Abstract
The transcription factor, CCAAT enhancer binding protein alpha (C/EBPα), is crucial for granulopoiesis and is deregulated by various mechanisms in acute myeloid leukemia (AML). Mutations in the CEBPA gene are reported in 10% of human patients with AML. Even though the C/EBPα mutants are known to display distinct biologic function during leukemogenesis, the molecular basis for this subtype of AML remains elusive. We have recently showed the significance of deregulation of C/EBPα-regulated microRNA (miR) in AML. In this study, we report that miR-34a is a novel target of C/EBPα in granulopoiesis. During granulopoiesis, miR-34a targets E2F3 and blocks myeloid cell proliferation. Analysis of AML samples with CEBPA mutations revealed a lower expression of miR-34a and elevated levels of E2F3 as well as E2F1, a transcriptional target of E2F3. Manipulation of miR-34a reprograms granulocytic differentiation of AML blast cells with CEBPA mutations. These results define miR-34a as a novel therapeutic target in AML with CEBPA mutations.
Introduction
Acute myeloid leukemia (AML) is characterized by gene mutations, chromosomal aberrations, and epigenetic modifications.1 Transcription factors have been discovered to be key targets of mutation in AML.2 CCAAT enhancer binding protein alpha (C/EBPα) is one of the major regulators in granulopoiesis.2 During granulopoiesis, C/EBPα regulates differentiation at multiple steps, including the transition from the common myeloid progenitor to the granulocytic-macrophage progenitor.3 A growing number of studies indicate that C/EBPα is down-regulated by various mechanisms in AML, suggesting C/EBPα is a myeloid tumor suppressor.4 Mutations in the CEBPA gene are present in approximately 10% of AML cases.5 Reported mutations of CEBPA include frame-shift mutations at the N-terminus, which result in the truncated form of C/EBPα (C/EBPα-p30), as well as point mutations at the C-terminus.5 These mutations result in proteins that fail to induce granulopoiesis6 and have the potential to induce leukemia in mouse models.7,8
C/EBPα induces myeloid differentiation via 2 major steps: (1) up-regulation of myeloid-specific genes necessary for granulocytic maturation and (2) inhibition of myeloid cell proliferation.2,9 Loss of one of these functions results in a block of granulocytic differentiation. Different mechanisms have been reported for C/EBPα-mediated inhibition of cell-cycle machinery.4,5 During granulopoiesis, inhibition of E2F members has been shown as a unique mechanism through which C/EBPα inhibits cell cycle progression.2,5 Interestingly, loss of C/EBPα-mediated E2F inhibition has been shown to be instrumental in the leukemic transformation process in AML with CEBPA mutations.7 We have recently shown that C/EBPα targets E2F1 via miR-223, and that this pathway is deregulated in different subtypes of AML.10 We have also reported that mutated C/EBPα (C/EBPα-p30) cooperates with E2F1 to block granulocyte differentiation in AML with CEBPA mutations.11 Given the importance of deregulation of the C/EBPα-E2F pathway in AML, understanding the mechanism of regulation of E2F activity by C/EBPα is critical in the development of novel therapeutic agents in AML.
microRNAs (miRNAs) function as key regulators of gene expression programs.12 microRNAs control various tumor suppressors and oncogenes, thereby contributing major roles in different steps of carcinogenesis.13 microRNA-34a (miR-34a) is a widely expressed microRNA and is regulated by the tumor suppressor, p53.14 miR-34a is down-regulated in a variety of tumors.14 These findings suggest that miR-34a acts as a tumor suppressor in various tissues. miR-34a expression correlates with CEBPA mutations in AML.15 However, there has been no report that shows any specific function of miR-34a in granulopoiesis. We investigated the role of miR-34a in granulopoiesis and in AML with CEBPA mutations. Here, we report that C/EBPα directly regulates miR-34a during granulopoiesis. miR-34a blocks myeloid cell cycle progression by inhibiting E2F3. Interestingly, miR-34a was observed to be down-regulated in AML samples with CEBPA mutations. We also observed that E2F3 protein levels, as well as protein levels of E2F1, a major transcriptional target of E2F3, were elevated in AML samples with CEBPA mutations. Taken together, our study provides evidence that deregulation of the C/EBPα-miR-34a-E2F3 axis forms the molecular basis for AML with CEBPA mutations.
Methods
Patient samples
AML blast cells were obtained from the Children's Oncology Group Myeloid Reference Bank at Fred Hutchinson Cancer Research Center, Seattle, WA; University Hospital of Munich, Munich, Germany; University of Lille Medical School, Lille, France; and University Hospital of Münster, Münster, Germany. The study protocols used for AML patient sample collection were approved by the ethics committees of the participating centers. All patients provided written informed consent in accordance with the Declaration of Helsinki. Mononuclear cells from bone marrow were enriched by Ficoll gradient centrifugation. Human umbilical cord blood samples were collected after full-term delivery with informed consent of the mothers from University Hospital of Halle, Halle, Germany. Hematopoietic CD34+ cells were isolated from cord-blood samples using CD34+ selection kit as previously described.16 After isolation of CD34+ cells, the percentage of cells positive for CD34 antigen was analyzed by fluorescence-activated cell sorting analysis using phycoerythrin (PE)–conjugated mouse anti–human CD34 antibody (BD Biosciences) and was found to be approximately 82%.
Molecular analysis
Cell cultures
K562-C/EBPα-p42-ER, K562-C/EBPα-p30-ER, K562-C/EBPα-BRM2-ER, and K562-ER cells were maintained in RPMI 1640 without phenol red supplemented with 10% charcoal-treated fetal bovine serum (FBS), 1% penicillin-streptomycin, and 2 μg/mL puromycin.6 Kasumi-6 cells were cultured in RPMI 1640 supplemented with 20% FBS, 1% penicillin-streptomycin, and 2 ng/mL GM-CSF (colony-stimulating factor)19 ; AML blast cells were cultured in Iscove modified Dulbecco medium supplemented with 20% FBS, 1% penicillin-streptomycin, and 20mM HEPES; NB4 cells were cultured in RPMI, supplemented with 10% FBS and 1% penicillin-streptomycin.
For differentiation experiments, CD34+ cells were seeded at 1 × 105/mL in Iscove modified Dulbecco medium supplemented with 10% FBS, 1% penicillin-streptomycin, and then cultured for 16 days in the presence of a cytokine cocktail: days 1-5: recombinant human stem-cell factor (50 ng/mL), fms-like tyrosine kinase 3 (100 ng/mL), interleukin-3 (IL-3; 5 ng/mL), GM-CSF (5 ng/mL), and G-CSF (30 ng/mL); days 5-8: IL-3 (5 ng/mL) and G-CSF (30 ng/mL); and days 8-16: G-CSF (30 ng/mL). On days 5, 8, 11, and 14, cells were harvested, washed, and expanded with fresh media supplemented with cytokines.
For differentiation of K562-C/EBPα-ER cell lines, cells (1 × 106) were induced with 5μM β-estradiol (Sigma-Aldrich) dissolved in ethanol. NB4 cells (1 × 106) were induced by the addition of 1μM of retinoic acid (Sigma-Aldrich) dissolved in dimethyl sulfoxide.
RNA isolation and miRNA detection
Total RNA from cells was extracted using the TRIzol method. The miRNA quantification was done with the TaqMan miRNA Detection kit (Applied Biosystems) using 100 ng of RNA in a Rotor-Gene RG-3000 cycler (Corbett Research) by the comparative CT method using U6 expression for normalization. Corresponding reverse-transcription (RT) and PCR primers for miRNA-34a, miR-223, miR-181a, and U6 were obtained from Applied Biosystems. All reactions were performed in triplicate.
DNA constructs and cloning
The luciferase vector containing wild-type and mutant E2F3 3′untranslated region (UTR) has been published.20 miR-34a promoter construct #1 (PM-34a-K1/2) was published before.21 miR-34a promoter constructs (2-5) were amplified from human genomic DNA and cloned into KpnI and XhoI digested pGL3 basic vector. Primer sequences are provided in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). miR-34a promoter construct #6 was generated using site-directed mutagenesis of the miR-34a promoter, construct #4, using the QuikChange Site-Directed Mutagenesis kit (Stratagene), according to the manufacturer's instructions. Primer sequences are provided in supplemental Table 1.
Luciferase assays
To test whether miR-34a directly targets E2F3, Kasumi-6 cells were transiently transfected with 0.7 μg of the E2F3 3′UTR reporter constructs (wild-type and mutant), 0.1 μg of Renilla construct, and 0.2 μg of miR-34a-pCDNA by Effectene transfection reagent (QIAGEN), as described by the manufacturer. Firefly luciferase activities from the promoter constructs and Renilla luciferase activity from the internal control plasmid were determined 24 hours after transfection using the Dual-Luciferase Reporter Assay System (Promega). Values were normalized using Renilla luciferase.
To find the regulation of miR-34a promoter by C/EBPα, Kasumi-6 cells were transiently transfected with 0.7 μg of the miR-34a promoter constructs (miR-34a promoters 1-6), 0.1 μg of Renilla construct, and 0.2 μg of control or C/EBPα-pCDNA3 vector by Effectene transfection reagent. Promoter activity was done as described in the previous paragraph.
Lentiviral transfections
Lentiviral transfections were done as reported before.21
Cell-cycle analysis
For cell-cycle analysis, Kasumi-6 cells were transfected with control or miR-34a-pCDNA vectors. Two days later, cells were fixed in cold ethanol, washed, resuspended in phosphate-buffered saline containing 50 μg/mL propidium iodide and 50 μg/mL RNAase A, and analyzed by flow cytometry on a FACScan (BD Biosciences).
Chromatin immunoprecipitation
The cross-linking of proteins to DNA was accomplished by the addition of 1% formaldehyde for 10 minutes to cultured cells at 37°C. After sonication, the chromatin was immunoprecipitated with 5 μg of anti-C/EBPα and anti–immunoglobulin G (IgG; Santa Cruz Biotechnology) antibodies at 4°C overnight. Primer sequences are provided in supplemental Table 1.
Cell differentiation
Cell differentiation was assessed by light microscopy morphologic examination of Wright-Giemsa–stained cytospins, quantitative real-time PCR (Q-RT-PCR) for G-CSFR and M-CSFR, and FACS analysis using PE-conjugated mouse anti–human CD11b, CD14, and CD41 antibodies (BD Biosciences) as previously described.11
Annexin V assay
The annexin V assay was carried out in conjunction with 7-amino-actinomycin D staining according to the manufacture's protocol (BD Pharmingen).
Statistical analysis
We used Student t tests to determine the statistical significance of experimental results. A P value of .05 or less was considered significant. The results were represented as the mean ± SD from 3 independent experiments.
Results
C/EBPα-p42 up-regulates miR-34a during granulopoiesis
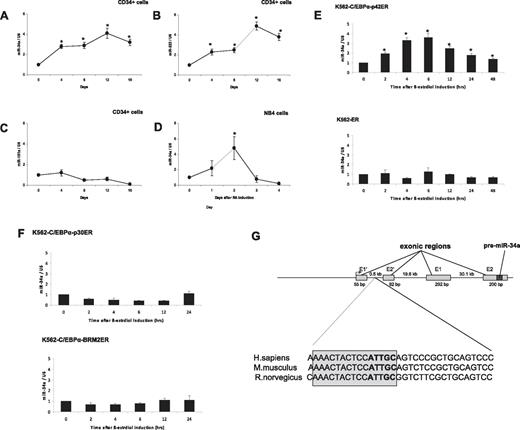
Recent work has shown that miR-34a expression correlates with CEBPA mutations in AML.15 To assess the role of miR-34a in granulopoiesis, we analyzed expression levels of this microRNA during differentiation of cord blood–derived CD34+ hematopoietic progenitor cells (see “Patient samples” for details). CD34+ cells were cultured in the presence of a sequential cytokine cocktail, which has been shown to induce granulopoiesis.16 We observed granulocytic differentiation of CD34+ cells, as evaluated by morphology and granulocytic marker (supplemental Figure 1). miR-34a expression was analyzed by real-time RT-PCR. We observed a gradual increase of miR-34a expression during granulocytic differentiation and maturation (Figure 1A). We also analyzed the expression levels of miR-223, a microRNA that has been shown to be up-regulated, and miR-181a, which has been shown not to be regulated, during granulopoiesis.23 As reported before, we found that miR-223 expression increased during granulocytic differentiation without any up-regulation in the expression levels of miR-181a (Figure 1B-C). To further understand the regulation of miR-34a during granulopoiesis, we analyzed its expression during retinoic acid (RA)–induced differentiation of NB4 cells. As shown in Figure 1D, we observed that RA was able to induce the expression of miR-34a. Interestingly, the miR-34a levels returned to basal levels after 2 days. This is similar to miR-223 levels in NB4 cells during RA-induced granulopoieisis.10 This suggests that during RA-induced differentiation, myeloid microRNAs are up-regulated only at earlier time points. To understand the regulation of miR-34a by C/EBPα proteins, we used an inducible cell-line model, K562-C/EBPα-ER. We selected this cell line because these cells do not have endogenous C/EBPα and have been reported as a good model for granulocytic differentiation in the context of C/EBPα proteins.6 K562-C/EBPα-ER cell lines were established by stably transfecting K562 cells with a plasmid encoding an estrogen-inducible C/EBPα (p42 form as well as mutants) estrogen receptor fusion protein.6 We observed that β-estradiol treatment of K562-C/EBPα-p42-ER cells induces granulocytic differentiation (supplemental Figure 2). Our data suggest that induction of C/EBPα leads to up-regulation of miR-34a expression in K562-C/EBPα-p42-ER cells (Figure 1E top). The induction of miR-34a was found to be high at 6 hours, which returned to basal levels at later time points. We have observed a similar pattern of regulation of miR-223 in K562-C/EBPα-p42-ER cells.10 C/EBPα induction was able to differentiate K562-C/EBPα-p42-ER cells in a very short time interval (4 days).6 So, this pattern of expression of miR-34a could be associated with C/EBPα expression, because C/EBPα is not expressed at terminal stages of granulopoiesis. We did not observe any up-regulation of miR-34a in the control cell line, K562-ER (Figure 1E bottom). CEBPA mutations comprise N-terminal frame-shift mutations and C-terminal point muta-tions (supplemental Figure 3). We next investigated how these mutants regulate miR-34a. We observed that these 2 mutants (N-terminal mutant K562-C/EBPα-p30-ER and C-terminal mutant K562-C/EBPα-BRM2-ER) failed to up-regulate miR-34a (Figure 1F). These data suggest that up-regulation of miR-34a during granulopoiesis is a specific function of C/EBPα-p42.
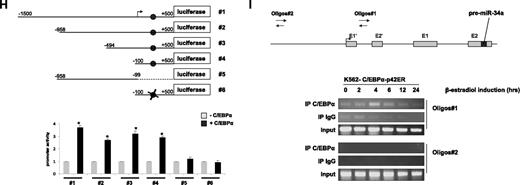
C/EBPα-p42 regulates miR-34a during granulopoiesis. (A-C) Hematopoietic CD34+ cells were cultured as discussed in “Cell cultures.” Total RNA was isolated at different time points and analyzed by quantitative real-time RT-PCR with oligos for miR-34a (A), miR-223 (B), and miR-181a (C). Data are represented as mean ± SD from 3 independent experiments. *P < .05. (D) NB4 cells were induced with retinoic acid (1μM) for respective time points. Total RNA was analyzed by quantitative real-time RT-PCR with oligos for miR-34a. Data are represented as mean ± SD from 3 independent experiments. *P < .05. (E) K562-C/EBPα-p42-ER and K562-ER cells were induced with β-estradiol (5μM) for respective time points. Total RNA was analyzed by quantitative real-time RT-PCR with oligos for miR-34a. Data are represented as mean ± SD from 3 independent experiments. *P < .05. (F) K562-C/EBPα-p30-ER and K562-C/EBPα-BRM2-ER cells were induced with β-estradiol (5μM) for respective time points. Total RNA was analyzed by quantitative real-time RT-PCR with oligos for miR-34a. Data are represented as mean ± SD from 3 independent experiments. (G) Schematic representation of the miR-34a genomic region and phylogenic conservation of genomic region in the first intron of miR-34a in humans, mice, and rats. The conserved region is shown by sequences in gray box, and the C/EBPα site is shown in bold letters. (H) Luciferase reporter assays were performed in Kasumi-6 cells using indicated reporters and C/EBPα. Cells were transfected with corresponding firefly luciferase vectors (miR-34 Promoter constructs #1 to #6, Renilla luciferase reporter construct as control vector, and C/EBPα-pCDNA3 vector. Luciferase activity was measured 24 hours later. Bars represent promoter activity for the corresponding vectors. Data are represented as mean ± SD from 3 independent experiments. *P < .05. (I) Chromatin derived from K562-C/EBPα-p42-ER cells was immunoprecipitated with anti-C/EBPα and IgG antibodies. Recovered DNA was PCR amplified with primers specific for C/EBPα-binding amplicon (oligos 1) and the nonbinding amplicon (oligos 2).
C/EBPα-p42 regulates miR-34a during granulopoiesis. (A-C) Hematopoietic CD34+ cells were cultured as discussed in “Cell cultures.” Total RNA was isolated at different time points and analyzed by quantitative real-time RT-PCR with oligos for miR-34a (A), miR-223 (B), and miR-181a (C). Data are represented as mean ± SD from 3 independent experiments. *P < .05. (D) NB4 cells were induced with retinoic acid (1μM) for respective time points. Total RNA was analyzed by quantitative real-time RT-PCR with oligos for miR-34a. Data are represented as mean ± SD from 3 independent experiments. *P < .05. (E) K562-C/EBPα-p42-ER and K562-ER cells were induced with β-estradiol (5μM) for respective time points. Total RNA was analyzed by quantitative real-time RT-PCR with oligos for miR-34a. Data are represented as mean ± SD from 3 independent experiments. *P < .05. (F) K562-C/EBPα-p30-ER and K562-C/EBPα-BRM2-ER cells were induced with β-estradiol (5μM) for respective time points. Total RNA was analyzed by quantitative real-time RT-PCR with oligos for miR-34a. Data are represented as mean ± SD from 3 independent experiments. (G) Schematic representation of the miR-34a genomic region and phylogenic conservation of genomic region in the first intron of miR-34a in humans, mice, and rats. The conserved region is shown by sequences in gray box, and the C/EBPα site is shown in bold letters. (H) Luciferase reporter assays were performed in Kasumi-6 cells using indicated reporters and C/EBPα. Cells were transfected with corresponding firefly luciferase vectors (miR-34 Promoter constructs #1 to #6, Renilla luciferase reporter construct as control vector, and C/EBPα-pCDNA3 vector. Luciferase activity was measured 24 hours later. Bars represent promoter activity for the corresponding vectors. Data are represented as mean ± SD from 3 independent experiments. *P < .05. (I) Chromatin derived from K562-C/EBPα-p42-ER cells was immunoprecipitated with anti-C/EBPα and IgG antibodies. Recovered DNA was PCR amplified with primers specific for C/EBPα-binding amplicon (oligos 1) and the nonbinding amplicon (oligos 2).
To better understand the direct regulation of miR-34a by C/EBPα-p42, we next examined putative C/EBPα binding sites in the promoter region of miR-34a using Transcription Element Search software (www.cbil.upenn.edu/tess). We found several C/EBPα-p42 binding sites in the upstream, as well as in the intronic regions of miR-34a (data not shown). Interestingly, the C/EBPα-p42 binding site in the first intronic region of miR-34a was found to be conserved in humans, mice, and rats (Figure 1G). To identify the C/EBPα-p42 binding site necessary for miR-34a expression, we cloned several genomic fragments of miR-34a into a promoterless luciferase reporter plasmid. The different constructs were cotransfected with C/EBPα-p42 in Kasumi-6 cells, a myeloid leukemia cell line established from the bone marrow cells of a patient having AML with CEBPA mutation.19 Analysis of luciferase activity in these cells showed that C/EBPα is able to transactivate miR-34a (miR-34a#1 to miR-34a#4, Figure 1H and supplemental Figure 4; supplemental Figure 5). Removal of the C/EBPα-p42 binding site by truncation or mutation abolished transcriptional activity (Figure 1H and supplemental Figure 4 #5 and #6). We conclude that the C/EBPα-p42 binding site located in the first intron of miR-34a is the critical binding site for C/EBPα-p42. Because p53 regulates miR-34a through a distal element,24 we analyzed miR-34a transactivation by C/EBPα-p42 in this region. We did not observe any transactivation of miR-34a by C/EBPα-p42 in this region (data not shown).
We next investigated whether C/EBPα-p42 directly binds to the miR-34a element, which we found to be necessary for C/EBPα-p42 regulation in promoter assays. To answer this, we performed chromatin immunoprecipitation (ChIP) experiments in K562-C/EBPα-p42-ER cells. Cells were treated with β-estradiol for various time points, and chromatin fragments were immunoprecipitated with an anti-C/EBPα antibody. DNA from the immunoprecipitates were amplified by PCR using primers (oligos 1) located near the C/EBPα binding site as well as primers located further upstream of the transcription starting site (oligos 2). C/EBPα-p42 was found to be associated at the region that encompasses oligos 1 (Figure 1I). We found that the C/EBPα binding at the miR-34a promoter is high at 4 and 6 hours, which correlates with miR-34a expression levels in the same cell line (Figure 1E top). We did not find any binding of C/EBPα-p42 when the genomic region upstream of the transcription starting site (oligos 2) was PCR amplified (Figure 1I). Taken together, these data suggest that C/EBPα-p42 interacts with the miR-34a genomic region in vivo, and that this binding correlates with the expression of miR-34a.
E2F3 is a direct target of miR-34a during granulopoiesis
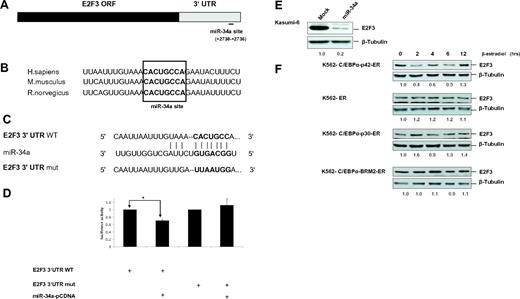
Even though E2F3 has been shown to be a target of miR-34a,25 there has been no report that delineates how miR-34a regulates E2F3 during granulopoiesis. The miR-34a binding site in E2F3 is located in the 3′UTR region and is conserved in humans, mice, and rats (Figure 2A-B). Because inhibition of E2F activity is a major pathway for myeloid cell proliferation by C/EBPα-p42,2,5 we hypothesized that E2F3 would be the most significant target of miR-34a in granulopoiesis. To find whether E2F3 would be a potential target of miR-34a during granulopoiesis, we conducted luciferase reporter assays in Kasumi-6 cells with construct having E2F3 3′UTR of the E2F3 gene with a putative miR-34a binding site (E2F3 3′UTR WT; Figure 2C). A control vector containing a mutated miR-34a binding site was also generated (E2F3 3′UTR mut). Overexpression of miR-34a leads to a decrease in luciferase activity of the E2F3 3′UTR (Figure 2D). miR-34a did not affect the reporter activity of the vector containing the mutated miR-34a binding site, suggesting a miR-34a-specific inhibition of E2F3 (Figure 2D). To validate E2F3 as a target of miR-34a, we analyzed E2F3 protein levels during the overexpression of miR-34a in Kasumi-6 cells. miR-34a overexpression leads to marked down-regulation of E2F3 protein levels (Figure 2E). Because C/EBPα-p42 directly regulates miR-34a (Figure 1) and miR-34a targets E2F3, we analyzed the potential of C/EBPα proteins in regulating E2F3 protein levels. Induction of C/EBPα-p42 is able to down-regulate E2F3 protein levels (Figure 2F). The finding that E2F3 levels are down-regulated at earlier time points correlates with our finding that miR-34a is up-regulated at earlier time points in K562-C/EBPα-p42-ER cells (Figure 1E top). Meanwhile, mutants of C/EBPα (C/EBPα-p30 and C/EBPα-BRM2) fail to down-regulate E2F3 protein levels (Figure 2F). Collectively, these data suggest that miR-34a regulation by C/EBPα-p42 plays a critical role in the regulation of E2F3 protein levels during granulopoiesis.
E2F3 is a direct target of miR-34a during granulopoiesis. (A) Schematic representation of miR-34a binding site in the human E2F3 3′UTR. The numbers (+2730 to +2736) represent the nucleotides relative to the termination codon of human E2F3. (B) Conservation of miR-34a binding site in E2F3 3′UTR in human, mouse, and rat genomes. (C) Sequences of predicted miR-34a binding site of E2F3. (D) Luciferase assays in Kasumi-6 cells transfected with E2F3 3′UTR constructs (wild type and mutant) and miR-34a-pCDNA. Bars represent luciferase activity for the corresponding vectors. Data are represented as mean ± SD from 3 independent experiments. *P < .05. (E) Kasumi-6 cells were transfected with control and miR-34a-pCDNA vectors. Total protein was analyzed by Western blot analysis with anti-E2F3 antibody. Values below the gel image indicate the E2F3 protein levels normalized to β-tubulin. (F) K562-C/EBPα-p42-ER, K562-ER, K562-C/EBPα-p30-ER, and K562-C/EBPα-BRM2-ER cells were induced with ß-estradiol (5μM) for respective time points. Total protein was analyzed by Western blot analysis with anti-E2F3 antibody. Values below the gel image indicate the E2F3 protein levels normalized to β-tubulin.
E2F3 is a direct target of miR-34a during granulopoiesis. (A) Schematic representation of miR-34a binding site in the human E2F3 3′UTR. The numbers (+2730 to +2736) represent the nucleotides relative to the termination codon of human E2F3. (B) Conservation of miR-34a binding site in E2F3 3′UTR in human, mouse, and rat genomes. (C) Sequences of predicted miR-34a binding site of E2F3. (D) Luciferase assays in Kasumi-6 cells transfected with E2F3 3′UTR constructs (wild type and mutant) and miR-34a-pCDNA. Bars represent luciferase activity for the corresponding vectors. Data are represented as mean ± SD from 3 independent experiments. *P < .05. (E) Kasumi-6 cells were transfected with control and miR-34a-pCDNA vectors. Total protein was analyzed by Western blot analysis with anti-E2F3 antibody. Values below the gel image indicate the E2F3 protein levels normalized to β-tubulin. (F) K562-C/EBPα-p42-ER, K562-ER, K562-C/EBPα-p30-ER, and K562-C/EBPα-BRM2-ER cells were induced with ß-estradiol (5μM) for respective time points. Total protein was analyzed by Western blot analysis with anti-E2F3 antibody. Values below the gel image indicate the E2F3 protein levels normalized to β-tubulin.
miR-34a is down-regulated in AML with CEBPA mutations
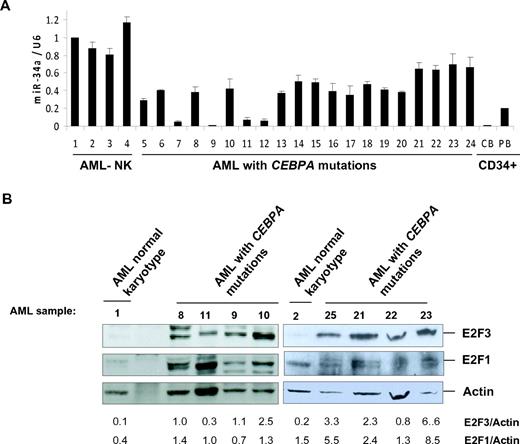
Because miR-34a is directly regulated by C/EBPα-p42, we hypothesized that this microRNA could be down-regulated in AML with CEBPA mutations. To understand the regulation of miR-34a in AML, the expression levels of miR-34a was quantified in diagnostic samples of AML samples. Genetic and morphologic features of AML samples used are shown in Table 1. We found a significant down-regulation of miR-34a expression in AML samples with CEBPA mutations, in comparison to AML samples without CEBPA mutations (Figure 3A). These results confirm the significance of miR-34a regulation during granulopoiesis and in AML with CEBPA mutations. CEBPA gene mutations in AML are reported as mono- and biallelic and characterized by distinct gene-expression patterns.26 However, we did not observe any correlation between distinct CEBPA mutations and down-regulation of miR-34a in patient samples with CEBPA mutations. Expression levels of miR-34a were found to be lowest in CD34+ cells (Figure 3A).
Genetic and morphologic characteristics of AML patient samples used for microRNA-34a analysis and Western blot analysis
| Patient no. . | Morphology by FAB . | % blasts in bone marrow . | Karyotype . | CEBPA mutation status . | Age (y) . | WBC count (109/L) . | FLT3 mutation status . |
|---|---|---|---|---|---|---|---|
| 1 | M1 | 95 | Normal | Nil | 70 | 12.3 | Positive |
| 2 | M2 | 60 | Normal | Nil | 60 | 1.8 | Negative |
| 3 | M4 | 80 | Normal | Nil | 79 | 27.0 | Negative |
| 4 | M5 | 95 | Normal | Nil | 35 | 174.5 | Positive |
| 5 | M4 | 90 | Normal | Double | 45 | 105.0 | Negative |
| 6 | M2 | 85 | Normal | Double | 64 | 759.0 | Positive |
| 7 | M2 | 90 | Normal | Double | 63 | 308.0 | Negative |
| 8 | M4 | 55 | del(12)(p11p12), t(17;18) (q24;, q11)[20] | Single | 35 | 16.4 | Negative |
| 9 | M2 | 55 | Normal | Double | 56 | 60.9 | Negative |
| 10 | M1 | 81 | Normal | Double | 12 | 29.5 | Negative |
| 11 | M2 | 65 | Normal | Double | 16 | 9.1 | Negative |
| 12 | M2 | 72 | Normal | Single | 51 | 45.0 | Negative |
| 13 | Unclassified | 86 | Normal | Double | 9 | 37.3 | Negative |
| 14 | M2 | 47 | Normal | Double | 6 | 19.8 | Negative |
| 15 | M2 | 81 | Normal | Double | 5 | 23.4 | Negative |
| 16 | M1 | 94 | Normal | Double | 12 | 160.6 | Negative |
| 17 | M4 | 58 | Normal | Double | 14 | 122.7 | Negative |
| 18 | M1 | 70 | Normal | Double | 11 | 20.4 | Negative |
| 19 | Unknown | Unknown | Normal | Double | 34 | 32.0 | Positive |
| 20 | M2 | 85 | Normal | Double | 48 | 72.0 | Negative |
| 21 | M1 | 85 | Normal | Double | 61 | 20.1 | Negative |
| 22 | M2 | 58 | Normal | Single | 72 | 4.9 | Negative |
| 23 | M1 | Unknown | Normal | Single | 73 | 34.3 | Positive |
| 24 | M2 | 72 | Normal | Single | 51 | 45.0 | Negative |
| 25 | M2 | 49 | Normal | Double | 31 | 4.3 | Negative |
| Patient no. . | Morphology by FAB . | % blasts in bone marrow . | Karyotype . | CEBPA mutation status . | Age (y) . | WBC count (109/L) . | FLT3 mutation status . |
|---|---|---|---|---|---|---|---|
| 1 | M1 | 95 | Normal | Nil | 70 | 12.3 | Positive |
| 2 | M2 | 60 | Normal | Nil | 60 | 1.8 | Negative |
| 3 | M4 | 80 | Normal | Nil | 79 | 27.0 | Negative |
| 4 | M5 | 95 | Normal | Nil | 35 | 174.5 | Positive |
| 5 | M4 | 90 | Normal | Double | 45 | 105.0 | Negative |
| 6 | M2 | 85 | Normal | Double | 64 | 759.0 | Positive |
| 7 | M2 | 90 | Normal | Double | 63 | 308.0 | Negative |
| 8 | M4 | 55 | del(12)(p11p12), t(17;18) (q24;, q11)[20] | Single | 35 | 16.4 | Negative |
| 9 | M2 | 55 | Normal | Double | 56 | 60.9 | Negative |
| 10 | M1 | 81 | Normal | Double | 12 | 29.5 | Negative |
| 11 | M2 | 65 | Normal | Double | 16 | 9.1 | Negative |
| 12 | M2 | 72 | Normal | Single | 51 | 45.0 | Negative |
| 13 | Unclassified | 86 | Normal | Double | 9 | 37.3 | Negative |
| 14 | M2 | 47 | Normal | Double | 6 | 19.8 | Negative |
| 15 | M2 | 81 | Normal | Double | 5 | 23.4 | Negative |
| 16 | M1 | 94 | Normal | Double | 12 | 160.6 | Negative |
| 17 | M4 | 58 | Normal | Double | 14 | 122.7 | Negative |
| 18 | M1 | 70 | Normal | Double | 11 | 20.4 | Negative |
| 19 | Unknown | Unknown | Normal | Double | 34 | 32.0 | Positive |
| 20 | M2 | 85 | Normal | Double | 48 | 72.0 | Negative |
| 21 | M1 | 85 | Normal | Double | 61 | 20.1 | Negative |
| 22 | M2 | 58 | Normal | Single | 72 | 4.9 | Negative |
| 23 | M1 | Unknown | Normal | Single | 73 | 34.3 | Positive |
| 24 | M2 | 72 | Normal | Single | 51 | 45.0 | Negative |
| 25 | M2 | 49 | Normal | Double | 31 | 4.3 | Negative |
FAB indicates French-American-British; and WBC, white blood count.
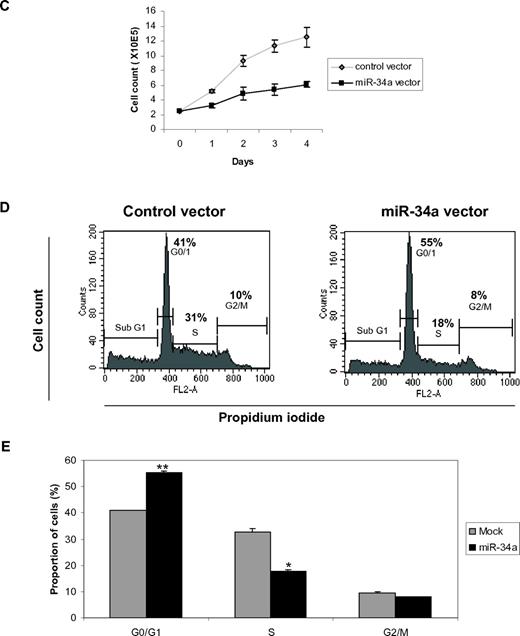
miR-34a functions as a tumor suppressor in AML with CEBPA mutations. (A) Quantitative real-time RT-PCR for miR-34a was carried out using bone marrow cells derived from AML patients. Values were normalized with U6. AML-NK, AML with normal karyotype; CB, cord blood; PB, peripheral blood. Data are represented as mean from 3 experiments. (B) Western blot analysis for E2F3 and E2F1 were carried out using bone marrow cells derived from AML patients. Values below the gel image indicate the E2F3 and E2F1 protein levels normalized to actin. (C) Growth curve of Kasumi-6 cells transfected with control or miR-34a.pCDNA vectors. Data are represented as mean ± SD from 3 independent experiments. (D) Flow cytometry of propidium iodide–stained Kasumi-6 cells transfected with control or miR-34a.pCDNA vectors from a representative experiment. (E) Cell-cycle profile of Kasumi-6 cells 2 days after transfection with control or miR-34a.pCDNA vectors. Data are represented as mean ± SD from 3 independent experiments. *P < .05, **P < .001.
miR-34a functions as a tumor suppressor in AML with CEBPA mutations. (A) Quantitative real-time RT-PCR for miR-34a was carried out using bone marrow cells derived from AML patients. Values were normalized with U6. AML-NK, AML with normal karyotype; CB, cord blood; PB, peripheral blood. Data are represented as mean from 3 experiments. (B) Western blot analysis for E2F3 and E2F1 were carried out using bone marrow cells derived from AML patients. Values below the gel image indicate the E2F3 and E2F1 protein levels normalized to actin. (C) Growth curve of Kasumi-6 cells transfected with control or miR-34a.pCDNA vectors. Data are represented as mean ± SD from 3 independent experiments. (D) Flow cytometry of propidium iodide–stained Kasumi-6 cells transfected with control or miR-34a.pCDNA vectors from a representative experiment. (E) Cell-cycle profile of Kasumi-6 cells 2 days after transfection with control or miR-34a.pCDNA vectors. Data are represented as mean ± SD from 3 independent experiments. *P < .05, **P < .001.
Because E2F3 is a potential target of miR-34a (Figure 2) and miR-34a is down-regulated in AML with CEBPA mutations (Figure 3A), we hypothesized that E2F3 is overexpressed in AML with CEBPA mutations. We analyzed E2F3 protein levels by Western blot analysis in AML samples. We observed that E2F3 protein is overexpressed in AML samples with CEBPA mutations (Figure 3B). This shows an inverse correlation between miR-34a and E2F3 in AML with CEBPA mutations. Because E2F3 is demonstrated to regulate the E2F1 gene at the transcriptional level,27 we hypothesized that overexpression of E2F1 might occur as a consequence of miR-34a down-regulation in AML with CEBPA mutations. To verify this, we analyzed E2F1 protein by Western blot analysis in AML samples. We observed overexpression of E2F1 protein (Figure 3B) in AML samples with CEBPA mutations.
miR-34a inhibits myeloid cell proliferation
C/EBPα-p42 has been shown to inhibit cell proliferation.28,29 Because miR-34a is regulated by C/EBPα-p42 during granulopoiesis (Figure 1) and miR-34a targets E2F3 (Figure 2), we hypothesized that miR-34a could regulate myeloid proliferation. To answer this, we overexpressed miR-34a in Kasumi-6 cells. We observed that miR-34a overexpression in Kasumi-6 cells inhibited myeloid cell proliferation (Figure 3C). We also observed that miR-34a can impede myeloid cell proliferation in U-937 cells (supplemental Figure 6). To better understand the regulation of myeloid cell proliferation by miR-34a, we analyzed cell-cycle profiles during miR-34a overexpression in Kasumi-6 cells. miR-34a was overexpressed in Kasumi-6 cells, and cell-cycle profile was analyzed 2 days after miR-34a overexpression by propidium iodide staining. We found that miR-34a was able to block the transition from the G0/G1 to S phase of cell-cycle progression, as shown by the accumulation of cells in the G0/G1 phase and reduction of cells in the S phase during miR-34a overexpression (Figure 3D-E). It is important to mention that C/EBPα-p42 displays a similar effect in the regulation of cell proliferation.29 Our data suggest that miR-34a could have a major role in the inhibition of myeloid cell cycle machinery by C/EBPα-p42. Taken together, these findings show that miR-34a acts as a tumor suppressor in AML with CEBPA mutations.
Overexpression of miR-34a in AML blast cells leads to granulopoiesis
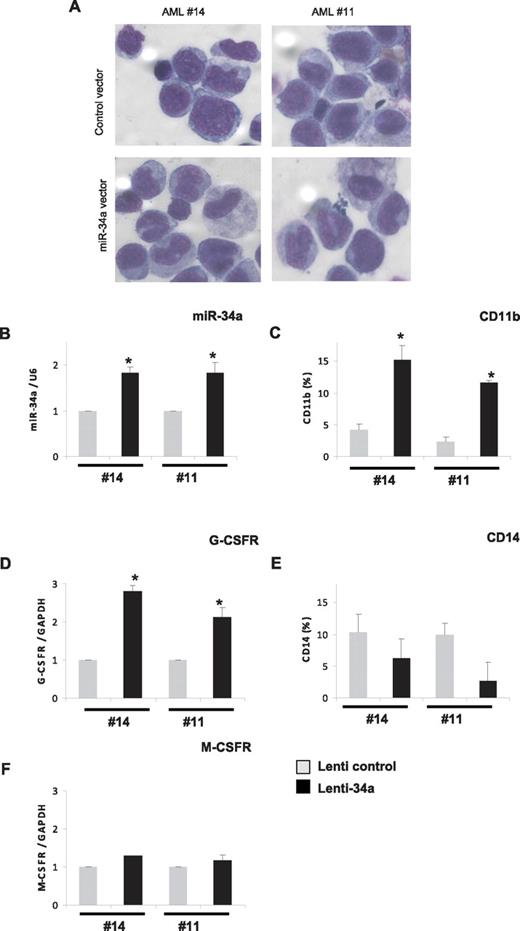
Because our data indicate that miR-34a is a major player during granulopoiesis, which is down-regulated in AML with CEBPA mutations, we hypothesized that overexpression of miR-34a could induce differentiation in AML blast cells isolated from AML patients with CEBPA mutations. To verify this, we overexpressed miR-34a via lentiviral vectors, as reported before,21 in primary blast cells isolated from AML patients with CEBPA mutations (14 and 11 of Table 1). As determined by GFP expression, efficient transduction with both control and miR-34a lentiviral was obtained in AML blast cells (49%-50% and 56%-61%, respectively; data not shown). The overexpression of miR-34a resulted in granulocytic differentiation of AML blast cells, as assessed by morphology and by the increased expression of myeloid markers, such as CD11b and G-CSFR (Figure 4A-D). We did not observe any up-regulation in the expression levels of CD14, M-CSFR, and CD41 (Figure 4E-F and data not shown), suggesting miR-34a overexpression specifically leads to granulocytic differentiation in AML blast cells with CEBPA mutations. Because miR-34a has been shown to induce apoptosis,14 we analyzed the ability of miR-34a in apoptosis induction in myeloid cells. Kasumi-6 cells were transfected with control or miR-34a vector and analyzed apoptosis 24 hours later. Our data show that miR-34a did not induce apoptosis in Kasumi-6 cells (supplemental Figure 7). Taken together, our data suggest that miR-34a expression has major functions in myeloid differentiation, and manipulation of miR-34a levels can re-establish myeloid differentiation in primary AML blast cells with CEBPA mutations.
Overexpression of miR-34a in AML blast cells leads to granulopoiesis. Bone marrow cells derived from AML patients with CEBPA mutation (14 and 11 of Table 1) were transfected with control or miR-34a lenti viral vectors. Cells were cultured for 6 days and collected for morphologic, immunophenotypic, and myeloid marker expression analysis. (A) Morphologic analysis by light microscopy of Wright-Giemsa–stained AML blast cells. (B) miR-34a expression levels in blast cells transfected with lentiviral miR-34a vector, in comparison with control vector, as analyzed by real-time RT-PCR analysis. (C,E) CD11b (C) and CD14 (E) expression levels in blast cells transfected with lenti viral miR-34a vector, in comparison with control vector, as analyzed FACS analysis. (D,F) G-CSFR (D) and M-CSFR (F) expression levels in blast cells transfected with lenti viral miR-34a vector, in comparison with control vector, as analyzed by real-time RT-PCR analysis. Data are represented as mean ± SD from 3 independent experiments. *P < .05.
Overexpression of miR-34a in AML blast cells leads to granulopoiesis. Bone marrow cells derived from AML patients with CEBPA mutation (14 and 11 of Table 1) were transfected with control or miR-34a lenti viral vectors. Cells were cultured for 6 days and collected for morphologic, immunophenotypic, and myeloid marker expression analysis. (A) Morphologic analysis by light microscopy of Wright-Giemsa–stained AML blast cells. (B) miR-34a expression levels in blast cells transfected with lentiviral miR-34a vector, in comparison with control vector, as analyzed by real-time RT-PCR analysis. (C,E) CD11b (C) and CD14 (E) expression levels in blast cells transfected with lenti viral miR-34a vector, in comparison with control vector, as analyzed FACS analysis. (D,F) G-CSFR (D) and M-CSFR (F) expression levels in blast cells transfected with lenti viral miR-34a vector, in comparison with control vector, as analyzed by real-time RT-PCR analysis. Data are represented as mean ± SD from 3 independent experiments. *P < .05.
Discussion
An emerging number of studies show the complex, heterogeneous picture of the development of acute myeloid leukemia. Recent studies suggest that a simple loss of a critical regulator is not the direct cause for AML; rather, it is the modulation of functions of such critical players that plays an important role in leukemogenesis. Deregulation of C/EBPα function has been shown to be a major event in several different steps of leukemogenesis.2,4 Most patients having CEBPA mutations carry mutations in both alleles—one allele with an N-terminal mutation and one with a C-terminal mutation. However, homozygous C- or N-terminal mutations are reported rarely in AML.30 This suggests that both mutations are needed for the development of leukemia. Interestingly, the N-terminal CEBPA mutation has been reported to predispose the development of the C-terminal mutation.31 In addition, patients with CEBPA mutations retain the same mutation signature at the time of diagnosis and relapse.32,33 This suggests that CEBPA mutations are early events during leukemogenesis. Even though the complete absence of C/EBPα has been shown to result in block of granulocytic differentiation in mouse models, these animals do not develop leukemia.34 This indicates that a complete loss of C/EBPα function cannot contribute to the development of AML. Rather, specific modulation of basal C/EBPα function is necessary for leukemia development. Recent reports show that C/EBPα-mutant proteins can induce leukemia and possess different leukemogenic properties, which are instrumental in the development of AML.7,8 However, despite the relevance of C/EBPα mutants in the induction of AML, little is known how these mutations affect myeloid cell cycle machinery, which could have profound functions in myeloid cell cycle progression, differentiation, and development of AML. The complex nature of CEBPA mutations in AML indicates the necessity to elucidate the molecular mechanisms behind different stages of leukemogenesis.
It is becoming clear that deregulation of C/EBPα-mediated myeloid cell cycle regulation plays a crucial role in the development of AML.7,8 During granulopoiesis, C/EBPα inhibits E2F activity through its C-terminal domain, which mediates direct protein-protein interaction with E2F, as well as its N-terminal domain, which mediates E2F repression.6,35-37 Disruption of any of these functions of C/EBPα results in an accumulation of myeloid progenitors and block of granulocytic differentiation.6,35,36 Interestingly, the major mutations reported for C/EBPα are distributed in the N- and C-terminal domains, signifying the relevance of these domains of C/EBPα in leukemogenesis. These C/EBPα mutants fail to induce granulopoiesis and are defective in inhibiting myeloid cell proliferation.6 The N-terminal mutant, C/EBPα-p30, possesses the lineage commitment function of C/EBPα.7 However, this mutant form fails to control the proliferation of myeloid progenitor cells and induces AML.7 The C-terminal mutant increases the proliferation of long-term hematopoietic stem cells (HSCs), which results in the expansion of premalignant HSCs and induces AML.8 These studies show the importance of the proper regulation of myeloid progenitor proliferation as the turning point in leukemogenesis.
Our data show that C/EBPα mutants fail to induce miR-34a (Figure 1F) and miR-34a is down-regulated in AML with CEBPA mutations (Figure 3A). Because E2F3 is a target of miR-34a (Figure 2) and miR-34a controls myeloid proliferation (Figure 3C-E), a lack of miR-34 results in an accumulation of E2F3 and E2F1 (Figure 3A-B). This suggests that a lack of miR-34a could result in the aberrant proliferation of myeloid progenitors. This could be one explanation for the increased HSC expansion observed in CEBPA mutation. Further studies are needed to show the relationship between miR-34a deregulation and the expansion of the myeloid progenitor pool in leukemogenesis in the setting of CEBPA mutation. Emerging evidence indicates that leukemia-initiating mutations happen in HSCs and result in a population of preleukemic HSCs or preleukemic committed progenitors, which have aberrant self-renewal capacity.38 Loss of C/EBPα results in enhanced self-renewal of HSCs and overexpression of Bmi-1.3 Bmi-1 has been shown to increase self-renewal and enhance repopulating activity of HSCs.39 Interestingly, BMI1 is a target gene of E2F1 and is up-regulated in certain tumors.40 These findings show the relevance of our finding that E2F1 as well as E2F3 are overexpressed in AML with the CEBPA mutation. Whether E2F3 or E2F1 overexpression during CEBPA mutations can confer self-renewal to HSCs or committed progenitors awaits further experiments.
E2F-family transcription factors are important players in cell cycle progression and cancer.41 They are composed of 8 members, of which E2F1-E2F3 are considered transcriptional activators and E2F4-E2F8 are considered transcriptional repressors.41 E2F activators have been shown to regulate genes necessary for G1- to S-phase transition and DNA replication.41 E2F3 has been shown to have a crucial role in cell proliferation.42 The E2F3 transgenic mouse shows the development of spontaneous skin tumors.43 Because E2F3 is one of the key regulators of the E2F1 gene,27 overexpression of E2F3 could also affect E2F1 status in the cell. The E2F1 transgenic mouse displays impaired terminal differentiation of megakaryocytes, which results in thrombocytopenia.44 Deregulated E2F3 and E2F1 expression is reported in a variety of cancers, including lung, breast, ovary, and prostate.41 In addition, E2F1 transgenic mice are predisposed to the development of spontaneous tumors.45 Amplification of either the E2F1 or the E2F3 gene locus has been demonstrated as a frequent event in different cancers.41 All these findings suggest that deregulation of E2F3 as well as E2F1 could play major roles in the pathogenesis of various tumors. Distinct E2F members have been shown to play important roles in lymphomagenesis.46 E2F1 has been shown to block granulopoiesis.47 We have recently shown that E2F1 cooperates with C/EBPα-p30 in up-regulating PIN1, an oncoprotein up-regulated in AML with CEBPA mutations.11 We have also reported that E2F1 transcriptionally represses miR-223, a key microRNA regulated by C/EBPα during granulopoiesis.10 E2F1 regulates c-Myc, which has been shown to block C/EBPα.48 All these findings, together with our data showing that E2F3 and E2F1 are overexpressed in AML with CEBPA mutation (Figure 3B), underlines deregulation of E2F function by C/EBPα as instrumental in AML with CEBPA mutations.
miR-223 is another microRNA involved in granulopoiesis.23 We have shown that this C/EBPα-regulated microRNA could target E2F1 during granulopoiesis.10 However, miR-223 null animals did not display a complete block of differentiation.49 This suggests that other microRNAs could be compensating partially for miR-223 function during the absence of miR-223. Further experiments using genetic models are needed to show the relative contribution of microRNAs, including miR-223 and miR-34a, in granulopoiesis. miR-34a is a well-characterized microRNA in various tissues and is controlled by the tumor suppressor, p53.14 miR-34a is down-regulated in a variety of tumors, including neuroblastoma, lung cancer, and colorectal cancer.14 Even though this microRNA has been shown to be involved in chronic lymphoid leukemia50 and megakaryocytic development,21 little is known about this microRNA in granulopoiesis. Our study reveals novel insights into the function of miR-34a in granulopoiesis.
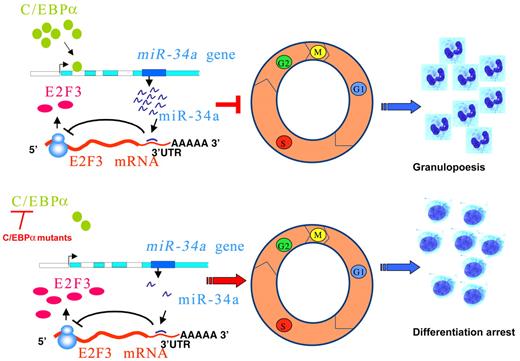
In summary, we propose a model in which miR-34a plays a critical role in regulating the myeloid differentiation program orchestrated by C/EBPα (Figure 5 top). This could result in down-regulation of E2F3, which, in turn, inhibits myeloid cell proliferation, resulting in granulopoiesis. In AML with CEBPA mutations, loss of function of C/EBPα results in a block of miR-34a regulation and lack of inhibition of E2F3, which results in increased proliferation of myeloid progenitors (Figure 5 bottom). Our data demonstrate the deregulation of the C/EBPα-miR-34a-E2F3 axis as a critical phenomenon in AML with CEBPA mutations. Manipulation of miR-34a could offer novel treatment strategies in AML with CEBPA mutations.
Schematic representation of regulation of granulopoiesis and AML by C/EBPα–miR-34a-E2F3 axis. During granulopoiesis (top panel), C/EBPα transactivates miR-34a, which, in turn, leads to E2F3 repression and inhibition of cell-cycle progression, resulting in myeloid differentiation. During CEBPA mutations in AML (bottom panel), low activity of C/EBPα fails to transactivate miR-34a, which results in lack of E2F3 inhibition. Overexpressed E2F3, together with E2F1, could accelerate myeloid cell-cycle progression and results in block of granulocytic differentiation.
Schematic representation of regulation of granulopoiesis and AML by C/EBPα–miR-34a-E2F3 axis. During granulopoiesis (top panel), C/EBPα transactivates miR-34a, which, in turn, leads to E2F3 repression and inhibition of cell-cycle progression, resulting in myeloid differentiation. During CEBPA mutations in AML (bottom panel), low activity of C/EBPα fails to transactivate miR-34a, which results in lack of E2F3 inhibition. Overexpressed E2F3, together with E2F1, could accelerate myeloid cell-cycle progression and results in block of granulocytic differentiation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank J. Liebermann, H. Hermeking, M. Esteller, J. T. Mendell, J. Khan, J. Nevins, P. Chartrand, A. Lund, and M. Watanabe for DNA constructs. We also thank the Children's Oncology Group Acute Myeloid Leukemia Reference Laboratory for providing diagnostic specimens and F. Navarro for advice on lentiviral transfections.
This work was supported by grants from the National Cancer Institute (U10 CA98543 and U10 CA98413) to S.M.; National Institutes of Health (R01 HL56745) to D.G.T. and D.F.G., Wilhem Sander, Roux, Carreras and Krebshilfe to G.B.
National Institutes of Health
Authorship
Contribution: J.A.P. and P.S.P. performed experiments and analyzed the data; P.A.H., C.P., O.N., S.M., S.K.B., and C.M.-T. provided AML patient samples; P.S.P., V.D., P.A.H., C.P., C.M.T., and D.G.T. commented on the manuscript; M.C. discussed the data; J.A.P. wrote the manuscript and designed the research; and G.B. supervised the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gerhard Behre, Department of Internal Medicine IV–Oncology and Hematology, Martin Luther University Halle-Wittenberg, Ernst-Grube-Str 40, Halle 06120, Germany; e-mail: gerhard.behre@medizin.uni-halle.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal