The recent shift to the use of stem cells mobilized by granulocyte colony-stimulating factor (G-CSF) for hematopoietic transplantation has increased chronic graftversus-host disease (GVHD), although the mechanisms of this are unclear. We have found that G-CSF invokes potent type 17 rather than type 1 or type 2 differentiation. The amplification of interleukin-17 (IL-17) production by G-CSF occurs in both CD4 and CD8 conventional T cells and is dependent on, and downstream of, G-CSF–induced IL-21 signaling. Importantly, donor IL-17A controls the infiltration of macrophages into skin and cutaneous fibrosis, manifesting late after transplantation as scleroderma. Interestingly, donor CD8 T cells were the predominant source of IL-17A after transplantation and could mediate scleroderma independently of CD4 T cells. This study provides a logical explanation for the propensity of allogeneic stem cell transplantation to invoke sclerodermatous GVHD and suggests a therapeutic strategy for intervention.
Introduction
Granulocyte colony-stimulating factor (G-CSF) is increasingly described as an immunomodulatory agent for a diverse range of diseases; and although the cytokine is usually associated with the regulation of immune responses, clear evidence exists that it can also exacerbate pathology in some settings. A major use of G-CSF is in the mobilization of stem cells for use in allogeneic hemopoietic stem cell transplantation (SCT). Graft-versus-host disease (GVHD) remains a major complication after SCT, and in the acute form is established as a Th1 (ie, interferon-γ [IFN-γ])–mediated disease that impairs survival of transplantation recipients.1 The chronic form is less well characterized but has features of an autoimmune disease. The use of G-CSF–mobilized stem cell grafts clinically has led to rapid hematopoietic reconstitution, improved leukemia eradication, similar levels of acute GVHD, but increased chronic GVHD.2 In murine models, donor pretreatment with G-CSF attenuates acute GVHD.3 The mechanism of this effect has been suggested, at least in part, to be the result of the promotion of Th2 differentiation and regulatory mechanisms, both within T cells and antigen-presenting cells.4 The induction of Th2 differentiation after G-CSF has been in the setting of low doses, and at higher doses this effect is lost.4 Furthermore, the mechanism by which G-CSF alters chronic GVHD remains elusive, largely because the pathogenesis of this disease is unclear, although recent evidence suggests that donor B cells and pathogenic autoantibodies contribute by driving a fibrogenic response.5,6 Here we examined the ability of G-CSF to influence Th17 differentiation and the subsequent effects on GVHD.
Methods
Mice
Female B6 (H-2b, CD45.2+) or B6 Ptprca, (H-2b, CD.45.1+), BALB/c (H-2d, CD45.2+), BALB/B (H-2b, CD45.2+), DBA/2 (H-2d, CD45.2+), and B6D2F1 (H-2b/d, CD45.2+) mice were purchased from the Animal Resources Center. Interleukin-10 (IL-10)−/−, IFN-γ−/−, TNF−/−, IL-6−/−, Jα18−/−, γδ−/− mice (B6, H-2b), and IL-4−/− (BALB/c, H-2d) mice were bred and supplied by the Herston Medical Research Center. IL-17A−/− B6 and BALB/c mice were supplied by Dr Iwakura, University of Tokyo, Japan, and the IL-17R−/− mice (B6, H-2b) were supplied by Amgen. IL-21R−/− B6 mice were supplied by M.J.S. Mice were backcrossed at least 9 generations, housed in sterilized microisolator cages, and received acidified autoclaved water (pH 2.5) after transplantation. All mice experiments were performed in accordance with, and under the approval of, the Queensland Institute of Medical Research Animal Ethics Committee.
mAbs
The following monoclonal antibodies (mAbs) were purchased from BioLegend: phycoerythrin (PE)–conjugated CD3 (145-2C11), Armenian hamster IgG isotype control (HTK888), anti-IL-17A (TC11-18H10.1), and IgG2b isotype control; allophycocyanin-conjugated CD8 (53-6.7), anti–IFN-γ (XMG1.2), and IgG2b isotype control; PE-Cy7-conjugated CD8 (53-6.7) and IgG2b isotype control; and Alexa Fluor 647–conjugated anti-FoxP3 (150D) and mouse IgG1 control. PE-conjugated IL-21 (mhaix21) was purchased from eBioscience. Purified mAbs against CD3 (KT3), CD19 (HB305), Gr1 (RB6-8C5), Thy1.2 (HO-13-4), Ter119, and fluorescein isothiocyanate–conjugated CD4 (GK1.5) and IgG2b (Mac5) isotype control were produced in-house.
Cytokine mobilization
Recombinant human G-CSF was given subcutaneously at 10 μg/animal daily (or as otherwise stated) for 4 days.
Cell preparation, culture, and cytokine analysis
Unfractionated splenocytes from naive or G-CSF–treated animals were cultured at an equivalent of 2.5 × 105 CD3+ T cells/mL with CD3 (2C11, 2 μg/mL), or concanavalin A (2.5 μg/mL) and tissue culture supernatants collected after 72 hours. IL-17A and IL-17F within tissue culture supernatants were determined by enzyme-linked immunosorbent assay (BioLegend and R&D Systems, respectively), and all other cytokines were determined by cytokine bead array (BD Biosciences) as per the manufacturer's instructions. In some experiments, before culture, cell subsets were selected or depleted by labeling splenocytes with mAbs as indicated, followed by separation and collection of the labeled or unlabeled cells by FACS sorting (MoFlo, Dako Cytomation). For intracellular cytokine staining, naive splenocytes were cultured with CD3 (2C11, 2 μg/mL) and IL-23 (10 ng/mL, eBioscience) for 48 hours with brefeldin (BioLegend) added for the last 2 hours. For analysis of cytokine production after SCT by intracellular cytokine staining, cells were stimulated for 4 hours with phorbol myristate acetate (50 ng/mL) and ionomycin (500 ng/mL) with brefeldin added for the last 2 hours. The cells were then surface-labeled with CD4-fluorescein isothiocyanate and CD8-PeCy7 and processed for intracellular staining of IL-17 and IFN-γ using a BD cytofix/cytoperm kit (BD Biosciences) as per the manufacturer's instructions. Labeled cells were analyzed by flow cytometry (FACSCanto, BD Biosciences). For transplantation, total T-cell depletion (TCD) was performed by incubating splenocytes with hybridoma supernatants containing anti-CD4 (RL172.4), anti-CD8 (TIB211), and Thy1.2 (HO-13-4) monoclonal antibodies followed by incubation with rabbit complement (Cedarlane Laboratories) as previously described.7 Resulting cell suspensions contained less than 1% contamination of viable CD3+ T cells.
Real-time PCR
Total RNA was extracted and prepared from unfractionated or sort-purified cell populations, and RORγt, IL-21, and IL-6 mRNA levels were determined using TaqMan Gene Expression Assays (Applied Biosystems) as recently described.8 All measurements were normalized against the expression of the housekeeping gene β2-microglobulin.
SCT
Mice were transplanted as described previously.7,9,10 Briefly, on day −1, recipient mice received 900 to 1100 cGy total body irradiation (137Cs source at 108 cGy/min), split into 2 doses separated by 3 hours to minimize gastrointestinal toxicity. Donor splenocytes (10-25 × 106/per animal) were injected intravenously on day 0. Non-GVHD control groups were injected with TCD splenic grafts. In bone marrow transplantation (BMT) experiments, B6 recipients received BALB/c grafts containing 107 bone marrow cells with or without 5 × 106 purified splenic T cells. Transplanted mice were monitored daily, and those with GVHD clinical scores11 of 6 were killed and the date of death registered as the next day in accordance with institutional animal ethics committee guidelines.
Histopathology and immunostaining of GVHD target organs
At various times after transplantation, GVHD target tissue was harvested, formalin-preserved, embedded in paraffin, and processed to generate 5-μm-thick sections. Hematoxylin and eosin-stained sections of gut (small bowel), lung, liver, and skin were examined in a blinded fashion (by A.D.C.) using a semiquantitative scoring system for GVHD as previously published.8 Scores on tissue taken 28 days after transplantation were added to provide a total score of 28 for gut, 18 for lung, 40 for liver, and 24 for skin. For quantification of chronic GVHD in the skin more than 30 days after transplantation, blinded hematoxylin and eosin samples were scored from 0 to 4 for epidermal inflammation and dermal inflammation (summed to give a cutaneous inflammation score of 8), dermal fibrosis and subcutaneous fibrosis (summed to give a total total fibrosis score of 8), subcutaneous fibrosis (total score = 4), and loss of subcutaneous fat (total score = 4). In addition, Masson staining of collagen was performed on the same skin samples. Immunostaining was performed on formalin-preserved paraffin-embedded 3- to 4-μm tissue sections using a standard 2-step immunoperoxidase method. Primary antibodies were F4/80 (Abcam) and CD3 (Biocare Medical). Neutrophils were labeled using a standard histochemical Leder stain (chloroacetate esterase). Images were acquired using an Olympus BX51 microscope (Olympus Japan) and Evolution MP 5.0 Camera and QCapture software (QImaging).
Statistical analysis
Survival curves were plotted using Kaplan-Meier estimates and compared by log-rank analysis. P value less than .05 was considered statistically significant. An unpaired 2-tailed Mann-Whitney U test was used to evaluate significant differences between groups in cytokine and mRNA studies. Data are mean plus or minus SEM.
Results
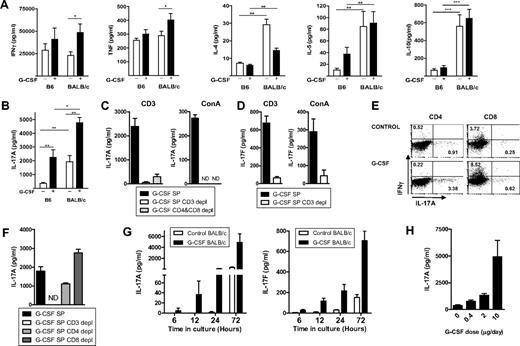
We first compared the effect of donor treatment with G-CSF on Th1, Th2, and regulatory cytokine production in B6 and BALB/c mice, the latter of which are known to be more responsive to G-CSF.12 Surprisingly, the classic Th1 cytokines IFN-γ and tumor necrosis factor (TNF) were either not affected or actually increased slightly after G-CSF administration; and consistent with previous reports using high G-CSF doses,3,4,13,14 there was no clear induction of Th2 differentiation by G-CSF in either strain (Figure 1A). Notably, BALB/c mice produced significantly higher levels of Th2 (IL-4, IL-5, and IL-10) cytokines than B6 mice irrespective of G-CSF treatment. We therefore studied the Th17 pathway that has not been explored previously in this setting. Surprisingly, G-CSF invoked IL-17A secretion in both mouse strains (Figure 1B), and this was confirmed to be secreted almost entirely by CD3+ T cells in these assays (Figure 1C). IL-17F was also produced by in these cultures, again predominantly by CD3+ T cells (Figure 1D). G-CSF induction of IL-17A occurred in both CD4+ and CD8+ T-cell populations (Figure 1E), although its production was enhanced in the absence of CD8+ T cells (Figure 1F), suggesting a regulatory role for this population, possibly via their enhanced IFN-γ production. Importantly, the amplification of IL-17A and IL-17F generation after G-CSF administration was evident within 6 hours of T-cell activation (Figure 1G), suggesting that type 17 differentiation had been induced during mobilization within the donor. Consistent with this, the degree of IL-17 differentiation was proportional to the dose of G-CSF used during mobilization (Figure 1H).
Stem cell mobilization with G-CSF enhances IL-17A production by splenic CD4+ and CD8+ T cells. (A) Th1 (IFN-γ and TNF), Th2 (IL-4, IL-5), and IL-10 and (B) IL-17A in culture supernatants generated by splenocytes from naive or G-CSF–mobilized WT B6 or BALB/c mice stimulated with CD3 for 72 hours. Histograms represent mean ± SEM (n = 6-12 individual animals/group) of data from 2 or 3 replicate experiments. *P < .05. **P < .01. ***P < .001. (C) IL-17A generation from splenocytes stimulated with CD3 or concanavalin A for 72 hours from G-CSF–mobilized B6 mice FACS sort depletion of CD3+ or CD4+ and CD8+ T-cell populations. Whole spleen and sorted cell populations were plated in triplicate, and histograms represent mean ± SEM from one of 2 replicate experiments. ND indicates not detected. (D) IL-17F in culture supernatants were generated as in panel C. (E) Flow cytometric analysis of intracellular IL-17A and IFN-γ production in CD4+ and CD8+ T cells after culture of B6 splenocytes for 48 hours in the presence of CD3 and IL-23. Numbers within quadrants represent frequency of IL-17A– or IFN-γ–producing cells within CD4 or CD8 compartments. (F) IL-17A generation by splenocytes stimulated with CD3 for 72 hours from G-CSF–mobilized B6 mice ± FACS sort depletion of CD3+, CD4+, or CD8+ T-cell populations. Sorted cell populations were plated in triplicate, and histograms represent mean ± SEM from one of 2 identical experiments. (G) Time course of IL-17A and IL-17F generation in vitro. Splenocytes from nonmobilized or G-CSF–mobilized donors (4 per group) were stimulated as in panel B and IL-17A and IL-17F determined in culture supernatants taken at the time points indicated. (H) Donors (4 per group) were mobilized with a range of G-CSF doses (micrograms per day) as indicated and IL-17A determined in culture supernatants 72 hours later.
Stem cell mobilization with G-CSF enhances IL-17A production by splenic CD4+ and CD8+ T cells. (A) Th1 (IFN-γ and TNF), Th2 (IL-4, IL-5), and IL-10 and (B) IL-17A in culture supernatants generated by splenocytes from naive or G-CSF–mobilized WT B6 or BALB/c mice stimulated with CD3 for 72 hours. Histograms represent mean ± SEM (n = 6-12 individual animals/group) of data from 2 or 3 replicate experiments. *P < .05. **P < .01. ***P < .001. (C) IL-17A generation from splenocytes stimulated with CD3 or concanavalin A for 72 hours from G-CSF–mobilized B6 mice FACS sort depletion of CD3+ or CD4+ and CD8+ T-cell populations. Whole spleen and sorted cell populations were plated in triplicate, and histograms represent mean ± SEM from one of 2 replicate experiments. ND indicates not detected. (D) IL-17F in culture supernatants were generated as in panel C. (E) Flow cytometric analysis of intracellular IL-17A and IFN-γ production in CD4+ and CD8+ T cells after culture of B6 splenocytes for 48 hours in the presence of CD3 and IL-23. Numbers within quadrants represent frequency of IL-17A– or IFN-γ–producing cells within CD4 or CD8 compartments. (F) IL-17A generation by splenocytes stimulated with CD3 for 72 hours from G-CSF–mobilized B6 mice ± FACS sort depletion of CD3+, CD4+, or CD8+ T-cell populations. Sorted cell populations were plated in triplicate, and histograms represent mean ± SEM from one of 2 identical experiments. (G) Time course of IL-17A and IL-17F generation in vitro. Splenocytes from nonmobilized or G-CSF–mobilized donors (4 per group) were stimulated as in panel B and IL-17A and IL-17F determined in culture supernatants taken at the time points indicated. (H) Donors (4 per group) were mobilized with a range of G-CSF doses (micrograms per day) as indicated and IL-17A determined in culture supernatants 72 hours later.
To understand the effect of the Th1/Th2 cytokine milieu controlling Th-17 differentiation during stem cell mobilization with G-CSF, we studied IL-17A and IL-17F generation in the absence of prototypic Th1/Th2 cytokines known to be important in transplantation outcome. In the absence of IFN-γ, TNF, IL-10, or IL-17R, the basal level of secretion of both IL-17A and IL-17F from untreated B6 mice was increased. However, only the presence of IFN-γ appeared to suppress G-CSF–induced production of both IL-17A and IL-17F (Figure 2A-B). Interestingly, in the absence of IL-17 signaling, G-CSF induction of IL-17A, but not IL-17F, was enhanced above that from wild-type (WT; IL-17R+/+) T cells (Figure 2A-B). The presence or absence of IL-4 had no effect on IL-17A (Figure 2C) or IL-17F secretion (data not shown). Thus, none of the Th1/Th2 cytokines examined appeared responsible for the effects of G-CSF on inducing IL-17A generation.
G-CSF–induced IL-17A secretion is independent of Th1/Th2 cytokines and IL-21 dependent. (A) IL-17A and (B) IL-17F in culture supernatants generated by splenocytes from naive or G-CSF–mobilized WT or respective knockout B6 mice stimulated with CD3 for 72 hours. (C) IL-17A in culture supernatants generated by splenocytes from naive or G-CSF–mobilized WT or BALB/c.IL-4−/− mice stimulated with CD3 for 72 hours. Splenocytes from individual animals were cultured separately (n = 3-7 individual animals/condition pooled from 2 or 3 replicate experiments). Data represent mean ± SEM. *P < .05, **P < .01, naive vs G-CSF–treated. (D-E) IL-17A in culture supernatants generated by splenocytes from naive or G-CSF–mobilized (D) WT, Jα18−/− or (E) γδ−/− B6 mice (n = 5-10 per group from replicate experiments) stimulated with CD3 for 72 hours. NS indicates not significant. (F) TGF-β1 in culture supernatants generated by splenocytes from naive or G-CSF–mobilized BALB/c.WT mice stimulated with CD3 for 72 hours. (G) Real-time polymerase chain reaction (PCR) analysis of IL-6 mRNA expression (left) by nonstimulated splenocytes from naive or G-CSF–treated B6 or BALB/c mice. Data represent pooled data from duplicate experiments with n = 3 or 4 mice/group. IL-6 protein secretion (right) by splenocytes stimulated with CD3 for 72 hours from naive or G-CSF–treated B6 or BALB/c mice. Histogram represents pooled data (n = 9-11 individual animals/condition) from 3 replicate experiments. *P < .05, **P < .01, ***P < .001 for marked groups. (H) IL-17A in culture supernatants generated by splenocytes from naive or G-CSF–mobilized WT, IL-6−/− or (I) IL-12/23p40−/− or (J) B6.IL-21R−/− mice stimulated with CD3 for 72 hours. Histograms represent combined data (n = 7-10 individual animals/condition) pooled from replicate experiments. (K) Real-time PCR analysis of IL-21 mRNA expression in freshly isolated splenocytes (T0) or those cultured for 24 hours with CD3 (T24) from naive (white bars) or G-CSF–treated (black bars) B6 mice. Splenocytes from individual animals were cultured separately. (Left graph) Combined data (n = 3 or 4 individual animals/condition) from replicate experiments. (Right graph) IL-21 mRNA expression levels in FACS-sorted cell subsets from T24 cultures represented as combined data (n = 3-6 individual animals/condition) from replicate experiments. Data are mean ± SEM. (L) Real-time PCR analysis of IL-21 mRNA expression in splenocytes cultured for 24 hours with CD3 from WT or IL-17A−/− donors. Combined data (n = 4 or 5 individual animals/condition) from replicate experiments.
G-CSF–induced IL-17A secretion is independent of Th1/Th2 cytokines and IL-21 dependent. (A) IL-17A and (B) IL-17F in culture supernatants generated by splenocytes from naive or G-CSF–mobilized WT or respective knockout B6 mice stimulated with CD3 for 72 hours. (C) IL-17A in culture supernatants generated by splenocytes from naive or G-CSF–mobilized WT or BALB/c.IL-4−/− mice stimulated with CD3 for 72 hours. Splenocytes from individual animals were cultured separately (n = 3-7 individual animals/condition pooled from 2 or 3 replicate experiments). Data represent mean ± SEM. *P < .05, **P < .01, naive vs G-CSF–treated. (D-E) IL-17A in culture supernatants generated by splenocytes from naive or G-CSF–mobilized (D) WT, Jα18−/− or (E) γδ−/− B6 mice (n = 5-10 per group from replicate experiments) stimulated with CD3 for 72 hours. NS indicates not significant. (F) TGF-β1 in culture supernatants generated by splenocytes from naive or G-CSF–mobilized BALB/c.WT mice stimulated with CD3 for 72 hours. (G) Real-time polymerase chain reaction (PCR) analysis of IL-6 mRNA expression (left) by nonstimulated splenocytes from naive or G-CSF–treated B6 or BALB/c mice. Data represent pooled data from duplicate experiments with n = 3 or 4 mice/group. IL-6 protein secretion (right) by splenocytes stimulated with CD3 for 72 hours from naive or G-CSF–treated B6 or BALB/c mice. Histogram represents pooled data (n = 9-11 individual animals/condition) from 3 replicate experiments. *P < .05, **P < .01, ***P < .001 for marked groups. (H) IL-17A in culture supernatants generated by splenocytes from naive or G-CSF–mobilized WT, IL-6−/− or (I) IL-12/23p40−/− or (J) B6.IL-21R−/− mice stimulated with CD3 for 72 hours. Histograms represent combined data (n = 7-10 individual animals/condition) pooled from replicate experiments. (K) Real-time PCR analysis of IL-21 mRNA expression in freshly isolated splenocytes (T0) or those cultured for 24 hours with CD3 (T24) from naive (white bars) or G-CSF–treated (black bars) B6 mice. Splenocytes from individual animals were cultured separately. (Left graph) Combined data (n = 3 or 4 individual animals/condition) from replicate experiments. (Right graph) IL-21 mRNA expression levels in FACS-sorted cell subsets from T24 cultures represented as combined data (n = 3-6 individual animals/condition) from replicate experiments. Data are mean ± SEM. (L) Real-time PCR analysis of IL-21 mRNA expression in splenocytes cultured for 24 hours with CD3 from WT or IL-17A−/− donors. Combined data (n = 4 or 5 individual animals/condition) from replicate experiments.
We next analyzed the contribution of known IL-17-producing cell subsets for their contribution to Th17 responses during G-CSF mobilization. Interestingly, IL-17 generation was still promoted in the absence of either type I NKT cells (Figure 2D) or γ-δ T cells (Figure 2E), although it was diminished in the absence of the former. Thus, IL-17 production after G-CSF administration was predominantly from conventional CD4+ and CD8+ T-cell populations (consistent with Figure 1E) in addition to type I NKT cells. To understand the possible Th17 cofactors induced by G-CSF, we next studied the most widely reported cytokines critical to this differentiation pathway, TGF-β and IL-6. TGF-β was not produced in a differential fashion in response to G-CSF (Figure 2F), and the inhibition of TGF-β with the 1D11 antibody had no effect on IL-17 generation (data not shown). Interestingly, both IL-6 mRNA and protein were induced by mobilization with G-CSF (Figure 2G), but this was not responsible for the induction of IL-17 because it still occurred in IL-6−/− donors (Figure 2H). Similarly, IL-17 generation was also promoted in the absence of IL-23 and IL-12 (Figure 2I). Finally, the absence of IL-21 signaling completely prevented the enhancement of IL-17 production, confirming this as the dominant pathway invoked by G-CSF (Figure 2J). Notably, the induction of IL-21 by G-CSF was predominantly within the CD4+ T-cell compartment (Figure 2K), and this was independent of IL-17A itself (Figure 2L). Thus, G-CSF invoked Th17 differentiation, initially by the induction of IL-21 from donor CD4 T cells.
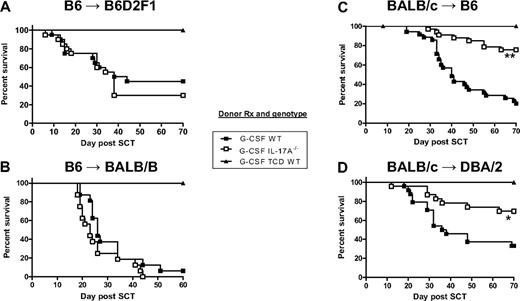
We next studied the effects of Th17 generation on transplantation outcome when using G-CSF–mobilized grafts. The mobilization and transplantation of grafts from WT or IL-17A−/− B6 donors resulted in identical GVHD outcomes in models of GVHD to both major histocompatibility complex (Figure 3A) and minor histocompatibility antigens (Figure 3B). In contrast, the transplantation of grafts from IL-17A−/− BALB/c donors resulted in attenuated GVHD in both systems (Figure 3C-D). These findings were next analyzed by histopathology. In recipients of B6 donor grafts, IL-17A promoted cutaneous GVHD with increased levels of both inflammation and fibrosis in the skin of WT grafts, but GVHD was not altered in other organs (Figure 4A,C). In the recipients of BALB/c donor grafts, cutaneous GVHD was again IL-17A-dependent, but GVHD pathology was also reduced in the lung and liver of recipients of IL-17A−/− grafts (Figure 4B-C), consistent with the survival advantage seen in these animals. Thus, the severity of GVHD was always seen to be IL-17A-dependent in skin but IL-17A-independent in the gastrointestinal tract, regardless of the strain of the donor used. The differential data generated with the 2 donor strains probably reflect the effect of IL-17A in conjunction with the background levels of other Th2 and Tc2 cytokines generated. Thus, the GVHD generated using BALB/c donors is less severe and later in onset because of the time taken to generate lethal levels of visceral GVHD. In contrast, severe gastrointestinal GVHD is invoked within a week when using B6 donors, and disease severity in this organ is the principal determinant of transplantation outcome.15 To study GVHD in the absence of G-CSF–mobilized grafts, we transplanted BALB/c bone marrow and purified T cells into lethally irradiated B6 recipients. Although these BMT recipients received the same T-cell doses as those receiving G-CSF–mobilized grafts, GVHD mortality was minimal (Figure 5A). However, clinical scores (Figure 5A) and cutaneous GVHD were significantly lower in recipients of IL-17A−/− grafts (Figure 5B-C). Furthermore, recipients of nonmobilized WT splenocytes had similar mortality to G-CSF–mobilized IL-17A−/− grafts, and both were significantly reduced compared with G-CSF–mobilized WT grafts (day 70 survival 69% and 76%, respectively, vs 20%; P < .01). Thus, GVHD was exacerbated by G-CSF mobilization in an IL-17A–dependent fashion, but IL-17A is still pathogenic in the absence of G-CSF.
IL-17A enhances GVHD mortality in models using BALB/c donors. Survival by Kaplan-Meier analysis of lethally irradiated (A) B6D2F1 or (B) BALB.B mice (n = 16-20 /group) transplanted with grafts from G-CSF–mobilized B6.WT or B6.IL-17A−/− donors. G-CSF–treated TCD B6.WT grafts (n = 7 or 8) were used as non-GVHD controls. Data combined from 2 experiments for each model. Lethally irradiated (C) B6 (n = 33-35/group) or (D) DBA/2 (n = 23 or 24 /group) mice were transplanted with grafts from G-CSF–mobilized WT or IL-17A−/− BALB/c donors. G-CSF–treated TCD WT grafts (n = 12-15) were used as non-GVHD controls. **P < .001 WT vs IL-17A−/− in BALB/c → B6 model. *P < .012 WT vs IL-17A−/− in BALB/c → DBA/2 model. Data combined from 3 or 4 experiments for each model.
IL-17A enhances GVHD mortality in models using BALB/c donors. Survival by Kaplan-Meier analysis of lethally irradiated (A) B6D2F1 or (B) BALB.B mice (n = 16-20 /group) transplanted with grafts from G-CSF–mobilized B6.WT or B6.IL-17A−/− donors. G-CSF–treated TCD B6.WT grafts (n = 7 or 8) were used as non-GVHD controls. Data combined from 2 experiments for each model. Lethally irradiated (C) B6 (n = 33-35/group) or (D) DBA/2 (n = 23 or 24 /group) mice were transplanted with grafts from G-CSF–mobilized WT or IL-17A−/− BALB/c donors. G-CSF–treated TCD WT grafts (n = 12-15) were used as non-GVHD controls. **P < .001 WT vs IL-17A−/− in BALB/c → B6 model. *P < .012 WT vs IL-17A−/− in BALB/c → DBA/2 model. Data combined from 3 or 4 experiments for each model.
IL-17A mediates cutaneous GVHD. Lethally irradiated (A) B6D2F1 (n = 6) or (B) B6 mice (n = 5 or 6) were transplanted with G-CSF–mobilized grafts from WT or IL-17A−/− B6 donors or WT or IL-17A−/− BALB/c donors, respectively. Non-GVHD controls (n = 3) received T-cell–depleted G-CSF WT grafts. Tissue injury expressed as a mean composite score was determined by semiquantitative histology as described in “Histology and immunostaining of GVHD target organs.” **P < .01, WT vs IL-17A−/−. (C) Representative images (original magnification ×400) of hematoxylin and eosin–stained paraffin sections of tissues taken 28 days after transplantation.
IL-17A mediates cutaneous GVHD. Lethally irradiated (A) B6D2F1 (n = 6) or (B) B6 mice (n = 5 or 6) were transplanted with G-CSF–mobilized grafts from WT or IL-17A−/− B6 donors or WT or IL-17A−/− BALB/c donors, respectively. Non-GVHD controls (n = 3) received T-cell–depleted G-CSF WT grafts. Tissue injury expressed as a mean composite score was determined by semiquantitative histology as described in “Histology and immunostaining of GVHD target organs.” **P < .01, WT vs IL-17A−/−. (C) Representative images (original magnification ×400) of hematoxylin and eosin–stained paraffin sections of tissues taken 28 days after transplantation.
IL-17A is generated predominantly from donor CD8 T cells after transplantation. (A) Survival by Kaplan-Meier analysis of lethally irradiated B6 recipients (n = 20 per group) transplanted with bone marrow and purified splenic T cells from nonmobilized BALB/c.WT or BALB/c.IL-17A−/− donors. A non-GVHD control group received TCD bone marrow only (n = 7). Data combined from 2 replicate experiments. Final clinical scores from week 7 after BMT are also shown. (B) Semiquantitative histopathology of skin 7 weeks after BMT (n = 9 per GVHD group and n = 2 in TCD group). **P < .01, P < .05, WT vs IL-17A−/−. (C) Representative histopathology at week 7 after BMT (original magnification ×200). (D) Lethally irradiated B6 recipients received nonmobilized WT splenocytes or G-CSF–mobilized splenocytes from WT or IL-17A−/− BALB/c donors (n = 5-14 per group). Seven days after SCT, splenic T cells were stimulated for 12 hours with CD3 and cytokine levels determined in culture supernatants. **P < .01, *P < .05, G-CSF–mobilized WT and IL-17A−/− vs nonmobilized. ND indicates not detected. (E) Ratio of effector (CD4+FoxP3neg) to regulatory (CD4+FoxP3+) T cells in spleen of respective B6 recipients of BALB/c grafts. (F) Representative plots of IL-17A and IFN-γ secretion in splenic (SP) and inguinal lymph node (LN) cells 7 days after SCT. (Right) The number of respective cytokine-producing CD4 and CD8 T cells per spleen (n = 4 per group, one of 3 experiments). *P < .05, CD8 vs CD4. (G) Relative expression of RORγt in sort-purified donor CD4 and CD8 T cells 7 days after SCT (n = 5 per group). (H) Representative plots of IL-17A and IL-21 secretion in splenic CD4 and CD8 T cells 7 days after SCT.
IL-17A is generated predominantly from donor CD8 T cells after transplantation. (A) Survival by Kaplan-Meier analysis of lethally irradiated B6 recipients (n = 20 per group) transplanted with bone marrow and purified splenic T cells from nonmobilized BALB/c.WT or BALB/c.IL-17A−/− donors. A non-GVHD control group received TCD bone marrow only (n = 7). Data combined from 2 replicate experiments. Final clinical scores from week 7 after BMT are also shown. (B) Semiquantitative histopathology of skin 7 weeks after BMT (n = 9 per GVHD group and n = 2 in TCD group). **P < .01, P < .05, WT vs IL-17A−/−. (C) Representative histopathology at week 7 after BMT (original magnification ×200). (D) Lethally irradiated B6 recipients received nonmobilized WT splenocytes or G-CSF–mobilized splenocytes from WT or IL-17A−/− BALB/c donors (n = 5-14 per group). Seven days after SCT, splenic T cells were stimulated for 12 hours with CD3 and cytokine levels determined in culture supernatants. **P < .01, *P < .05, G-CSF–mobilized WT and IL-17A−/− vs nonmobilized. ND indicates not detected. (E) Ratio of effector (CD4+FoxP3neg) to regulatory (CD4+FoxP3+) T cells in spleen of respective B6 recipients of BALB/c grafts. (F) Representative plots of IL-17A and IFN-γ secretion in splenic (SP) and inguinal lymph node (LN) cells 7 days after SCT. (Right) The number of respective cytokine-producing CD4 and CD8 T cells per spleen (n = 4 per group, one of 3 experiments). *P < .05, CD8 vs CD4. (G) Relative expression of RORγt in sort-purified donor CD4 and CD8 T cells 7 days after SCT (n = 5 per group). (H) Representative plots of IL-17A and IL-21 secretion in splenic CD4 and CD8 T cells 7 days after SCT.
We next analyzed T-cell differentiation after SCT. Recipients of G-CSF–mobilized grafts secreted higher levels of IL-17A, IL-17F, and IL-21 after SCT, and increases in IL-17F and IL-21 were maintained in the absence of IL-17A (Figure 5D), confirming that the type 17 response induced by G-CSF was not IL-17A-dependent. As expected, in the absence of IL-17A, donor T cells secreted more IFN-γ and IL-4 after restimulation (Figure 5D), effectively excluding these cytokines as causative in cutaneous GVHD (because even with high levels, the absence of IL-17A was protective). Numbers of FoxP3+ Treg were similar between groups (Figure 5E). Most importantly, there was a 2-fold increase in the proportion of IL-17A-secreting cells in CD8+ compared with CD4+ T-cell fractions (Figure 5F). However, because of the much higher numbers of CD8+ T cells, the CD8+IL-17+ T cells outnumbered CD4+IL-17+ T cells by 10- to 20-fold in both in the B6 → B6D2F1 (Figure 5F) and the BALB/c → B6 system (data not shown). Furthermore, donor CD8 T cells were polyfunctional such that all IL-17A-producing cells also made IFN-γ (Figure 5F), and RORγt levels were highest in the recipients of G-CSF–mobilized donor CD8 T cells (Figure 5G). Surprisingly, donor CD8+ T cells were also a major source of IL-21 after SCT (Figure 5H). Thus, classic paradigms of T-cell differentiation are not adhered to after SCT. Together, these data suggest that the protection from skin GVHD seen in the absence of donor IL-17A is the result of its effects as an effector molecule rather than its influence on T-cell differentiation.
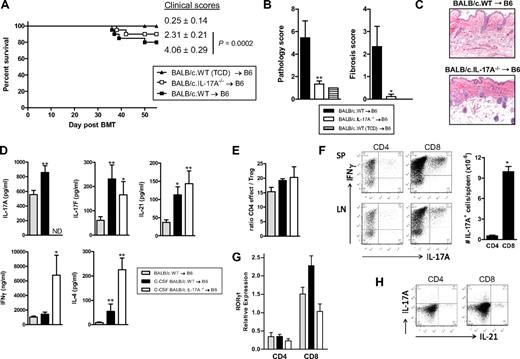
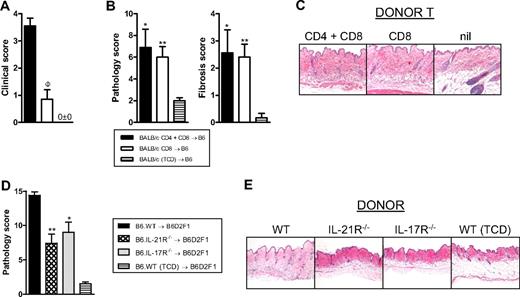
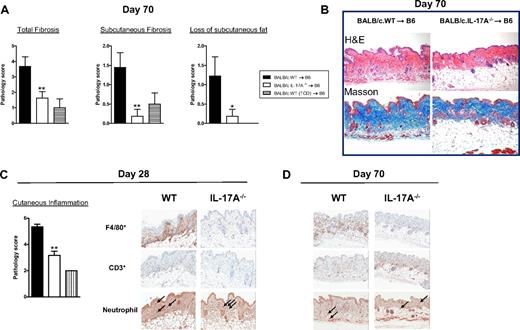
Autoantibodies (antinuclear Ab) were generated only at low levels after SCT in these models and did not correlate with chronic GVHD or the presence of IL-17A because they were highest in TCD recipients with no clinical or histologic evidence of GVHD (data not shown). Because most IL-17A was derived from CD8+ T cells after SCT, we undertook transplantations in the presence or absence of donor CD4+ T cells. In the absence of CD4+ T cells, GVHD appeared minimal because clinical scores were similar to recipients of T cell-depleted grafts (Figure 6A). Surprisingly, however, the recipients of CD8+ T cells only had similar levels of cutaneous GVHD (Figure 6B), including fibrosis (Figure 6B-C), to recipients of both CD4+ and CD8+ T cells. Thus, cutaneous GVHD and fibrosis can develop in a CD4-independent fashion. We next confirmed that IL-21 and IL-17 signaling within donor cells contributed to cutaneous GVHD because recipients of either IL-21R−/− or IL-17R−/− grafts developed significantly less pathology (Figure 6D-E). In the course of these studies, we noted that the skin of recipients of BALB/c grafts was developing fibrosis in the context of inflammation 4 weeks after SCT and demonstrated overt thickening and lichenoid changes late after transplantation (> day 60). We thus hypothesized that these animals were developing scleroderma. Histologic analysis of the skin of these animals confirmed features of moderate to severe cutaneous and subcutaneous fibrosis extending into subcutaneous fat in recipients of WT grafts, but this was absent in recipients of IL-17A−/− grafts (Figure 7A-B). In contrast, there were reduced levels of inflammation within the skin in recipients of IL-17A−/− grafts 28 days after transplantation (Figure 7C), but by day 70 there were only low levels of inflammatory infiltrate in recipients of either WT or IL-17A−/− grafts (data not shown). We next investigated the nature of the inflammatory response within the skin of SCT recipients developing scleroderma. Surprisingly, T-cell infiltration occurred to similar levels in the skin of recipients of both WT and IL-17A−/− grafts (Figure 7C-D). In contrast, the major effect of IL-17A appeared to be as a chemoattractant for macrophages because F4/80+ cells were markedly increased in the skin of WT grafts but largely absent in recipients of IL-17A−/− grafts (Figure 7C-D). Thus, the generation of IL-17A from the donor graft was not required for the generation of an inflammatory T-cell infiltrate within skin during GVHD but was important for macrophage infiltration and the subsequent fibrotic response late after transplantation. In addition, hepatic GVHD remained attenuated at day 70 in recipients of IL-17A−/− grafts (pathology scores, 5.7 ± 0.6, IL-17A−/− vs 11.6 ± 1.3, WT; P = .013).
Cutaneous GVHD is CD4-independent but requires signaling through the donor IL-17 and IL-21 receptor. (A) Clinical scores 6 weeks after transplantation of lethally irradiated B6 recipients (n = 10 per group) of G-CSF–mobilized BALB/c.WT grafts containing both CD4 and CD8 T cells or equivalent numbers of CD8 T cells only. A non-GVHD control group received TCD G-CSF–mobilized splenocytes only (n = 6). ΦP < .001, CD4 plus CD8 vs CD8. (B) Semiquantitative histopathology of skin 6 weeks after BMT (n = 9 or 10 per GVHD group and n = 6 in TCD group). **P < .01, *P < .05, CD4 plus CD8 T and CD8 T vs TCD, respectively. (C) Representative histopathology at week 6 after BMT (original magnification ×200). (D) Semiquantitative skin histopathology 5 weeks after transplantation in lethally irradiated B6D2F1 recipients (n = 5-9 per group) transplanted with G-CSF–mobilized B6.WT, B6.IL-21R−/− or B6.IL-17R−/− splenocytes. A non-GVHD control group received B6.WT T-cell-depleted G-CSF–mobilized splenocytes only (n = 4). *P < .05, WT vs IL-21R−/− and IL-17R−/−. (E) Representative histopathology at week 7 after BMT (original magnification ×100).
Cutaneous GVHD is CD4-independent but requires signaling through the donor IL-17 and IL-21 receptor. (A) Clinical scores 6 weeks after transplantation of lethally irradiated B6 recipients (n = 10 per group) of G-CSF–mobilized BALB/c.WT grafts containing both CD4 and CD8 T cells or equivalent numbers of CD8 T cells only. A non-GVHD control group received TCD G-CSF–mobilized splenocytes only (n = 6). ΦP < .001, CD4 plus CD8 vs CD8. (B) Semiquantitative histopathology of skin 6 weeks after BMT (n = 9 or 10 per GVHD group and n = 6 in TCD group). **P < .01, *P < .05, CD4 plus CD8 T and CD8 T vs TCD, respectively. (C) Representative histopathology at week 6 after BMT (original magnification ×200). (D) Semiquantitative skin histopathology 5 weeks after transplantation in lethally irradiated B6D2F1 recipients (n = 5-9 per group) transplanted with G-CSF–mobilized B6.WT, B6.IL-21R−/− or B6.IL-17R−/− splenocytes. A non-GVHD control group received B6.WT T-cell-depleted G-CSF–mobilized splenocytes only (n = 4). *P < .05, WT vs IL-21R−/− and IL-17R−/−. (E) Representative histopathology at week 7 after BMT (original magnification ×100).
Incidence of scleroderma in the presence or absence of donor-derived IL-17. (A) Lethally irradiated recipient B6 mice were transplanted with grafts from G-CSF–treated WT (n = 9) or IL-17A−/− (n = 11) BALB/c donors. Non-GVHD recipients received T-cell-depleted WT grafts (n = 4). Histologic scoring of parameters of scleroderma 70 days after transplantation as detailed in “Histology and immunostaining of GVHD target organs.” Data represent mean ± SEM pooled from 2 replicate experiments. (B) Representative images (original magnification ×400) of hematoxylin and eosin or Masson (which stains collagen blue) stained paraffin sections of skin taken 70 days after transplantation. (C-D) Lethally irradiated recipient B6 mice were transplanted with grafts from G-CSF–treated WT or IL-17A−/−BALB/c donors (n = 6 per group). Non-GVHD recipients received T-cell-depleted WT grafts (n = 3). Histologic scoring of parameters of inflammation in skin 28 days after transplantation as detailed in “Histology and immunostaining of GVHD target organs.” Representative images of cellular infiltration within skin from B6 recipients of BALB/c grafts from G-CSF–treated WT or IL-17A−/− donors at day 28 and day 70 after SCT. Macrophages (F4/80+) and T cells (CD3+) are stained brown. Neutrophils are stained red (arrow) by chloroacetate esterase Leder stain. **P < .01, *P < .05, WT vs IL-17A−/− grafts.
Incidence of scleroderma in the presence or absence of donor-derived IL-17. (A) Lethally irradiated recipient B6 mice were transplanted with grafts from G-CSF–treated WT (n = 9) or IL-17A−/− (n = 11) BALB/c donors. Non-GVHD recipients received T-cell-depleted WT grafts (n = 4). Histologic scoring of parameters of scleroderma 70 days after transplantation as detailed in “Histology and immunostaining of GVHD target organs.” Data represent mean ± SEM pooled from 2 replicate experiments. (B) Representative images (original magnification ×400) of hematoxylin and eosin or Masson (which stains collagen blue) stained paraffin sections of skin taken 70 days after transplantation. (C-D) Lethally irradiated recipient B6 mice were transplanted with grafts from G-CSF–treated WT or IL-17A−/−BALB/c donors (n = 6 per group). Non-GVHD recipients received T-cell-depleted WT grafts (n = 3). Histologic scoring of parameters of inflammation in skin 28 days after transplantation as detailed in “Histology and immunostaining of GVHD target organs.” Representative images of cellular infiltration within skin from B6 recipients of BALB/c grafts from G-CSF–treated WT or IL-17A−/− donors at day 28 and day 70 after SCT. Macrophages (F4/80+) and T cells (CD3+) are stained brown. Neutrophils are stained red (arrow) by chloroacetate esterase Leder stain. **P < .01, *P < .05, WT vs IL-17A−/− grafts.
Discussion
G-CSF is widely used in SCT, and its effects on immunity have been widely studied in both preclinical and clinical settings. Early preclinical studies have demonstrated the ability of G-CSF to invoke Th2 responses without impairment of cytotoxic T-cell responses after transplantation.3,16 This phenotype has been widely associated with a separation of GVHD and graft-versus-leukemia effects in murine studies.17,–19 Reductions in Th1 cytokine profiles have been seen in the peripheral blood of healthy donors,20 and increases in the Th2 transcription factor GATA3 have also been described.21 However, additional insights from preclinical and clinical models suggest that the Th2 paradigm may be an oversimplification because G-CSF also appears to induce effects on regulatory T cells and antigen-presenting cells with clear downstream effects on GVHD.14,22,23 In addition, any effects on Th2 differentiation are lost at higher doses of G-CSF.7 Importantly, IL-12, IL-4, and TGF-β appear to be the master regulators of Th1, Th2, and Treg differentiation, respectively, and so G-CSF would be expected to invoke responses in these cytokines. However, although these cytokines impact on GVHD,24,,,–28 their modification in response to G-CSF is highly variable.
G-CSF signals via its surface receptor to invoke Stat3 phosphorylation and in turn is negatively regulated by the suppressor of cytokine signaling-3 molecule.29 Other cytokine pathways using the same cell signaling pathways include IL-6, IL-10, IL-21, and IL-23.30 Here we demonstrate the ability of G-CSF to invoke Th17 responses, which appear to dominate over effects on Th1/Th2 differentiation. The differentiation of Th17 cells is complex but is promoted by IL-6 and TGF-β, which induce the RORγt transcription factor.31 Importantly, it is now clear that Th17 differentiation may occur independently of IL-6 and probably TGF-β also.32,33 The Th17 phenotype is stabilized by IL-23 and subsequently amplified by IL-21.34 Of these important cofactors, we found IL-21 to be the critical cytokine controlling the induction of Th17 generation by G-CSF. IL-21 is produced largely by NK cells, NKT cells, and follicular helper CD4 T cells.34 The generation of IL-21 from donor cells early after stimulation was predominantly from CD4 T cells; but after SCT, donor CD8 cells became a major source of IL-21, which is produced independently of IL-17A and presumably is capable of maintaining Tc17 T cells in isolation. These findings would appear to be highly relevant clinically as 2 recent reports have also demonstrated an increase in IL-17A secretion from CD4 T cells in donors mobilized with G-CSF.35,36 Our study provides mechanistic data and outlines the important functional consequences of this in regard to transplantation outcome.
The ability of IL-17A to influence GVHD outcome in relation to acute GVHD is somewhat controversial. The first study to report the use of IL-17A−/− donors suggested that IL-17A attenuated severe acute GVHD because of the suppression of Th1 differentiation.37 A subsequent report using the same mouse strain combinations suggested a transient protection from CD4-dependent acute GVHD but concluded that overall the cytokine was redundant.38 A third report suggested that in vitro generated B6-derived Th17 cells were capable of inducing lethal GVHD, particularly within lung and skin.39 In our hands, the mobilization of B6 donors with G-CSF augments IL-17A secretion, but the presence or absence of this cytokine plays no role in GVHD mortality or the induction of visceral organ-specific pathology. However, cutaneous GVHD was IL-17A dependent. In these systems, significant and early Th1-dependent gastrointestinal tract injury occurs, which largely dictates transplantation outcome.15 More recent studies from Yi et al40 using an array of B6-derived cytokine knockout donor mice have expanded our previous studies,8 confirming that GVHD within gastrointestinal tract and liver is largely Th1 (IFN-γ) dependent, whereas that in skin is more Th2 (IL-4) and Th17 (IL-17) dependent. Interestingly, lung GVHD appeared almost exclusively Th2 dependent. This may be consistent with the pattern of GVHD seen in these studies where we used BALB/c donors, which are known to generate preferential Th2 (and now Th17) cytokine responses and are also more sensitive to G-CSF.12 However, the fact that skin GVHD occurred in an IL-17A-dependent and CD4-independent fashion in the context of predominantly CD8-dependent IL-17A generation after SCT provides a more logical explanation for the discrepancies in the literature to date. The IL-21 dependence of a CD8+ driven response is also entirely consistent. Interestingly, we have recently demonstrated that the pathogenic effects of G-CSF administered after BMT are also CD8-dependent.41 Grafts from BALB/c donors induced GVHD in a more delayed fashion than B6 donors and in this setting, GVHD mortality, as well as hepatic, lung, and cutaneous organ pathology, was demonstrated to be IL-17A dependent. Thus, the pattern of GVHD organ involvement seen after transplantation is probably more complex than the dominance of a single cytokine and most probably reflects the balance of all type 1, type 2, and type 17 effectors in both CD4+ and CD8+ T cells, in addition to CD8+ T-cell cytolytic pathways and specific donor-host antigenic disparities.
In contrast to acute GVHD, chronic GVHD is a poorly understood process and appears to involve donor B cells5 and autoantibody generation,6 which can induce the fibrogenic pathways characteristic of scleroderma, although the latter concept is also somewhat controversial.42,43 Additional animal data suggest that this process may be the result of a failure of thymic negative selection44 because of the thymus being a major target of GVHD itself. Importantly, the process does not appear to be a clear Th1- or Th2-dependent process, and the dominant role of TGF-β as an effector molecule of fibrosis24 suggests that this cytokine may be involved in driving the pathologic T-cell differentiation responsible for disease. Importantly, the regulatory and Th17 pathways best fulfill this requirement for TGF-β, and the generation of additional cytokines during GVHD (eg, IL-6, IL-21) will inherently promote Th17 generation over the purely regulatory T-cell pathway of differentiation. Within the GVHD target organs, IL-17A probably acts directly, as suggested by the requirement for the IL-17R, and/or as a chemoattractant. The latter may attract other cell types via the induction of additional cytokines (eg, IL-6 and IL-8) and chemokines (eg, CXCL1 and CXCL10),45 which may in turn attract monocytes and macrophages that are known to be important sources of TGF-β and thus mediators of fibrosis.24,46 It is important to note that scleroderma is the major clinical burden of disease late after allogeneic SCT, and here we demonstrate this process to be IL-17-dependent, regardless of the model tested.
These studies provide new insight into the ability of G-CSF to modify T cell-dependent diseases. Paradoxically, we demonstrate that immune responses in G-CSF–treated donors are characterized by regulatory4,9,47 and type 17 differentiation, explaining the divergent effects of G-CSF mobilization on acute and chronic GVHD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Leukemia Foundation of Australia and the National Health and Medical Research Council. G.R.H. and M.J.S. are National Health and Medical Research Council Fellows. K.P.A.M. is a National Health and Medical Research Council R. D. Wright Fellow.
Authorship
Contribution: G.R.H. and K.P.A.M. designed studies and helped write the manuscript; S.D.O., R.D.K., A.V., N.C.R., K.A.M., and Y.A.W. performed experiments; A.L.D. performed experiments and generated reagents; M.J.S. helped design experiments and provided vital reagents; Y.I. and J.T. provided vital reagents; and A.D.C. performed all blinded histologic analyses.
Conflict-of-interest disclosure: J.T. is employed by a company with a commercial interest in reagents that are relevant to this study. The remaining authors declare no competing financial interests.
Correspondence: Kelli P. A. MacDonald, Bone Marrow Transplantation Laboratory, Queensland Institute of Medical Research, 300 Herston Rd, Herston, Queensland 4006, Australia; e-mail: kelli.macdonald@qimr.edu.au.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal