Abstract
FcγRI is the sole high-affinity immunoglobulin G (IgG) receptor on leukocytes. Its role in immunity and the clearance of opsonized particles has been challenged, as the receptor function may well be hindered by serum IgG. Here, we document immune complex binding by FcγRI to be readily enhanced by cytokine stimulation, whereas binding of monomeric IgG only modestly increased. Enhanced immune complex binding was independent of FcγRI surface expression levels. FcγRI, saturated with prebound IgG, was found capable of effective immune complex binding upon cytokine stimulation. Cytokine-enhanced binding was observed across a variety of immune complexes, including huIgG3- or mIgG2a-opsonized red blood cells, rituximab- or ofatumumab-opsonized B-cell lymphoma, and cetuximab-opsonized glioblastoma cells. This study contributes to our understanding of how FcγRI can participate in the clearance of opsonized particles despite saturation by monomeric IgG.
Introduction
Fc receptors are receptors for immunoglobulins that play a central role in immunity. On human leukocytes, a myriad of Fcγ receptors are expressed; among these receptors, FcγRI is the only known high-affinity receptor for immunoglobulin G (IgG).1 FcγRI is constitutively expressed on monocytes, macrophages, and myeloid dendritic cells. Interferonγ (IFNγ) can enhance surface expression of FcγRI on these cells, and FcγRI expression can be induced on granulocytes by IFNγ or granulocyte colony–stimulating factor (G-CSF) stimulation. In contrast, cytokines such as interleukin-4 (IL-4), IL-10, and transforming growth factor-β (TGF-β) down-regulate activating Fc receptors, including FcγRI, and enhance expression of the inhibitory receptor FcγRIIb.2,3 In vitro, IFNγ treatment leads to increased Fcγ receptor–induced cytokine production.4
In vivo, the role of FcγRI in immunity remains unclear, as due to its high affinity FcγRI is believed to be saturated with monomeric IgG. This has led to the concept that prebound monomeric IgG prevents participation of FcγRI in clearing immune complexes by extravasated effector cells.5 Nevertheless, several in vivo studies documented a role for FcγRI, varying from a contribution during inflammation and autoimmune reactions,6-8 or, during monoclonal antibody (mAb)–based immunotherapy in melanoma and B-cell lymphoma models,9,10 to a malaria model in which transgenic expression of human FcγRI was central for effective mAb treatment.11 Furthermore, FcγRI can induce potent proinflammatory signaling compared with FcγRIIa,12 and can efficiently mediate both major histocompatibility complex class II (MHC-II) antigen presentation and cross-presentation.13-16
It remains unclear how FcγRI contributes to immune complex clearance in the presence of high IgG levels. For FcαRI17,18 and FcγRIIa19,20 it has been shown that cytokine stimulation can increase ligand binding of these receptors. This appears analogous to inside-out regulation described for integrins. Many integrins are expressed in a low-affinity binding state, which can be transformed to a high-affinity form upon cellular activation.21 We hypothesized that inside-out regulation could contribute to immune complex binding to FcγR occupied by IgG. Indeed, several studies support intracellular proteins interacting with FcγRI and possibly affecting ligand binding.22,23
In this study, we investigated the effect of cytokine stimulation on FcγRI ligand binding. Stimulation of FcγRI-expressing Ba/F3 cells and primary monocytes resulted in increased binding of immune complexes, whereas binding of monomeric IgG was only moderately enhanced. Upon cellular activation, FcγRI could readily bind immune complexes despite pre-engaged monomeric IgG. Taken together, these data might explain how FcγRI supports leukocyte interaction with immune complexes.
Methods
Antibodies and reagents
Anti–FcγRI-A647 was from BioLegend (clone 10.1). Unlabeled 10.1 mAb and mouse IgG1/IgG2a isotype were from eBioscience and BD Pharmingen, respectively. Anti–human Glycophorin A mouse IgG2a hybridoma supernatant was described previously;24 anti-RhD human IgG3 and IgG1 (clones BRAD3 and BIRMA D6, respectively) were from the International Blood Group Reference Laboratory. Human IgG1 anti-RhD clones LHM76/58 and ESD-1 were from Alba Bioscience. Unlabeled polyclonal human IgG3 and IgG1 were purchased from Sigma-Aldrich. Okadaic acid, LY294002, and U0126 were purchased from ALEXIS Biochemicals. Monoclonal antibody IV.3 was isolated from hybridoma supernatant, and IV.3 Fab fragments were made by Fusion Antibodies.
Cell lines and monocyte isolation
Ba/F3 cells were cultured in RPMI1640 medium (GIBCO) supplemented with 10% fetal calf serum, penicillin/streptomycin, and mouse IL-3, as described.18 A1207 and Daudi were kept in RPMI1640 medium supplemented with 10% fetal calf serum and penicillin/streptomycin.
The retroviral vector pMX FcγRI internal ribosome entry site (IRES) green fluorescent protein (GFP) was described previously.25 Amphotropic viral particles produced in HEK293T cells were used to transduce Ba/F3 cells. After transduction, Ba/F3-FcγRI cells were sorted on a FACSAria (BD Biosciences) on green fluorescent protein expression and further subcloned by limited dilution. For all experiments where subclones are shown, results were repeated with a polyclonal line and/or with at least 2 subclones.
Primary monocytes were from healthy donors. Magnetic-activated cell sorting (MACS)–isolated monocytes were obtained from peripheral blood mononuclear cells (PBMCs) using CD14-beads (Miltenyi Biotec).
Red blood cells (RBCs) were from ficoll-separated blood. RBCs were kept in Alsever buffer (Sigma-Aldrich) for a maximum of 1 week at 4°C.
Monomeric IgG binding
Ba/F3-FcγRI cells were stimulated as described in “FACS-based EA rosette assay.” Next, cells were washed and incubated with various amounts of mouse IgG2a or huIgG3. After a 1-hour incubation on ice, cells were washed and stained with goat F(ab′)2 anti–mouse IgG (or anti–human IgG) phycoerythrin (PE; SouthernBiotech). After washing, cells were fixed with 1% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) and analyzed on a FACSCanto II.
FACS–based EA rosette assay
Fluorescence-activated cell sorting (FACS)–based erythrocytes coated with antibody (EA) rosette assay was adapted from Beekman et al.26 Briefly, Ba/F3-FcγRI cells were starved overnight in RPMI1640 containing 1% fetal calf serum (FCS; RPMI 1% FCS). The next day, cells were stimulated for various time points with 30 ng/mL recombinant mouse IL-3 (PeproTech) in RPMI 1% FCS or in RPMI 1% FCS alone (medium control). RBCs were fluorescently labeled with PKH26 (Sigma-Aldrich) according to manufacturer's protocol, opsonized on ice with mIgG2a anti-glycophorin or huIgG3 anti–Rhesus D, and subsequently washed and resuspended in RPMI EDTA (ethylenediaminetetraacetic acid; RPMI1640, 1% FCS, 10mM EDTA). In a 96-well plate, 1 × 105 Ba/F3 cells/well were combined with 1 × 106 RBCs/well in RPMI EDTA and incubated for 1 hour at 4°C on a shaker. Cells were washed once with cold PBS and resuspended in cold PBS containing 1% PFA. Rosetting was analyzed on a FACSCanto II (BD Biosciences). It was shown previously that bovine IgG does not bind to human FcγRI.27
Blocking mAb 10.1 or isotype control was added at 20 μg/mL to Ba/F3-FcγRI cells before rosette assay and were kept present during assay at 10 μg/mL.
Rosette assays using IgG coupled to tosyl-activated Dynabeads was adapted from Bracke et al.28 Briefly, human IgG1 was coupled to tosyl-activated Dynabeads-M450 (Invitrogen) as described by the manufacturer. IgG-coupled dynabeads were then added to Ba/F3-FcγRI cells in RPMI EDTA in a ratio of 3.5 beads/cell. Dynabeads were found sufficiently autofluorescent in the PE channel to discriminate between Ba/F3 cells, beads, and Ba/F3 cell–bound beads. Rosettes were visualized on a LSM710 confocal microscope (Zeiss) equipped with a transmission photomultiplier tube for differential interference contrast (DIC) imaging. Pinhole opening was set to maximum.
For competition assays with monomeric ligand, Ba/F3-FcγRI cells were incubated with various concentrations of polyclonal huIgG3 on ice (Sigma-Aldrich). Unbound IgG was removed by washing Ba/F3-FcγRI cells before EA rosette assay. For inhibition experiments, Ba/F3- FcγRI cells were treated for 30 minutes with dimethyl sulfoxide (DMSO), 20μM LY294002, 20μM U-0126, or 1μM okadaic acid (OA) and stimulated with IL-3 in the presence of the inhibitor. Subsequently, cells were washed and subjected to EA rosette assay.
For measuring binding to tumor cells, tumor cell lines were fluorescently labeled with Lavacell (Activemotif), an amino reactive dye, and subsequently opsonized with 0.25 μg/mL rituximab (Roche) or ofatumumab (Genmab/GlaxoSmithKline) for Daudi or cetuximab (Merck) for A1207. After washing, the opsonized cells were incubated with Ba/F3-FcγRI for 1 hour at 4°C on a shaker. Cells were washed once with cold PBS and fixed in PBS containing 1% PFA. Binding was measured on a FACSCanto II and visualized on a LSM710 confocal microscope (Zeiss) equipped with a transmission photomultiplier tube for differential interference contrast imaging. Pinhole opening was set to maximum.
Binding assay with primary monocytes
For inhibition of inside-out signaling, monocytes were isolated as described in the previous “Cell lines and monocyte isolation” paragraph. After overnight incubation with 400 U/mL IFNγ, monocytes were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) or CellTracker Violet (Invitrogen) and treated with OA for 30 minutes at 37°C. Monocytes were plated out at 1 × 105 cells per well in RPMI EDTA containing 5 μg/mL IV.3 antigen-binding fragments (Fabs). After 10 minutes incubation on ice, 1 × 106 opsonized RBCs were added. Cells were allowed to bind for 1 hour at at 4°C on a shaker. After fixation in 1% PFA, binding was analyzed on a FACSCanto II.
For short-term stimulation with interleukins, PBMCs were isolated using ficoll. Cells were allowed to rest for 1 hour in 1% FCS RPMI at 37°C. Next, PBMCs were stimulated with either IL-4 (500 U/mL), IL-6 (200 U/mL), or tumor necrosis factor (TNF)α and IFNγ (500 and 400 U/mL, respectively) for 1 hour in 1% FCS RPMI at 37°C. Subsequently, PBMCs were allowed to bind to PKH26-labeled opsonized erythrocytes for 1 hour at 4°C in a 96-well V-bottom plate. After incubation, the plate was spun down and CD14-fluorescein isothiocyanate (FITC) in PBS with 5% normal mouse serum (NMS) was added to each well. The amount of monocyte binding to RBCs was scored as the percentage of CD14, PKH26 double positive events.
Statistical analysis
Data analysis was performed using Microsoft Excel 2007 and GraphPad Prism 4 software. Sigmoidal dose-response curves were used to fit rosette-binding curves data. Fitted data were tested using the F-test (comparing curves between treatments). Blocking experiments were tested using unpaired Student t test. Error bars depict SDs.
Results
lL-3 stimulation does not affect surface expression of FcγRI on Ba/F3-FcγRI cells
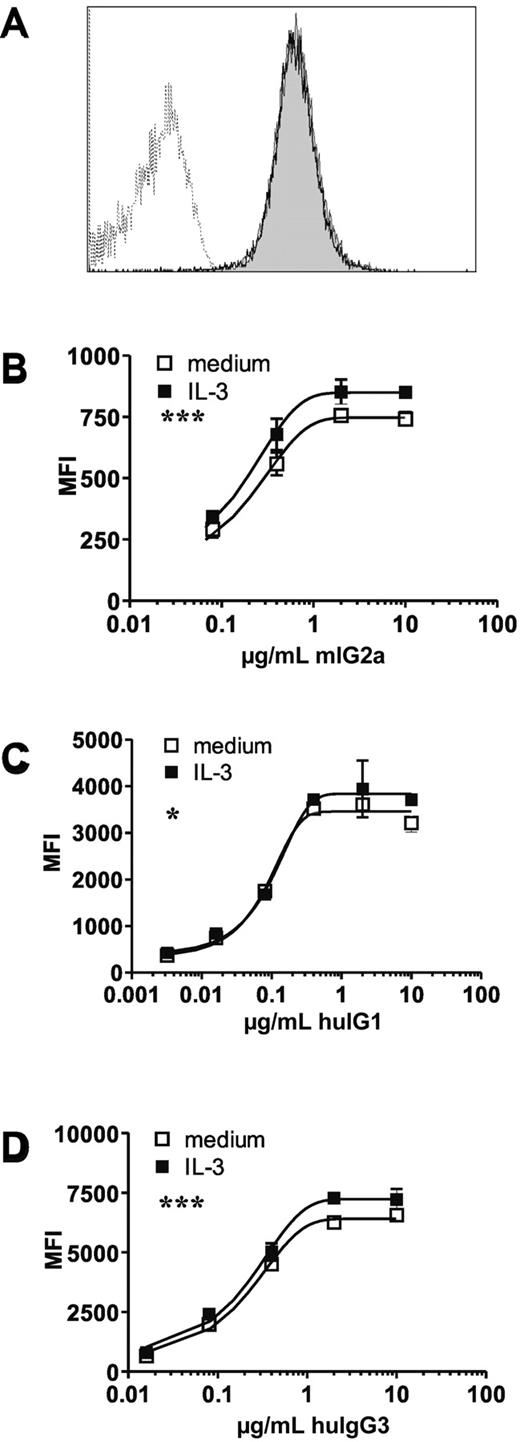
To determine whether FcγRI is regulated inside out, we used the IL-3–dependent Ba/F3 model. Parental Ba/F3 cells do not express any endogenous FcγR receptors but do express the FcRγ chain (Ho et al29 and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). One-hour IL-3-stimulation of an FcγRI-transduced Ba/F3 subclone (Ba/F3-FcγRI) did not affect FcγRI surface expression levels (Figure 1A). Similar results were obtained in a polyclonal line (data not shown). These findings allowed us to perform ligand-binding studies independent of changes in receptor surface expression.
IL-3 stimulation has no effect on FcγRI surface expression and slightly increases monomeric IgG binding. Ba/F3-expressing cells were starved overnight and subsequently restimulated with IL-3 for 1 hour. (A) FcγRI surface expression on a starved (open histogram) and restimulated (closed histogram) subclone of FcγRI-transduced Ba/F3 cells. Dotted line represents isotype control. (B-D) Binding of monomeric mouse IgG2a (B), human IgG1 (C), or human IgG3 (D) to FcγRI expressing Ba/F3 cells. Open squares represent starved cells, filled squares represent IL-3 restimulated Ba/F3 cells. Similar results were seen in 3 independent experiments. Datapoints represent means of at least 4 replicates. *P < .05, ***P < .001
IL-3 stimulation has no effect on FcγRI surface expression and slightly increases monomeric IgG binding. Ba/F3-expressing cells were starved overnight and subsequently restimulated with IL-3 for 1 hour. (A) FcγRI surface expression on a starved (open histogram) and restimulated (closed histogram) subclone of FcγRI-transduced Ba/F3 cells. Dotted line represents isotype control. (B-D) Binding of monomeric mouse IgG2a (B), human IgG1 (C), or human IgG3 (D) to FcγRI expressing Ba/F3 cells. Open squares represent starved cells, filled squares represent IL-3 restimulated Ba/F3 cells. Similar results were seen in 3 independent experiments. Datapoints represent means of at least 4 replicates. *P < .05, ***P < .001
Monomeric IgG binding is a unique characteristic of FcγRI. IL-3–treated Ba/F3-FcγRI cells showed a modest, albeit significant, increased binding to mouse IgG2a (mIgG2a), human IgG1, and human IgG3 (huIgG3; Figures 1B-D, respectively). These data demonstrate that short-term stimulation with IL-3 mildly affects monomeric IgG binding in the absence of effects on receptor surface expression levels.
Inside-out regulation of FcγRI enhances immune complex binding
To investigate whether treatment of Ba/F3-FcγRI cells with IL-3–altered immune complex (IC) binding, we used a FACS-based EA rosette assay. This method was previously shown to correlate with EA-rosette counting by light miscroscopy.26 An example of a FACS-based EA rosette assay and gating strategy is shown in supplemental Figure 2.
Upon stimulation with IL-3, Ba/F3-FcγRI cells exhibited increased binding to mIgG2a-IC compared with control cells, as detected by higher percentages of Ba/F3-FcγRI:RBC clusters (Figure 2A). Enhanced IC binding was observed as early as 15 minutes after stimulation (Figure 2C), reaching maximum levels by 1 hour. Blocking with FcγRI-specific mAb 10.1 demonstrated basal binding and IL-3–enhanced binding to be FcγRI dependent (Figure 2B).
Stimulation of primary monocytes and Ba/F3-FcγRI cells results in enhanced IC binding. Percentage of Ba/F3-FcγRI cells binding RBCs opsonized with IgG2a (A-C) or human IgG3 (D-E). Open squares represent starved cells, filled squares represent starved Ba/F3-FcγRI cells stimulated with IL-3 for 1 hour. (B) EA rosette assay in the presence of FcγRI-specific mAb 10.1 (10.1) or isotype control (iso). **P < .01; NS: not significant, t test. (C) Effect of duration of IL-3 stimulus on IC binding. *P < .05; **P < .01, t test. (D) Binding of 1 or more RBCs to Ba/F3 cells. (E) Binding of 2 or more RBCs to Ba/F3 cells. Datapoints represent the means of at least 3 replicates. Similar results were obtained in 3 independent experiments. ***P < .001. (F) Binding of Ba/F3-FcγRI cells to BSA or IgG1-coupled beads in the presence of 10.1 mAb (10.1) or isotype control (ISO). Similar results were obtained in 3 independent experiments. **P < .01, t test. (G) Rosetting of BSA and IgG1-coupled beads with Ba/F3-FcγRI cells visualized with fluorescence microscopy. Beads are detected in the red channel, Ba/F3-FcγRI in the green channel. (H) Binding of unopsonized (unopson) and opsonized (opson) erythrocytes to primary monocytes in the presence of 10.1mAb (10.1) or isotype control (iso). Data represent means of triplicates measured from 1 donor. Similar results were seen in 3 of 4 donors. *P < .05; **P < .01. (I) FcγRI (CD64) and CD11b expression on CD14+ gated monocytes. Shown is the fold increase in surface expression relative to unstimulated (medium) cells. Data represent mean from triplicates.
Stimulation of primary monocytes and Ba/F3-FcγRI cells results in enhanced IC binding. Percentage of Ba/F3-FcγRI cells binding RBCs opsonized with IgG2a (A-C) or human IgG3 (D-E). Open squares represent starved cells, filled squares represent starved Ba/F3-FcγRI cells stimulated with IL-3 for 1 hour. (B) EA rosette assay in the presence of FcγRI-specific mAb 10.1 (10.1) or isotype control (iso). **P < .01; NS: not significant, t test. (C) Effect of duration of IL-3 stimulus on IC binding. *P < .05; **P < .01, t test. (D) Binding of 1 or more RBCs to Ba/F3 cells. (E) Binding of 2 or more RBCs to Ba/F3 cells. Datapoints represent the means of at least 3 replicates. Similar results were obtained in 3 independent experiments. ***P < .001. (F) Binding of Ba/F3-FcγRI cells to BSA or IgG1-coupled beads in the presence of 10.1 mAb (10.1) or isotype control (ISO). Similar results were obtained in 3 independent experiments. **P < .01, t test. (G) Rosetting of BSA and IgG1-coupled beads with Ba/F3-FcγRI cells visualized with fluorescence microscopy. Beads are detected in the red channel, Ba/F3-FcγRI in the green channel. (H) Binding of unopsonized (unopson) and opsonized (opson) erythrocytes to primary monocytes in the presence of 10.1mAb (10.1) or isotype control (iso). Data represent means of triplicates measured from 1 donor. Similar results were seen in 3 of 4 donors. *P < .05; **P < .01. (I) FcγRI (CD64) and CD11b expression on CD14+ gated monocytes. Shown is the fold increase in surface expression relative to unstimulated (medium) cells. Data represent mean from triplicates.
Similar to mIgG2a-IC, Ba/F3-FcγRI cells showed higher levels of binding of huIgG3-IC upon IL-3 stimulation (Figure 2D). Furthermore, IL-3 stimulation led to a marked increased in binding of 2 or more opsonized RBCs to Ba/F3-FcγRI (Figure 2E). Binding to huIgG3-IC was FcγRI-dependent as determined by receptor blocking with FcγRI mAb 10.1 (data not shown).
We tested several IgG1 RhD clones (BIRMA D6, ESD-1, and LHM76/58) in the rosette assay. Despite considerable opsonization, IgG1 opsonized RBCs failed to bind to Ba/F3-FcγRI or primary monocytes (data not shown). This is in concordance with previous reports.30 It was suggested that in contrast to IgG1, the longer hinge region of IgG3 is capable of overcoming the forces between the negatively charged RBC and the effector cell. Therefore, we used IgG1-coupled beads (huIgG1-IC) to evaluate cytokine-enhanced binding of huIgG1-IC to Ba/F3-FcγRI. IgG1 coupled beads bound to Ba/F3-FcγRI cells, whereas bovine serum albumin (BSA)–coupled beads did not. IL-3 stimulation enhanced only binding of IgG1-coated beads, and binding was FcγRI–dependent, as blocking mAb 10.1 decreased binding to near background levels(Figure 2F). The formation of rosettes with IgG1-coupled beads and Ba/F3-FcγRI cells could clearly be observed with fluorescence microscopy (Figure 2G).
We next assessed whether FcγRI was similarly regulated in primary monocytes and tested the effect of several proinflammatory cytokines on IC binding. Stimulation with TNFα and IFNγ, a known macrophage stimulus,14 enhanced IC binding by primary monocytes. IC binding was inhibited by Ab 10.1, but not isotype control, supporting the involvement of FcγRI (Figure 2H and supplemental Figure 3A). Under these conditions, monocytes were positive for surface IgG. Upon stimulation, only slightly increased surface IgG levels were detected, suggesting that TNFα and IFNγ-enhanced IC binding to monocytes was not due to a decrease of prebound IgG to FcγRI after stimulation (supplemental Figure 3B). Furthermore, stimulation did not alter FcγRI surface expression levels but often (2 of 4 donors) coincided with increased CD11b expression levels (Figure 2I and supplemental Figure 3B). The cytokines IL-6 and IL-4 did not enhance the binding of IC to FcγRI. Taken together, these data suggested cytokine stimulation increases binding of multivalent ligands (IC) to FcγRI.
Cytokine stimulation enhances FcγRI binding to opsonized tumor cells
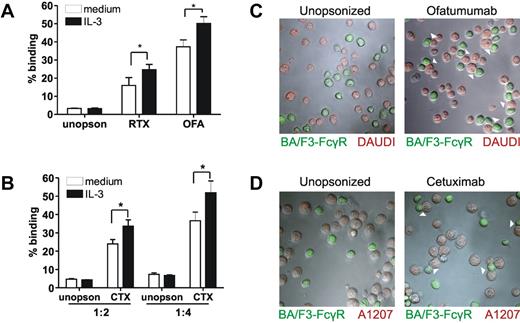
To date, rituximab (RTX) is one of the most successful monoclonal antibodies used in the clinic.31 Using RTX in combination with a B-cell lymphoma line (Daudi), we studied binding of Ba/F3-FcγRI to multivalent ligands. IL-3 stimulation led to increased binding of RTX-opsonized Daudi cells to Ba/F3-FcγRI (Figure 3A). IL-3 induced similar increased binding of Ba/F3-FcγRI to Daudi cells opsonized with ofatumumab, a newly developed human CD20 mAb (Figure 3A). Binding was FcγRI–dependent, as evidenced by blocking with mAb 10.1 (supplemental Figure 4). To investigate whether this effect was limited to B-cell lymphoma and CD20 mAb, we tested the human glioblastoma cell line A1207. IL-3 stimulation increased cetuximab-opsonized A1207-binding to Ba/F3-FcγRI (Figure 3B). Binding of Ba/F3-FcγRI to opsonized Daudi or A1207 was confirmed using fluorescence microscopy (Figure 3C-D, respectively).
Cellular activation leads to enhanced FcγRI-mediated binding of opsonized tumor celllines. (A) Percentage of Ba/F3- FcγRI cells binding to Daudi cells opsonized with Rituximab (RTX), Ofatumumab (OFA) or unopsonized (unopson) Daudi cells. E/T ratio was 1/2. (B) Percentage of Ba/F3- FcγRI cells binding to Cetuximab (CTX) or unopsonized (unopson) A1207. E/T ratio was either 1/2 or 1/4. Data represent the means of at least 3 replicates. FcγRI-blocking mAb 10.1 was used to investigate FcγRI-dependent binding (not shown) *P < .05. Experiment was repeated 3 times yielding similar results. (C-D) Binding of IL-3 stimulated Ba/F3-FcγRI to Ofatumumab-opsonized Daudi cells (C) or Cetuximab-opsonized A1207 cells (D) determined with fluorescence microscopy. Ba/F3-FcγRI cells are shown in green, Daudi and A1207 cells in red. Arrowheads denote contacts between Ba/F3-FcγRI and tumor cells.
Cellular activation leads to enhanced FcγRI-mediated binding of opsonized tumor celllines. (A) Percentage of Ba/F3- FcγRI cells binding to Daudi cells opsonized with Rituximab (RTX), Ofatumumab (OFA) or unopsonized (unopson) Daudi cells. E/T ratio was 1/2. (B) Percentage of Ba/F3- FcγRI cells binding to Cetuximab (CTX) or unopsonized (unopson) A1207. E/T ratio was either 1/2 or 1/4. Data represent the means of at least 3 replicates. FcγRI-blocking mAb 10.1 was used to investigate FcγRI-dependent binding (not shown) *P < .05. Experiment was repeated 3 times yielding similar results. (C-D) Binding of IL-3 stimulated Ba/F3-FcγRI to Ofatumumab-opsonized Daudi cells (C) or Cetuximab-opsonized A1207 cells (D) determined with fluorescence microscopy. Ba/F3-FcγRI cells are shown in green, Daudi and A1207 cells in red. Arrowheads denote contacts between Ba/F3-FcγRI and tumor cells.
FcγRI inside-out regulation is inhibited by the phosphatase inhibitor, okadaic acid
IL-3 stimulation of Ba/F3 cells leads to activation of the phosphatidylinositol-3-kinase–protein kinase B (PI-3K-PKB) and Raf/mitogen-activated protein kinase/extracellular-signal-regulated kinase (Raf/MEK/ERK) pathways. It has been shown that FcαRI inside-out regulation in Ba/F3 cells is dependent on PI-3K and protein phosphatase 2 (PP2a).17,18 We investigated whether similar signaling pathways underlie FcγRI inside-out regulation. In our model, both a PI3-Kinase inhibitor (LY294002)32 and a MEK1/2 inhibitor (U0126)33 did not affect IL-3–enhanced binding to mIgG2a-IC (Figure 4A). OA, a PP2a inhibitor,34 completely inhibited the IL-3en]increased binding.
OA inhibits cytokine-enhanced FcγRI IC binding on primary monocytes and Ba/F3- FcγRI cells. (A) Ba/F3-FcγRI cells were treated with DMSO, LY294002 (LY), U-0126 (U0) or OA and stimulated with IL-3. IC Binding was tested using mIgG2a-opsonized RBCs. Data represent means of at least 4 replicates. Similar results were obtained in 3 independent experiments. **P < .01; ***P < .01; NS: not significant. (B) IFNγ stimulated monocyte binding to mIgG2a-opsonized RBCs after OA treatment. ***P < .001, comparing 1μM OA treatment with DMSO fitted curve; NS: not significant, comparing 0.1μM OA treatment with DMSO treated fitted curve. (C) Effect of FcγRI blocking mAb 10.1 binding of mIgG2a-opsonized RBCs to FcγRI. Similar results were seen in 3 independent experiments.
OA inhibits cytokine-enhanced FcγRI IC binding on primary monocytes and Ba/F3- FcγRI cells. (A) Ba/F3-FcγRI cells were treated with DMSO, LY294002 (LY), U-0126 (U0) or OA and stimulated with IL-3. IC Binding was tested using mIgG2a-opsonized RBCs. Data represent means of at least 4 replicates. Similar results were obtained in 3 independent experiments. **P < .01; ***P < .01; NS: not significant. (B) IFNγ stimulated monocyte binding to mIgG2a-opsonized RBCs after OA treatment. ***P < .001, comparing 1μM OA treatment with DMSO fitted curve; NS: not significant, comparing 0.1μM OA treatment with DMSO treated fitted curve. (C) Effect of FcγRI blocking mAb 10.1 binding of mIgG2a-opsonized RBCs to FcγRI. Similar results were seen in 3 independent experiments.
To investigate whether FcγRI was similarly regulated on primary monocytes, we stimulated monocytes overnight with IFNγ before OA treatment. OA efficiently inhibited binding of opsonized RBCs to monocytes in a dose-dependent manner (Figure 4B). Importantly, treatment of OA did not affect FcγRI-expression levels (supplemental Figure 5). In these assays, binding of IC to FcγRII was prevented by the addition of FcγRII-specific IV.3 Fab before adding IC. FcγRI-dependent binding of opsonized RBC was confirmed with mAb 10.1 (Figure 4C). These data suggest that protein phosphatases play a role during FcγRI inside-out regulation.
Cytokine stimulation enhances binding of immune complexes to saturated FcγRI
Inside-out regulation of FcγRI in the Ba/F3 model mainly affected binding to multivalent ligands (immune complexes) rather than monomeric IgG. We hypothesized that this shift could contribute to immune complex binding of FcγRI already saturated by monomeric ligands.
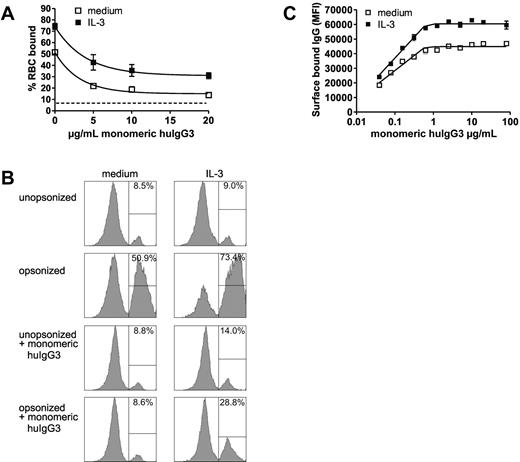
By saturating Ba/F3-FcγRI with monomeric IgG3 after stimulation, we studied whether opsonized RBCs were able to bind to IgG-occupied FcγRI. In unstimulated cells, preincubation with as little as 5 μg/mL monomeric IgG3 blocked RBC binding to Ba/F3-FcγRI cells to near background levels. Stimulation with IL-3, however, led Ba/F3-FcγRI to bind opsonized RBCs effectively (Figure 5A, B). Binding was lower compared with unoccupied FcγRI but quickly reached a plateau, which was maintained at saturating levels of monomeric IgG3 (20 μg/mL). At this concentration, FcγRI was saturated by IgG3 as evidenced by surface IgG staining (Figure 5C). These data document that cytokine stimulation can lead to FcγRI IC binding despite saturation of the receptor with monomeric IgG.
Effect of cellular activation on immune complexes binding to saturated FcγRI. (A) Binding of Ba/F3-FcγRI to huIgG3-opsonized RBCs (y-axis) after incubating Ba/F3-FcγRI with varying amounts of monomeric huIgG3 (x-axis). Dotted line represents binding of Ba/F3-FcγRI to unopsonized RBCs. (B) Percentage of Ba/F3-FcγRI binding to RBCs. One representative sample of triplicates is shown. (C) Amounts of cell-bound Ba/F3-FcγRI IgG3 after incubation with varying amounts of monomeric human IgG3 as detected by flow cytometry. Experiments were repeated 3 times yielding similar results.
Effect of cellular activation on immune complexes binding to saturated FcγRI. (A) Binding of Ba/F3-FcγRI to huIgG3-opsonized RBCs (y-axis) after incubating Ba/F3-FcγRI with varying amounts of monomeric huIgG3 (x-axis). Dotted line represents binding of Ba/F3-FcγRI to unopsonized RBCs. (B) Percentage of Ba/F3-FcγRI binding to RBCs. One representative sample of triplicates is shown. (C) Amounts of cell-bound Ba/F3-FcγRI IgG3 after incubation with varying amounts of monomeric human IgG3 as detected by flow cytometry. Experiments were repeated 3 times yielding similar results.
Discussion
The role of FcγRI in immunity remains unclear, as it is unknown how a high-affinity Fcγ receptor can bind immune complexes. Extravasating monocytes originate from an IgG rich milieu, and it is believed that due to the high affinity of FcγRI, this receptor may well be saturated with monomeric IgG. Only after de novo synthesis has FcγRI been postulated to contribute to the clearance of IgG-opsonized particles. Here, we show that FcγRI ligand–binding can be regulated inside out (Figures 2,Figure 3–4) and that this regulation impacts IC binding to a saturated receptor (Figure 5). Inside-out regulation represents a fast process, as IL-3 enhanced binding could be detected within 15 minutes after stimulation (Figure 2C). Cytokine-enhanced IC binding was observed with different ICs, including tumor cell lines opsonized with IgG1-therapeutic mAbs, as well as mouse IgG2a– and human IgG3–opsonized RBCs and human IgG1–coupled beads (Figures 2–3). Increased binding induced by cytokines was not associated with differences in receptor-surface expression levels. The presence of EDTA in our binding assays makes it unlikely that integrin interactions are involved during cytokine-enhanced IC binding. Furthermore, we did not observe increased binding to unopsonized cells upon stimulation.
Increased binding of ligand upon cellular activation was particularly striking when multivalent ligands (ICs) were used (Figure 3). Monovalent ligands showed a small increase in binding (Figure 1) upon stimulation. This could be due to small aggregates in our IgG preparations, as IgG dimers formed with mouse anti–human κ light-F(ab′)2 showed a small increase in binding upon stimulation (data not shown). Because the increase in binding was most apparent using multivalent ligands, we speculate that the main mechanism involved in regulating IC binding is likely due to alterations in receptor mobility and/or receptor clustering. Photobleach experiments with an FcαRI-YFP fusion protein suggested increased mobility of the receptor upon IL-3 stimulation in Ba/F3 cells (Honing et al, unpublished data). On the other hand, dimerization of FcγRI has been previously suggested35 and FcγRI has been shown to associate with plasma membrane microdomains.36 Indeed, FcγRI-binding capacity could be altered upon cholesterol depletion. A model where lipid raft localization and subsequent clustering affects ligand binding has also been suggested for FcγRIIA.37 It is important to note that the inside-out regulation described here is probably different from the active “trapping” of IC by FcγR as suggested by Dale et al,38 as our binding experiments were performed at 4°C. Cytokine-enhanced ligand binding was capable of inducing IC binding despite the presence of prebound IgG to FcγRI. It is possible that the affinity of FcγRI might differ between antigen-bound (in IC) and antigen-unbound IgG (prebound IgG to the receptor). However, it seems likely that such differences would be antigen dependent.
Several mouse models have shown involvement of FcγRI during immunotherapy. We found that ligand binding by mouse FcγRI is at least differently regulated compared with human FcγRI. Ligand binding to IC by mouse bone marrow–derived dendritic cells (DCs) and bone marrow–derived macrophages was not affected by cytokine stimulation or OA treatment (supplemental Figure 5). Thus, mouse FcγRI is not capable of inside-out regulation, or else it is dependent on different stimuli. Mouse and human FcγRI both bind IgGs with high affinity; however, they show only 19% sequence similarity with the intracellular, c-terminal tail.
Previously, dephosphorylation of FcαRI was shown to regulate ligand binding and to be dependent on PP2A.17 Unlike FcαRI, however, serine phosphorylation of the cytosolic tail of FcγRI α-chain is unlikely to play a role during inside-out regulation, because a truncated receptor lacking all serines was found to respond to cytokine stimulation (data not shown). Strikingly, PI-3K inhibition did not affect cytokine-enhanced IC binding to FcγR,I suggesting distinct mechanisms underlying FcγRI regulation compared with FcαRI.
In earlier reports, we published the interaction between the cytosolic tail of FcγRI and periplakin22,26 to affect receptor-ligand binding. Ba/F3-FcγRI cells did not show periplakin-dependent inside-out regulation, as truncation of FcγRI and overexpression of c-terminal periplakin did not affect cytokine-enhanced ligand binding (data not shown). This could be due to low expression levels of endogenous periplakin in Ba/F3 cells.
Overall, our data suggest that FcγRI saturated with monomeric IgG is capable of binding multivalent immune complexes. This finding contributes to the Fcγ receptor field, as it suggests that FcγRI can participate in clearance of IgG-opsonized particles despite saturation with monomeric ligand. Furthermore, it opens the field for targeted immunotherapy to this receptor. FcγRI-targeted immunotherapy can be achieved via bispecific antibodies or the use of aglycosylated IgGs to bind selectively to FcγRI.39 Studies using a chimeric receptor consisting of carcinoembryonic antigen–specific scFv fused to the cytosolic tail of FcγRI have demonstrated the potent cytotoxic effects mediated through this receptor.40 Direct targeting of FcγRI by expressing scFv-H22 on tumor cell lines showed similar results.41
Our data provide new insights into the role of FcγRI in biology, although the precise mechanism underlying FcγRI inside-out regulation needs to be elucidated. Furthermore, our finding that FcγRI can contribute to immune complex binding warrants further research into the efficacy and role of FcγRI during immunotherapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank G. Spierenburg and K. Gaiser for cell sorting, M. Jansen for assisting with mouse models, and P. Coffer for critically reading the manuscript.
C.E.v.d.P. was supported by a Dutch Science Fund (NWO-ALW2PJ/05 088), and P.B. was supported by a grant from the Association of International Cancer Research (AICR 06-368).
Authorship
Contribution: C.E.v.d.P., R.A.K., and P.B. designed and performed research and analyzed and interpreted data; J.A.v.d.L. and M.B. performed research; and C.E.v.d.P., J.G.J.v.d.W., and J.H.W.L. designed research and wrote the manuscript.
Conflict-of-interest disclosure: Ofatumumab is a product of Genmab/GlaxoSmithKline and J.G.J.v.d.W. is an employee of Genmab. The remaining authors declare no competing financial interests.
Correspondence: J. H. W. Leusen, University Medical Centre Utrecht, Department of Immunology KC02.085.2, Laboratory for Immunotherapy, Lundlaan 6, 3584 EA Utrecht, The Netherlands; e-mail: jleusen@umcutrecht.nl.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal