Abstract
Stromal cell derived factor-1 (SDF-1 or CXCL12) and its receptor CXCR4 are involved in the directional homing to the bone marrow niches and in peripheral mobilization of normal and transformed hematopoietic stem and myeloid progenitor cells. Elevated CXCR4 expression confers poor prognosis, whereas inhibition of CXCR4 signaling overcomes stroma-mediated chemoresistance in acute myeloid leukemia (AML). Here, we demonstrate that treatment with the pan-histone deacetylase inhibitor panobinostat (PS) depleted the mRNA and protein levels of CXCR4 in the cultured and primary AML cells. PS-induced acetylation of the heat shock protein (hsp) 90 reduced the chaperone association between CXCR4 and hsp90, directing CXCR4 to degradation by the 20S proteasome. PS treatment also depleted G protein–coupled receptor kinase 3, as well as attenuated the phosphorylation of AKT and ERK1/2 in AML cells, which was not affected by cotreatment with CXCL12. Compared with each agent alone, cotreatment with PS and CXCR4 antagonist AMD3100 or FC-131 synergistically induced apoptosis of cultured and primary AML cells. PS and FC-131 exerted more lethal effects on primary AML versus normal CD34+ bone marrow progenitor cells. These findings support the rationale to test the in vivo efficacy of PS in enhancing the lethal effects of CXCR4 antagonists against AML cells.
Introduction
Several mechanisms are involved in the interaction between the bone marrow microenvironment, consisting of extracellular matrix and the stromal cells and the normal and leukemia bone marrow stem and progenitor cells (BMPCs).1,2 This interaction determines the survival, self-renewal, chemoresistance, as well as peripheral blood mobilization of BMPCs.1,2 Among the molecular mechanisms, the interaction between the stroma cell–derived chemokine CXCL12 and its 7 transmembrane domain, G protein–coupled receptor CXCR4, is critical for activating signaling that determines the homing, survival, and mobilization of normal and leukemia BMPCs.3-6 Binding of CXCL12 promotes CXCR4 to be phosphorylated, internalized, and incorporated into lipid rafts, which also incorporate PI3K, FAK, and Src kinases, thus enabling the clustering and phosphorylation of the receptor and the effector kinases necessary for downstream G protein–dependent and –independent signaling.7-10 This leads to alteration in gene transcription and cell migration, as well as in promotion of survival and proliferation of normal and leukemia BMPCs.5,6 Overexpression of CXCR4 on human CD34+ progenitor cells increases their proliferation, migration, and NOD/SCID mouse repopulation potential.11,12 In contrast, reduced retention of hematopoietic cells was noted in the bone marrow microenvironment in CXCR4−/− mice.13 In addition, in acute myeloid leukemia (AML), high CXCR4 expression is associated with poor relapse-free and overall survival.14,15 Treatment with the CXCR4 antagonist AMD3100 alone inhibited proliferation and induced differentiation in AML cells.16 Clinical trials have demonstrated that treatment with AMD3100 alone or in combination with granulocyte colony-stimulating factor induces rapid peripheral blood mobilization of normal BMPCs.17-19 In addition, in a genetically engineered mouse model of acute promyelocytic leukemia, administration of AMD3100 increased the circulating acute promyelocytic leukemia cell count, and cotreatment of AMD3100 with chemotherapy decreased tumor burden and improved overall survival compared with mice treated with chemotherapy alone.20 A novel peptide CXCR4 antagonist was shown to strongly inhibit migratory and signaling responses to CXCL12 and overcome stroma-conferred chemoresistance to apoptosis in AML cells.21 Targeting the leukemia microenvironment with CXCR4 inhibition was demonstrated to overcome resistance to kinase inhibitors and chemotherapy.22,23 These reports have underscored the potential importance of CXCR4 as a therapeutic target in hematologic malignancies.24,25 Based on this, novel CXCR4 antagonists, including the inverse agonist FC-131, have been developed and tested preclinically.26,27
Histone acetyltransferases and histone deacetylases (HDACs) play a central role in modifying the acetylation status of the lysine residues of histone and nonhistone proteins.28 Treatment with pan-HDAC inhibitor, such as vorinostat or panobinostat (PS), induces hyperacetylation of histones and nonhistone proteins, including heat shock protein 90 (hsp90) in AML cells.29-31 Hyperacetylation of hsp90 inhibits its chaperone function and promotes the proteasomal degradation of hsp90 client proteins, including FLT-3, AKT, c-RAF, and JAK2, in AML cells.31,32 Concomitantly, HDAC inhibitor treatment has been shown to cause cell cycle growth arrest and apoptosis of AML cells.31,32 In a phase 1 clinical trial, we observed that treatment with PS reduced AML blast cell numbers in the blood of 7 of 11 patients.33 A recent study also showed that treatment with HDAC inhibitor lowers CXCR4 expression in human vascular endothelial cells.34 Taken together, these findings prompted us to determine the in vitro effects of clinically relevant levels of PS on surface expression of CXCR4 and its intracellular signaling in AML cells. Our findings demonstrate that CXCR4 is chaperoned by hsp90, and treatment with PS induces hyperacetylation and inhibition of chaperone association of hsp90 with CXCR4, resulting in depletion of CXCR4 levels and downstream signaling. We further demonstrate that cotreatment with PS significantly increased the anti-AML activity of anti-CXCR4 antagonists AMD3100 and FC-131 or anti-CXCR4 antibody MAB-172.
Methods
Reagents
PS and AUY922 were kindly provided by Novartis Pharmaceuticals AG. FC-131 was obtained from the School of Pharmaceutical Sciences, Kyoto University (Kyoto, Japan). SDF-1α (CXCL12) was purchased from PeproTech. Anti-CXCR4 antibody was purchased from Abcam (ab2074). Anti–G protein–coupled receptor kinase-3 (GRK3) was obtained from Millipore. Monoclonal anti-c-Myc 9E10 was obtained from Santa Cruz Biotechnology. Anti-pFAK (Tyr397) and anti-FAK were obtained from Cell Signaling. Anti-hsp90, anti-hsp70, anti–p-AKT (Ser473), anti-AKT, anti-p-ERK1/2, anti-ERK1/2, anti–c-Raf, and anti–β-actin were obtained as previously described.31,32 Polyclonal anti–hypoxia inducible factor-1α (HIF-1α) was obtained from Novus Biologicals. Affinity-purified polyclonal antibody against Ac-K69-hsp90 was generated by Alpha Diagnostic based on the synthetic 12–amino acid peptide flanking K69 (acetylated and unacetylated) ETLTDPSKLDSGK.35 CXCR4 for antibody blocking experiments (MAB-172) was obtained from R&D Systems.
Cell culture
The human AML cell line OCI-AML-3 was maintained in α-minimum essential medium with 1% penicillin/streptomycin, 1% nonessential amino acids, and 10% fetal bovine serum. HL-60 cells were obtained from ATCC and maintained in RPMI with 1% penicillin/streptomycin, 1% nonessential amino acids, and 10% fetal bovine serum. HEK-293 cells expressing myc-tagged CXCR4 were generated and cultured as previously described.36 For stabilization of HIF-1α in cells, cobalt chloride was added to a final concentration of 100μM, before treatment with PS. Logarithmically growing cell cultures were used for all experiments described.
Primary leukemia samples
Patient samples were obtained with informed consent in accordance with the Declaration of Helsinki as part of a clinical protocol approved by the Institutional Review Board at the Medical College of Georgia. As previously described,37 mononuclear cells were separated by Ficoll-Hypaque density-gradient centrifugation, washed, and resuspended in complete RPMI 1640. The purity of the blast population was confirmed to be ≥ 80% by morphologic evaluation of Wright-stained cytospun cell preparations. CD34+ mononuclear cells were purified from banked, delinked, and de-identified patient samples using immunomagnetic beads conjugated with an anti-CD34 antibody (StemCell Technologies).37
RNA isolation and reverse transcription PCR
Cells were harvested and total RNA was isolated using Trizol reagent (Invitrogen). Total RNA (2 μg) was reverse transcribed using a Superscript First-Stand Synthesis kit (Invitrogen). Resulting cDNAs were used for subsequent polymerase chain reaction (PCR) analysis of CXCR4, p21, p27, and β-actin. PCR reactions were performed using the following parameters: 94°C for 5 minutes followed by 35 cycles of 94°C (1 minute), 55°C (1 minute), and 72°C (1 minute). Amplified products were separated on a 2% agarose gel and recorded with an ultraviolet transilluminator. Horizontal scanning densitometry was performed with ImageQuant Version 5.2 (GE Healthcare), and the band intensity of each PCR product was compared with that of β-actin.
Detection and analysis of hsa-miR-146 in AML cells
For detection of hsa-miR-146a in AML OCI-AML3 cells, miRNAs were isolated with a kit from Applied Biosystems. Total RNA was reverse transcribed with a stem loop primer included in the TaqMan hsa-miR-146a microRNA assay following the manufacturer's protocol (Applied Biosystems). Expression of hsa-miR-146a was detected by quantitative PCR with a TaqMan probe specific to mature hsa-miR-146a. Relative expression of hsa-miR-146a was normalized against expression of 18S RNA.
Assessment of percentage nonviable cells
Untreated and drug-treated cells were stained with trypan blue (Sigma- Aldrich). The numbers of nonviable cells were determined by counting the cells that showed trypan blue uptake in a hemocytometer and reported as a percentage of untreated control cells, as previously described.37
Assessment of apoptosis and synergism
Untreated or drug-treated cells were stained with annexin V and propidium iodide. The percentage of apoptotic cells was determined by flow cytometry. To analyze synergism between PS and FC-131 or AMD3100 at inducing apoptosis, cells were treated with PS (5-50nM) and FC-131 (0.4-4.0μM) or AMD3100 (2.0-10.0μM) at a constant ratio for 48 hours. The percentage of apoptotic cells was determined by flow cytometry. The combination index (CI) and the fraction affected for each drug combination were obtained using the commercially available software Calcusyn Version 2.0 (Biosoft).32,37 CI values < 1.0 represent synergism of the 2 drugs in the combination.
Cell lysis and immunoblot analyses
After designated treatments, cells were centrifuged, washed with one time phosphate-buffered saline, resuspended in TNSEV lysis buffer (50mM Tris-HCl, pH 7.5, 2mM ethylenediaminetetraacetic acid, 150mM NaCl, 1mM sodium orthovanadate, 1% Nonidet P-40) containing 20 μg/mL aprotinin, 20 μg/mL leupeptin, 1mM phenylmethylsulfonyl fluoride, and 25mM NaF, and incubated on ice for 30 minutes. For detection of HIF-1α, cells were lysed in RIPA buffer (Sigma-Aldrich). Cell lysates were centrifuged at 17 000g for 15 minutes to remove the nuclear and cellular debris. A total of 100 μg of total cell lysates was used for sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Western blot analyses of CXCR4, hsp90, hsp70, pERK1/2, ERK1/2, pAKT (Ser 473), AKT, pGSK3β, GRK2/3, HIF-1α, and c-Raf were performed on the total cell lysates using specific antisera or monoclonal antibodies.32,37 The expression levels of β-actin were used as the loading control for the immunoblots. Densitometry was performed with ImageQuant Version 5.2 software (GE Healthcare).
Immunoprecipitation
Precleared cell lysates were incubated for 2 hours at 4°C with anti-CXCR4 (Rb), rat anti-hsp90, polyclonal GRK3, or anti-Myc 9E10. Protein-A or -G agarose beads, as appropriate, were added to the lysate, and the mixture was incubated overnight at 4°C. The next day, the immunoprecipitates were separated from unbound protein by centrifugation, washed 3 times in TNSEV lysis buffer, and eluted from the beads by boiling in 6 times sodium dodecyl sulfate sample buffer before immunoblot analysis.31,35
AKT kinase activity assay
Cells were treated with FC-131 and/or PS for 24 hours and then stimulated with SDF-1α at 100nM for 5 minutes. Cell lysates were prepared and an AKT kinase activity kit from Cell Signaling was used according to the manufacturer's protocol. AKT kinase activity was assessed by immunoblot analysis of phosphorylated GSK3β on a recombinant GSK3β substrate. Immunoblot analyses were also performed for pAKT and total AKT.
Flow cytometry
Cell surface expression of CXCR4 was measured by flow cytometry. After an acid wash (pH 3) to elute-bound CXCL12 or FC131 from the receptor,38 cells were incubated with a saturating concentration of phycoerythrin-conjugated anti-CXCR4 (BD Biosciences) and imaged on a FACSCalibur (BD Biosciences). phycoerythrin-conjugated anti-IgG2a served as the isotype control.
Statistics
Results are shown as mean plus or minus SE of at least 3 experiments. Data were analyzed using the paired Student t test. P values less than .05 were considered significant. All statistical sets were 2-sided.
Results
Treatment with PS attenuates the expression levels of CXCR4 mRNA and protein in cultured and primary AML cells
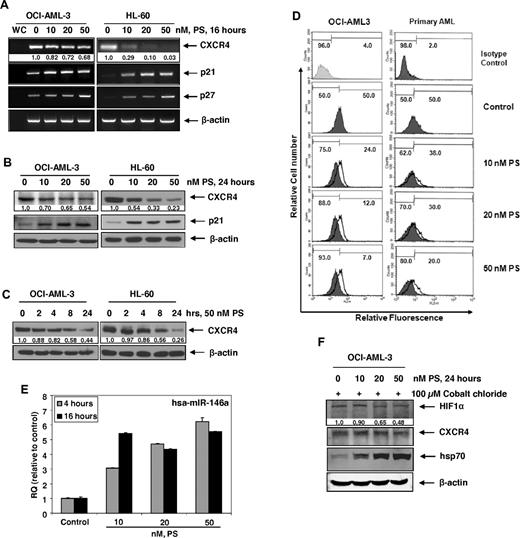
We first determined the effect of PS on the mRNA expression of CXCR4 in cultured AML OCI-AML3 and HL-60 cells. Treatment with PS (10-50nM) dose-dependently reduced the expression of CXCR4 mRNA while concomitantly inducing the mRNA expression of the cyclin-dependent kinase inhibitors p21 and p27 (Figure 1A). Depletion of CXCR4 mRNA was more pronounced after treatment with PS in HL-60 versus OCI-AML3 cells. Treatment with PS also depleted CXCR4 protein expression levels in a dose- and time-dependent manner, again more in HL-60 than OCI-AML3 cells (Figure 1B-C). Reduction in CXCR4 levels was noted within 4 hours with maximum depletion seen after 24 hours of treatment with PS (Figure 1C). These findings in human AML cells are consistent with the reported results demonstrating that PS treatment also reduces the mRNA and protein expression of CXCR4 in human umbilical vein endothelial cells.34 The specificity of the antibody used here for the detection of CXCR4 was confirmed by determining that treatment with the siRNA to CXCR4 for 48 hours depleted the levels of the same 42-kDa band noted as CXCR4 in Figure 1B-C (data not shown). Next, using flow cytometry, we determined whether PS treatment also down-regulated the cell surface expression of CXCR4 in the cultured and primary AML cells. Figure 1D demonstrates that treatment with PS depleted the surface expression of CXCR4 in both OCI-AML3 and primary AML cells, as indicated by the decrease in the relative fluorescence as well as the relative decline in the percentage of positive cells in the histograms. As CXCR4 has previously been determined to be posttranscriptionally repressed by hsa-miR-146a,39,40 we next determined the effects of PS treatment on the expression of hsa-miR146a in AML cells. As shown in Figure 1E, treatment with PS causes a dose- and time-dependent induction of hsa-miR-146a up to 6-fold greater than untreated controls in OCI-AML3 cells. Because HIF-1α regulates the transcription of CXCR4 in hypoxic conditions,41 we also determined the effects of PS treatment on the expression of HIF-1α in AML cells under chemically induced hypoxia. As Figure 1F demonstrates, treatment with PS dose-dependently depletes the expression levels of HIF-1α with concomitant down-regulation of CXCR4 expression in OCI-AML3 cells.
Treatment with PS depletes CXCR4 mRNA and protein expression in cultured and primary AML cells. (A) OCI-AML3 and HL-60 cells were treated with the indicated concentrations of PS for 16 hours. Then, total RNA was extracted and RT-PCR was performed for CXCR4, p21, and p27. A β-actin–specific PCR reaction served as a loading control for the amplification. (B) OCI-AML3 and HL-60 cells were treated with the indicated concentration of PS for 24 hours. After treatment, immunoblot analyses were performed for CXCR4 and p21 on the total cell lysates. The expression of β-actin in the lysates served as the loading control. (C) OCI-AML3 and HL-60 cells were treated with 50nM PS for the indicated times, and immunoblot analyses were performed as described in panel B. (D) OCI-AML3 and primary AML cells were treated with the indicated concentrations of PS for 24 hours. Then surface expression of CXCR4 was analyzed by flow cytometry using monoclonal phycoerythrin-conjugated 12G5 antibody. Values indicate the relative fluorescence of CXCR4 detected. (E) OCI-AML3 cells were treated with the indicated concentrations of PS for 4 and 16 hours. After treatment, total RNA was isolated and reverse transcribed with a specific stem loop primer for hsa-miR-146a. The resulting cDNAs were used for quantitative PCR with a TaqMan probe for hsa-miR-146a. Relative expression levels were normalized against 18S rRNA. (F) OCI-AML3 cells were treated with the indicated concentrations of PS for 24 hours in the presence of cobalt chloride. After treatment, cell lysates were prepared and immunoblot analyses were performed for HIF-1α, CXCR4, and hsp70. The expression levels of β-actin in the lysates served as the loading control.
Treatment with PS depletes CXCR4 mRNA and protein expression in cultured and primary AML cells. (A) OCI-AML3 and HL-60 cells were treated with the indicated concentrations of PS for 16 hours. Then, total RNA was extracted and RT-PCR was performed for CXCR4, p21, and p27. A β-actin–specific PCR reaction served as a loading control for the amplification. (B) OCI-AML3 and HL-60 cells were treated with the indicated concentration of PS for 24 hours. After treatment, immunoblot analyses were performed for CXCR4 and p21 on the total cell lysates. The expression of β-actin in the lysates served as the loading control. (C) OCI-AML3 and HL-60 cells were treated with 50nM PS for the indicated times, and immunoblot analyses were performed as described in panel B. (D) OCI-AML3 and primary AML cells were treated with the indicated concentrations of PS for 24 hours. Then surface expression of CXCR4 was analyzed by flow cytometry using monoclonal phycoerythrin-conjugated 12G5 antibody. Values indicate the relative fluorescence of CXCR4 detected. (E) OCI-AML3 cells were treated with the indicated concentrations of PS for 4 and 16 hours. After treatment, total RNA was isolated and reverse transcribed with a specific stem loop primer for hsa-miR-146a. The resulting cDNAs were used for quantitative PCR with a TaqMan probe for hsa-miR-146a. Relative expression levels were normalized against 18S rRNA. (F) OCI-AML3 cells were treated with the indicated concentrations of PS for 24 hours in the presence of cobalt chloride. After treatment, cell lysates were prepared and immunoblot analyses were performed for HIF-1α, CXCR4, and hsp70. The expression levels of β-actin in the lysates served as the loading control.
Treatment with PS or AUY922 induces acetylation of hsp90, inhibits the chaperone association of CXCR4 with hsp90, leading to proteasomal degradation of CXCR4
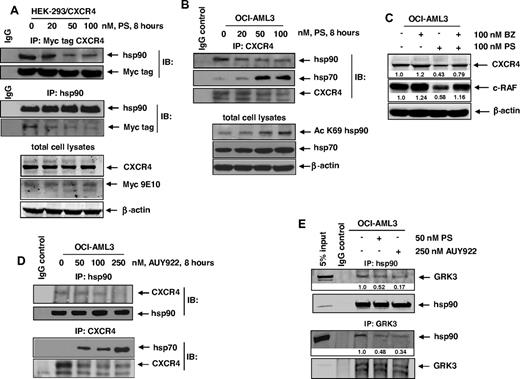
We have previously reported that, by inhibiting HDAC6, PS induces hyperacetylation of hsp90 and disruption of its chaperone function for HSF1 and other hsp90 client proteins.31,35 This leads to the proteasomal degradation of the signaling protein kinase hsp90 client proteins (eg, c-RAF, AKT, and CDK4).31,35 Next, we determined whether CXCR4 has chaperone association with hsp90 and whether PS treatment disrupted the binding of CXCR4 to hsp90. In HEK293 cells, with ectopic overexpression of a myc-tagged CXCR4, treatment with PS reduced the binding of myc-tagged CXCR4 and hsp90 (Figure 2A). Because the expression of the ectopic myc-tagged CXCR4 was driven by a cytomegalovirus promoter, immunoblot analysis of total cell lysates after PS treatment did not show as much decline in the levels of myc-tagged CXCR4 here as was seen in Figure 1B in the levels of the endogenous CXCR4. We confirmed that the endogenous CXCR4 also exhibited chaperone association with hsp90 in OCI-AML3 cells, which was disrupted after treatment with PS (Figure 2B). Concomitantly, PS treatment increased the levels of acetylated hsp90 as well as promoted binding of the endogenous CXCR4 to hsp70 in OCI-AML3 cells (Figure 2B). After PS treatment, reduced chaperone function of the acetylated hsp90 was also evidenced by an increase in the levels of hsp70 (Figure 2B). PS treatment–mediated reduced CXCR4 levels were partly the result of the proteasomal degradation because cotreatment with bortezomib, a proteasome inhibitor, partially restored PS-depleted CXCR4 levels (Figure 2C). A similar effect on the levels of c-RAF, a well-documented hsp90 client protein, was also noted (Figure 2C). To further confirm that CXCR4 is chaperoned by hsp90, we treated OCI-AML3 cells with the hsp90 inhibitor AUY922.42 As demonstrated in Figure 2D, treatment with AUY922 also inhibited the binding of CXCR4 to hsp90, thereby leading to depletion of CXCR4 while inducing hsp70 levels. We also determined the effects of treatment with PS or AUY922 treatment on the chaperone association of GRK3 with hsp90. As shown in Figure 2E, treatment with PS or AUY922 for as little as 4 hours significantly inhibits the association of GRK3 and hsp90 in AML cells, consistent with GRK3 being a client protein chaperoned by hsp90.
Treatment with PS or AUY922 depletes the binding of CXCR4 and GRK3 with hsp90 in AML cells. (A) HEK293/CXCR4 cells were treated with the indicated concentrations of PS for 8 hours. After treatment, cell lysates were prepared and Myc-tagged CXCR4 and hsp90 were immunoprecipitated. Immunoblot analyses were performed for Myc tag and hsp90 on the immunoprecipitates and total cell lysates. The expression levels of β-actin in the lysates served as the loading control. (B) OCI-AML3 cells were treated with the indicated concentrations of PS for 8 hours. At the end of treatment, cells were harvested and CXCR4 was immunoprecipitated from the total cell lysates. Immunoblot analyses were performed for CXCR4, hsp90, and hsp70 on the immunoprecipitates. Immunoblot analyses were also performed for acetylated K69 hsp90 and hsp70 in the total cell lysates. The expression levels of β-actin in the lysates served as the loading control. (C) OCI-AML3 cells were treated with the indicated concentrations of bortezomib (BZ) and PS for 8 hours. Then, total cell lysates were prepared and immunoblot analyses were performed for CXCR4 and c-RAF on the total cell lysates. The expression levels of β-actin in the lysates served as the loading control. (D) OCI-AML3 cells were treated with the indicated concentrations of AUY922 for 8 hours. At the end of treatment, cells were harvested and CXCR4 and hsp90 were immunoprecipitated from the total cell lysates. Immunoblot analyses were performed for CXCR4, hsp90, and hsp70 on the immunoprecipitates. (E) OCI-AML3 cells were treated with the indicated concentrations of PS or AUY922 for 4 hours. After treatment, hsp90 and GRK3 were immunoprecipitated from the total cell lysates. Immunoblot analyses were performed for GRK3 and hsp90 on the immunoprecipitates.
Treatment with PS or AUY922 depletes the binding of CXCR4 and GRK3 with hsp90 in AML cells. (A) HEK293/CXCR4 cells were treated with the indicated concentrations of PS for 8 hours. After treatment, cell lysates were prepared and Myc-tagged CXCR4 and hsp90 were immunoprecipitated. Immunoblot analyses were performed for Myc tag and hsp90 on the immunoprecipitates and total cell lysates. The expression levels of β-actin in the lysates served as the loading control. (B) OCI-AML3 cells were treated with the indicated concentrations of PS for 8 hours. At the end of treatment, cells were harvested and CXCR4 was immunoprecipitated from the total cell lysates. Immunoblot analyses were performed for CXCR4, hsp90, and hsp70 on the immunoprecipitates. Immunoblot analyses were also performed for acetylated K69 hsp90 and hsp70 in the total cell lysates. The expression levels of β-actin in the lysates served as the loading control. (C) OCI-AML3 cells were treated with the indicated concentrations of bortezomib (BZ) and PS for 8 hours. Then, total cell lysates were prepared and immunoblot analyses were performed for CXCR4 and c-RAF on the total cell lysates. The expression levels of β-actin in the lysates served as the loading control. (D) OCI-AML3 cells were treated with the indicated concentrations of AUY922 for 8 hours. At the end of treatment, cells were harvested and CXCR4 and hsp90 were immunoprecipitated from the total cell lysates. Immunoblot analyses were performed for CXCR4, hsp90, and hsp70 on the immunoprecipitates. (E) OCI-AML3 cells were treated with the indicated concentrations of PS or AUY922 for 4 hours. After treatment, hsp90 and GRK3 were immunoprecipitated from the total cell lysates. Immunoblot analyses were performed for GRK3 and hsp90 on the immunoprecipitates.
Treatment with PS inhibits CXCR4-mediated downstream signaling molecules in cultured and primary AML cells
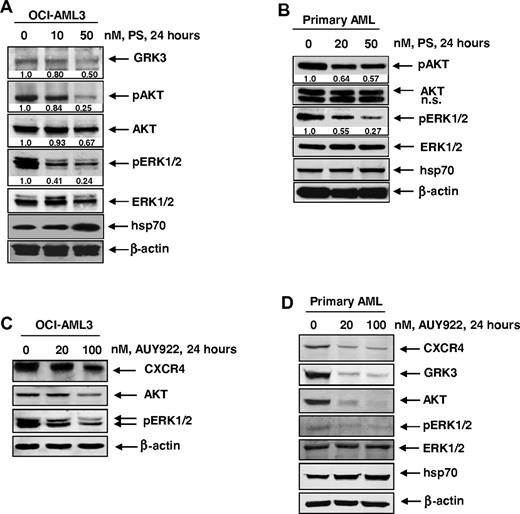
The GRK3 has been shown to specifically regulate CXCL12-promoted internalization and desensitization of CXCR4.43 GRKs, including GRK2 and GRK3, are chaperoned by hsp90 and involved in phosphorylation of signaling proteins downstream of CXCR4, such as AKT and ERK1/2.43,44 Therefore, we next determined the effects of PS treatment on the signaling proteins downstream of CXCR4 in the cultured and primary AML cells. Treatment with PS for 24 hours dose-dependently depleted the levels of GRK3, p-AKT, AKT, and p-ERK1/2 while concomitantly inducing hsp70 expression in OCI-AML3 cells (Figure 3A). Similar effects of PS were also observed in primary AML cells (Figure 3B). Because AUY922 treatment also disrupted the chaperone association of CXCR4 with hsp90, we determined the effect of AUY922 on CXCR4 and its downstream signaling. Figure 3C demonstrates that treatment with AUY922 dose-dependently depleted the levels of CXCR4, AKT, and p-ERK1/2 while simultaneously inducing the levels of hsp70 in OCI-AML3 cells. Similar effects of AUY922 were observed on CXCR4, GRK3, AKT, and p-ERK1/2 levels in primary AML cells (Figure 3D).
Treatment with PS or AUY inhibits CXCR4-mediated signaling through AKT and ERK1/2 in cultured and primary AML cells. (A) OCI-AML3 cells were treated with the indicated concentrations of PS for 24 hours. After treatment, cells were lysed and immunoblot analyses were performed for CXCR4, GRK3, pAKT (Ser 473), AKT, pERK1/2, ERK1/2, and hsp70. The expression of β-actin in the lysates served as the loading control. (B) Primary AML cells were treated with the indicated concentrations of PS for 24 hours. After treatment, cell lysates were prepared and immunoblot analyses were performed for pAKT (Ser 473), AKT, pERK1/2, ERK1/2, and hsp70. The expression of β-actin in the lysates served as the loading control. (C) OCI-AML3 cells were treated with the indicated concentrations of AUY922 for 24 hours. After treatment, cells were lysed and immunoblot analyses were performed for CXCR4, AKT, and pERK1/2. The expression of β-actin in the lysates served as the loading control. (D) Primary AML cells were treated with the indicated concentrations of AUY922 for 24 hours. After treatment, cell lysates were prepared and immunoblot analyses were performed for CXCR4, GRK3, AKT, pERK1/2, ERK1/2, and hsp70. The expression of β-actin in the lysates served as the loading control.
Treatment with PS or AUY inhibits CXCR4-mediated signaling through AKT and ERK1/2 in cultured and primary AML cells. (A) OCI-AML3 cells were treated with the indicated concentrations of PS for 24 hours. After treatment, cells were lysed and immunoblot analyses were performed for CXCR4, GRK3, pAKT (Ser 473), AKT, pERK1/2, ERK1/2, and hsp70. The expression of β-actin in the lysates served as the loading control. (B) Primary AML cells were treated with the indicated concentrations of PS for 24 hours. After treatment, cell lysates were prepared and immunoblot analyses were performed for pAKT (Ser 473), AKT, pERK1/2, ERK1/2, and hsp70. The expression of β-actin in the lysates served as the loading control. (C) OCI-AML3 cells were treated with the indicated concentrations of AUY922 for 24 hours. After treatment, cells were lysed and immunoblot analyses were performed for CXCR4, AKT, and pERK1/2. The expression of β-actin in the lysates served as the loading control. (D) Primary AML cells were treated with the indicated concentrations of AUY922 for 24 hours. After treatment, cell lysates were prepared and immunoblot analyses were performed for CXCR4, GRK3, AKT, pERK1/2, ERK1/2, and hsp70. The expression of β-actin in the lysates served as the loading control.
Cotreatment with PS disrupts SDF-1α-mediated internalization of CXCR4 and downstream signaling
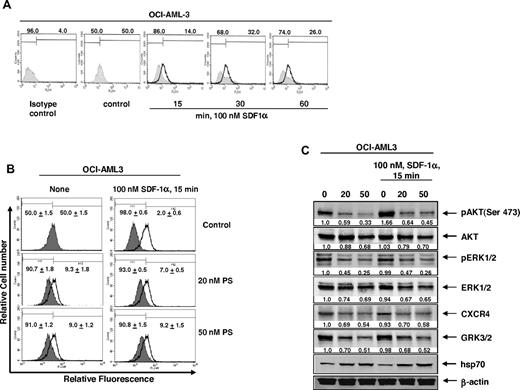
Exposure to SDF-1α (CXCL12), the ligand for CXCR4, stimulates the internalization of CXCR4, leading to the activation of downstream signaling through PI3/AKT and ERK1/2.7 Therefore, we next determined the effects of SDF-1α alone or in combination with PS on CXCR4 levels and signaling in AML cells. As shown in Figure 4A, compared with the control cells, a short treatment (15-60 minutes) with 100nM SDF-1α alone reduced the surface expression by promoting internalization of CXCR4 levels, as detected by flow cytometry using the conformation-specific CXCR4 (12G5) antibody.38 Treatment with PS also reduced the surface expression of CXCR4 to a similar extent as SDF-1α (Figure 4B). Cotreatment with SDF-1α and PS also showed similar reduction in the surface expression of CXCR4, although there was a slight increase with the combined treatment (Figure 4B). As has also been previously reported, exposure to SDF-1α rapidly (15 minutes) induced p-AKT levels (Figure 4C). However, pretreatment with PS reduced SDF-1α-mediated induction of p-AKT levels. In addition, PS-mediated decline in the levels of CXCR4, GRK3, AKT, p-ERK1/2, and ERK1/2 in AML cells was not affected by subsequent treatment with SDF-1α (Figure 4C). This suggests that the salutary effects of PS on disrupting CXCR4 levels and signaling are not compromised by cotreatment with SDF-1α.
Cotreatment with PS inhibits SDF-1α-mediated internalization of CXCR4 in AML cells. (A) OCI-AML3 cells were treated with 100nM SDF-1α for the indicated times. After SDF-1α stimulation, cells were collected and surface expression of CXCR4 was assessed by staining the cells with anti-CXCR4 12G5 antibody and flow cytometry. (B) OCI-AML3 cells were treated with the indicated concentrations of PS for 24 hours followed by stimulation with 100nM SDF-1α for 15 minutes. Then, cells were collected and surface expression of CXCR4 was assessed by staining the cells with anti-CXCR4 (12G5) antibody and flow cytometry. (C) Immunoblot analyses of pAKT, AKT, pERK1/2, ERK1/2, CXCR4, GRK3, and hsp70 in total cell lysates from OCI-AML3 cells treated with the indicated concentrations of PS for 24 hours followed by stimulation with SDF-1α for 15 minutes. The expression of β-actin in the lysates served as the loading control.
Cotreatment with PS inhibits SDF-1α-mediated internalization of CXCR4 in AML cells. (A) OCI-AML3 cells were treated with 100nM SDF-1α for the indicated times. After SDF-1α stimulation, cells were collected and surface expression of CXCR4 was assessed by staining the cells with anti-CXCR4 12G5 antibody and flow cytometry. (B) OCI-AML3 cells were treated with the indicated concentrations of PS for 24 hours followed by stimulation with 100nM SDF-1α for 15 minutes. Then, cells were collected and surface expression of CXCR4 was assessed by staining the cells with anti-CXCR4 (12G5) antibody and flow cytometry. (C) Immunoblot analyses of pAKT, AKT, pERK1/2, ERK1/2, CXCR4, GRK3, and hsp70 in total cell lysates from OCI-AML3 cells treated with the indicated concentrations of PS for 24 hours followed by stimulation with SDF-1α for 15 minutes. The expression of β-actin in the lysates served as the loading control.
Cotreatment with PS and CXCR4 antagonists or a blocking antibody for CXCR4 exerts synergistic antileukemia effects on cultured leukemic cells
We next determined the effect of PS and/or CXCR antagonists AMD3100 and FC-131 in AML cells. As has been previously reported,32 here we also determined that treatment with PS induced apoptosis of OCI-AML3 and HL-60 cells in a dose-dependent manner (data not shown). Cotreatment with AMD3100 (dose range, 2.0-10.0μM) or FC-131 (dose range, 0.5-4μM) and PS (5-50nM) induced synergistic apoptosis of OCI-AML3 cells (Figure 5A). We next compared the lethal effects of treatment with FC-131 and/or PS against primary AML cells derived from peripheral blood mononuclear cells and/or bone marrow aspirates from 8 patients with AML versus CD34+ normal bone marrow progenitor cell samples from 3 normal donors. Similar to the results in the cultured cell lines, cotreatment with FC-131 significantly enhanced PS-induced cell death of primary AML cells, whereas the combination was significantly less toxic to normal CD34+ cells, where it induced less than 20% cell death at 48 hours (Figure 5B). Notably, cotreatment with PS also significantly enhanced induced apoptosis of OCI-AML3 cells because of the anti-CXCR4 antibody MAB-172 (10 μg/mL; Figure 5C). We next evaluated the effect of FC-131 and PS on CXCR4 levels and signaling in AML cells. Similar to PS, treatment with FC-131 partially inhibited AKT kinase activity in OCI-AML3 cells, as measured by depletion of p-GSK3β levels (Figure 6). Cotreatment with FC-131 augmented PS-mediated depletion of AKT kinase activity represented by further decline in the levels of p-GSK3β, without affecting PS-mediated reduction in CXCR4, GRK3, p-AKT, AKT, p-ERK1/2, and ERK1/2 levels (Figure 6).
Treatment with AMD3100, FC-131, or a CXCR4 blocking antibody significantly increases PS-mediated lethality of AML cells. (A) Analysis of the dose-effect relationship for PS (5-50nM) and AMD3100 (2-10μM) or FC-131 (0.5-4μM) for the apoptotic effects after 48 hours of exposure in OCI-AML3 cells was performed according to the median dose effect method of Chou and Talalay. After this, the CI values were calculated using the percentage of apoptotic cells (fraction affected [FA]) by the 2 agents together. CI < 1, CI = 1, and CI > 1 represent synergism, additivity, and antagonism of the 2 agents, respectively. (B) Primary AML (n = 8) and CD34+ peripheral blood mononuclear cells from healthy donors were treated with the indicated concentrations of FC-131 and/or PS for 48 hours. Then, the percentages of nonviable cells were determined by trypan blue dye uptake in a hemocytometer. (C) OCI-AML3 cells were treated with the indicated concentrations of anti-CXCR4 MAB-172 antibody and/or PS for 48 hours. Then, the percentages of apoptotic cells were determined by annexin V and propidium iodide staining and flow cytometry. Columns represent the mean of 3 experiments. Bars represent the SEM.
Treatment with AMD3100, FC-131, or a CXCR4 blocking antibody significantly increases PS-mediated lethality of AML cells. (A) Analysis of the dose-effect relationship for PS (5-50nM) and AMD3100 (2-10μM) or FC-131 (0.5-4μM) for the apoptotic effects after 48 hours of exposure in OCI-AML3 cells was performed according to the median dose effect method of Chou and Talalay. After this, the CI values were calculated using the percentage of apoptotic cells (fraction affected [FA]) by the 2 agents together. CI < 1, CI = 1, and CI > 1 represent synergism, additivity, and antagonism of the 2 agents, respectively. (B) Primary AML (n = 8) and CD34+ peripheral blood mononuclear cells from healthy donors were treated with the indicated concentrations of FC-131 and/or PS for 48 hours. Then, the percentages of nonviable cells were determined by trypan blue dye uptake in a hemocytometer. (C) OCI-AML3 cells were treated with the indicated concentrations of anti-CXCR4 MAB-172 antibody and/or PS for 48 hours. Then, the percentages of apoptotic cells were determined by annexin V and propidium iodide staining and flow cytometry. Columns represent the mean of 3 experiments. Bars represent the SEM.
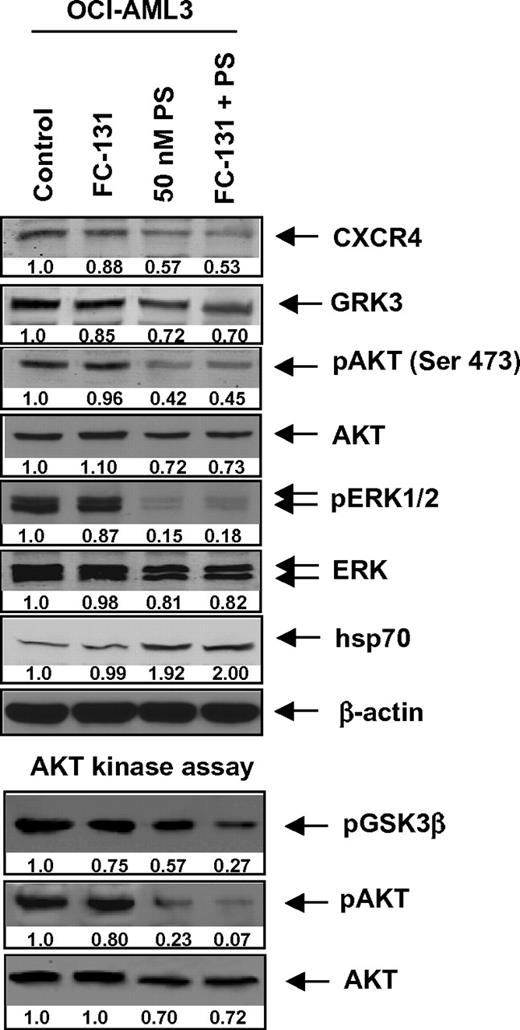
Treatment with FC-131 and/or PS inhibits CXCR4 signaling through AKT. OCI-AML3 cells were treated with 1 μM FC-131 and/or PS for 24 hours. Then, cells were harvested and immunoblot analyses were performed for CXCR4, GRK3, pAKT, AKT, pERK1/2, ERK1/2, and hsp70. The expression of β-actin in the lysates served as the loading control. Alternatively, activated AKT was immunoprecipitated from the total cell lysates of untreated and treated cells and used for AKT kinase activity assays. Immunoblot analyses were performed for phosphorylation of a GSK3β substrate as well as for pAKT and total AKT in the immunoprecipitates.
Treatment with FC-131 and/or PS inhibits CXCR4 signaling through AKT. OCI-AML3 cells were treated with 1 μM FC-131 and/or PS for 24 hours. Then, cells were harvested and immunoblot analyses were performed for CXCR4, GRK3, pAKT, AKT, pERK1/2, ERK1/2, and hsp70. The expression of β-actin in the lysates served as the loading control. Alternatively, activated AKT was immunoprecipitated from the total cell lysates of untreated and treated cells and used for AKT kinase activity assays. Immunoblot analyses were performed for phosphorylation of a GSK3β substrate as well as for pAKT and total AKT in the immunoprecipitates.
Discussion
Functional CXCR4 is expressed in AML cells and confers poor prognosis in AML.5,6,15 CXCR4 antagonists have been shown to inhibit the survival signaling resulting from tumor microenvironment and sensitize AML cells to anti-AML therapy.21-23 In the present studies, we demonstrate that treatment with the pan-HDAC inhibitor PS inhibits both the mRNA and protein expression of CXCR4 in cultured and primary AML cells. Although not elucidated here, it is possible that PS treatment modifies chromatin marks on the promoter of CXCR4, which enables transcriptional repression of CXCR4. Several transcriptional regulatory mechanisms have been described that regulate the expression of CXCR4.7 These include the activity of transcriptional factors (eg, NRF1, HIF-1, NF-κB, and YY1).7 In the present studies, we demonstrate that treatment with PS dose-dependently depletes the expression of HIF-1α, a known hsp90 client protein, in OCI-AML3 cells. Depletion of HIF-1α by PS treatment could partially explain the transcriptional down-regulation of CXCR4. Although methylation of the promoter and CXCR4 repression have been described in pancreatic cancer cells, this has not been reported in AML blasts.45 A recent report demonstrated that megakaryopoiesis is controlled by cascade pathway where the transcription factor PLZF inhibits miR-146a, which in turn up-regulates CXCR4 expression.40 In the present studies, we demonstrate that treatment with PS dose- and time-dependently induces the levels of hsa-miR146a in AML cells. These findings indicate a second potential mechanism leading to the down-regulation of CXCR4 in AML cells.
Findings presented here demonstrate, for the first time, that CXCR4 is chaperoned by hsp90, and PS induces the acetylation and inhibits the chaperone function of hsp90, thereby promoting the proteasomal degradation and depletion of CXCR4 in AML cells. Thus, PS treatment depletes the mRNA and promotes the post-translational degradation of CXCR4 in AML cells. Chaperone association of CXCR4 with hsp90 in AML cells was further confirmed by the observation that treatment with AUY922 also disrupted the binding of CXCR4 to hsp90 and depleted CXCR4 levels. Consistent with the previous report that GRKs, including GRK3, are also chaperoned by hsp90,43,44 findings presented here demonstrate that PS and AUY922 treatment also depletes GRK3 levels and inhibits GRK3 binding to hsp90 in AML cells. In addition, treatment with PS also lowered the levels of other signaling hsp90 client oncoproteins in AML cells, including AKT and c-RAF, which are activated by CXCL12 binding and activation of CXCR4.7,31,32 AUY922 exerted similar activity in the cultured and primary AML cells. Activation of JAK-STAT pathway has also been reported after activation of CXCR4.46 Treatment with PS has been shown to also down-regulate JAK-STAT signaling in human leukemia cells.47 Taken together, these findings indicate that PS treatment is capable of attenuating multiple CXCR4-mediated signaling mechanisms that are known to confer survival advantage, as well as confer chemoresistance in AML cells.21,22
It has been previously reported that, on CXCL12 activation, CXCR4 is rapidly phosphorylated and internalized, activating CXCR4 signaling.6,7 Our findings show that exposure to CXCL12 or PS also caused lowering of the surface expression of CXCR4 on AML cells. Because of its inhibitory effect on hsp90, treatment with PS also reduced the expression and activity of AKT and c-RAF independent of its effects on CXCR4. PS treatment lowered intracellular p-AKT levels and AKT kinase activity, determined against GSK3β as the AKT substrate. One possible mechanism for the down-regulation of AKT phosphorylation by HDAC or hsp90 inhibitor is that treatment with PS or AUY922 activates PP1 or another phosphatase, resulting in increased AKT dephosphorylation. Hsp90 inhibitors, such as geldanamycin, have been previously reported to increase PP1-mediated dephosphorylation of AKT in breast cancer cells.48 Importantly, PS-mediated inhibition of the signaling kinases is not affected or compromised by the exposure of AML cells to CXCL12. This indicates that the anti-AML activity of PS mediated by its effects on CXCR4 and the signaling kinases is not abrogated by the CXCL12-CXCR4 component of the signaling for survival and chemoresistance mediated by the bone marrow microenvironment against kinase inhibitors and chemotherapy.21-23 Other important mechanisms involved in the survival signaling of the bone marrow microenvironment niche are the VLA-4 integrins and CD44, which are expressed on AML cells that interact with stromal fibronectin and hyaluronan, respectively.1,2,5 Because CXCR4 has been shown to cooperate with VLA-4 during AML cell adhesion and migration, lowering of CXCR4 and disrupting its signaling may also undermine the VLA-4-fibronectin–mediated survival mechanism of the bone marrow microenvironment.5 CXCR4 antagonists, such as AMD3100 and FC-131, or anti-CXCR4 antibody, have all been shown to disrupt CXCL12-CXCR4 signaling and overcome resistance to chemotherapy and other antileukemia agents.21-23 Here we demonstrate that cotreatment with PS significantly enhanced apoptosis of cultured AML cells induced by the anti-CXCR4 antibody MAB-172. In addition, cotreatment with PS synergistically enhanced apoptosis induced by AMD3100 and FC-131 in cultured AML cells. This was associated with greater decline in p-AKT levels and AKT kinase activity resulting from the combination, compared with treatment with each agent alone. These molecular perturbations demonstrate that combined treatment with a CXCR4 antagonist and a pan-HDAC inhibitor could attenuate AML cell homing and migration stimulated by CXCL12. In addition, importantly, the in vitro cotreatment with PS and FC-131 was significantly more lethal against primary AML cells, compared with CD34+ normal BMPCs. These findings underscore the relative anti-AML selectivity of the combination of PS and a CXCR4 antagonist. Recent studies have demonstrated that a relatively longer exposure interval to the CXCR4 antagonist AMD3100 or anti-CXCR4 antibody, than those used in this study, arrested proliferation and induced features of differentiation in AML cells.16 It is also noteworthy that treatment with pan-HDAC inhibitors has also been noted to induce differentiation in AML cells.30 Therefore, it is possible that, when administered in the appropriate schedule, the combined treatment with PS and CXCR4 antagonist may also induce superior differentiation of AML cells, which may contribute to the overall anti-AML activity of the combination.
Recently, the CXCR4 antagonist plerixaflor (AMD3100) was approved for hematopoietic stem cell mobilization in combination with granulocyte colony-stimulating factor for stem cell transplantation.49 Because plerixaflor has been shown to chemosensitize AML cells after mobilization, it is now being evaluated clinically in the treatment of AML. PS is also currently in phase I/II trials in hematologic malignancies and has been demonstrated to be safe and active against AML, non-Hodgkin lymphoma, and Hodgkin disease.50 Although not studied here in vivo, the preclinical findings that plerixaflor and PS are synergistically active against AML cells highlight the strong rationale for future studies of the combination in the therapy of AML and AML mouse models. Because the CXCR4 antagonist also exerts antitumor effects against epithelial cancers, findings presented here support the rationale for testing the combination of PS with the CXCR4 antagonist against epithelial cancers, including breast, ovarian, and lung cancers.2,50-53
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.M., W.F., K.M.B., K.R., R.R., R.B., J.-M.N., Z.-X.W., and D.G.C. performed the in vitro studies with the cultured and primary AML cells; C.U. procured and assisted in performing the studies on primary AML cells; P.A., N.F., and S.C.P. provided important reagents for the in vitro studies in cultured and primary AML cells; and K.B. planned and supervised the in vitro studies and prepared the report.
Conflict-of-interest disclosure: P.A. is an employee of Novartis Institute for Biomedical Research Inc. K.B. has received clinical and laboratory research grants from Novartis Institute for Biomedical Research Inc. The remaining authors declare no competing financial interests.
Correspondence: Kapil Bhalla, The University of Kansas Cancer Center, Kansas University Medical Center, 3901 Rainbow Blvd, 4030 Robinson, Mail Stop 1027, Kansas City, KS 66160; e-mail: kbhalla@kumc.edu.
References
Author notes
A.M. and W.F. contributed equally to this study and should be considered co–first authors.

![Figure 5. Treatment with AMD3100, FC-131, or a CXCR4 blocking antibody significantly increases PS-mediated lethality of AML cells. (A) Analysis of the dose-effect relationship for PS (5-50nM) and AMD3100 (2-10μM) or FC-131 (0.5-4μM) for the apoptotic effects after 48 hours of exposure in OCI-AML3 cells was performed according to the median dose effect method of Chou and Talalay. After this, the CI values were calculated using the percentage of apoptotic cells (fraction affected [FA]) by the 2 agents together. CI < 1, CI = 1, and CI > 1 represent synergism, additivity, and antagonism of the 2 agents, respectively. (B) Primary AML (n = 8) and CD34+ peripheral blood mononuclear cells from healthy donors were treated with the indicated concentrations of FC-131 and/or PS for 48 hours. Then, the percentages of nonviable cells were determined by trypan blue dye uptake in a hemocytometer. (C) OCI-AML3 cells were treated with the indicated concentrations of anti-CXCR4 MAB-172 antibody and/or PS for 48 hours. Then, the percentages of apoptotic cells were determined by annexin V and propidium iodide staining and flow cytometry. Columns represent the mean of 3 experiments. Bars represent the SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/24/10.1182_blood-2010-05-284414/4/m_zh89991061570005.jpeg?Expires=1763508494&Signature=ARF2EpJ9DkHgkDZ7RrVcLXpWCSmFwn2sMow1thlOidQSxYO-B~wq3Us6mZHN2bq3n9jDrxqM4Rl7KsBfVzTcxhfWAcVHIZVmjJb0yN4n8z9ZzRGyaO4wupYd0uK0gSBtHJ9G~u5FdZIgR9xXUA-q48qmFs8tgidRCONWy2Uh17AzkVYM2DqkXPA6UKeEP4rekS7iEajONOJxI2RHXps2JHMsza5UxfctZFStiei7xtu6hsqbqmF6KaJQXiL5H4RPyyT1oTyuGNFXscaOK0IpS-gmetXsi6snQr7MAgXf8n8tmsn7Dp3ImtNlTjv9PWK8QfrG8a3m~np4yKmbSKZlIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)