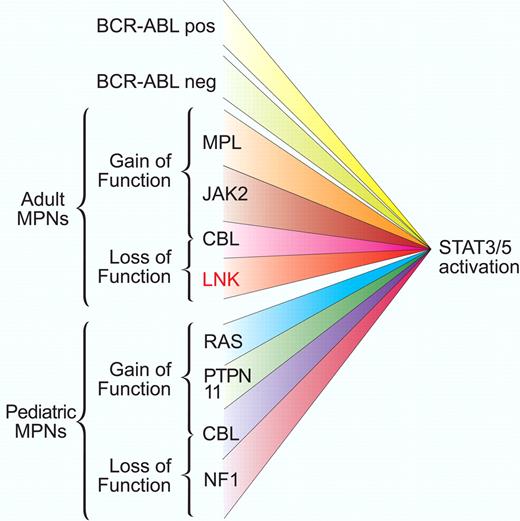
Gain-of-function and loss-of-function mutations associated with aberrant STAT activation. Adult and pediatric myeloproliferative disorders are characterized by dysregulated balance between activation and inactivation of the JAK-STAT pathway. Mutant kinase activation can occur from BCR-ABL translocation or in Philadelphia chromosome–negative patients from a short list of mutations in receptor and intracellular signaling components. Loss of negative regulator function such as Lnk, as described by Oh and colleagues,1 also facilitates JAK-STAT activation. This convergence of signaling suggests that JAK-STAT components may be good therapeutic targets for this broad class of disorders. Professional illustration by Paulette Dennis.
Gain-of-function and loss-of-function mutations associated with aberrant STAT activation. Adult and pediatric myeloproliferative disorders are characterized by dysregulated balance between activation and inactivation of the JAK-STAT pathway. Mutant kinase activation can occur from BCR-ABL translocation or in Philadelphia chromosome–negative patients from a short list of mutations in receptor and intracellular signaling components. Loss of negative regulator function such as Lnk, as described by Oh and colleagues,1 also facilitates JAK-STAT activation. This convergence of signaling suggests that JAK-STAT components may be good therapeutic targets for this broad class of disorders. Professional illustration by Paulette Dennis.
The reciprocal of increased activation of tyrosine kinases is decreased negative feedback regulation. Although descriptions of changes in methylation status of suppressor of cytokine signaling 2 (SOCS2) and SOCS3 have been observed, no mutations in these genes have yet been reported. Recent reports of frequent mutations in c-Cbl highlight the essential role of ubiquitin-mediated degradation of signaling components in the control of myeloproliferation.7,8 c-Cbl mutations are more complex than simple loss of function, because they still maintain adapter protein function and can facilitate signaling in a positive manner.9 In the current study by Oh et al,1 another link to activation of JAK-STAT is reported which is of immediate clinical interest. In mouse models it has been known that the inhibitory adapter protein Lnk is associated with erythropoietin and thrombopoietin signaling and is required for their down-modulation, and Lnk can bind to wild-type and mutant JAK2 and modulate growth. However, no mutations in Lnk had been described.
From a total of 33 JAK2V617F-negative MPN samples, Oh and colleagues found 2 patients, 1 with primary myelofibrosis and 1 with essential thrombocythemia with whole or partially inactivating mutations in Lnk. These mutations were cloned and tested biochemically in transfected BaF3 cells and shown to exhibit JAK/STAT activation with high levels of STAT3 and STAT5 activation. Interestingly, Lnk mutations did not evoke cytokine-independent growth but rather stimulated thrombopoietin and granulocyte colony-stimulating factor–induced growth. The authors did not explore the very interesting but more subtle differences between the mutants and it will be important to move deeper into understanding the biochemical consequences of these mutations on Lnk localization. The broader prevalence in a larger clinical sample size will also be needed to determine whether these mutations could occur in polycythemia vera alone or alongside JAK2 or MPL mutations and whether they are associated with additional subtypes and overlap syndromes proceeding to leukemic transformation.
A notable feature of the study is application of the sophisticated intracellular phospho-flow cytometry method to identify a unique STAT3/STAT5 double-positive population. It will be interesting to know how broadly this population occurs in other types of MPN and whether the phospho-STAT3/5 double-positive population confers greater diagnostic or prognostic power than phospho-STAT3 or phospho-STAT5 alone. The response of the STAT3/5 population to JAK inhibition suggests potential therapeutic opportunity at least for controlling proliferative and survival signaling.
Although the present study stopped short of functionally testing the Lnk mutations in a mouse model, the biochemical data suggest that Lnk mutations in vivo could behave much like those of the Lnk−/− mouse model that has been well characterized by several groups. Further study could provide unique insights into the signals required for pathogenesis and the role of Lnk in myelofibrosis. This interesting brief report provides additional diagnostic markers for human MPNs and again highlights the downstream activation of STAT5 in these disorders. A similar scenario has emerged in juvenile myelomonocytic leukemia where mutations in RAS, SHP-2, NF1, and c-CBL result in activated STAT5. Although a variety of signaling pathways including ERK and AKT are activated by these mutations, thus far the degree of phospho-STAT5 as determined by intracellular flow cytometry stands out as an attractive biomarker for patient prognosis.10 Additional interrogation of the JAK-STAT pathway in hematologic malignancies promises to reveal additional new insights into leukemogenesis and new approaches for targeted therapy.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal