Abstract
The generation of reactive oxygen species (ROS) by the nicotinamide adenine dinucleotide phosphate oxidase is an important mechanism by which neutrophils kill pathogens. The oxidase is composed of a membrane-bound cytochrome and 4 soluble proteins (p67phox, p40phox, p47phox, and GTP-Rac). These components form an active complex at the correct time and subcellular location through a series of incompletely understood mutual interactions, regulated, in part, by GTP/GDP exchange on Rac, protein phosphorylation, and binding to lipid messengers. We have used a variety of assays to follow the spatiotemporal assembly of the oxidase in genetically engineered primary mouse neutrophils, during phagocytosis of both serum- and immunoglobulin G-opsonized targets. The oxidase assembles directly on serum-Staphylococcus aureus–containing phagosomes within seconds of phagosome formation; this process is only partially dependent (∼ 30%) on PtdIns3P binding to p40phox, but totally dependent on Rac1/2 binding to p67phox. In contrast, in response to immunoglobulin G-targets, the oxidase first assembles on a tubulovesicular compartment that develops at sites of granule fusion to the base of the emerging phagosome; oxidase assembly and activation is highly dependent on both PtdIns3P-p40phox and Rac2-p67phox interactions and delivery to the phagosome is regulated by Rab27a. These results define a novel pathway for oxidase assembly downstream of FcR-activation.
Introduction
Neutrophils are a critical component of our innate immune system, primarily responsible for combating bacterial and fungal infections.1 One of the key weapons used by neutrophils to kill pathogens is the formation of reactive oxygen species (ROS) by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.2 The active neutrophil NADPH oxidase complex is composed of at least 6 proteins: gp91phox, p22phox, p40phox, p67phox, p47phox, and Rac1/2.3,4 The genes encoding these proteins can be mutated in an immune deficiency disorder called chronic granulomatous disorder.5 gp91phox, and p22phox form a tightly bound, intrinsic membrane protein called cytochrome b558, which is predominantly found in neutrophil granules and the plasma membrane.6 In resting neutrophils, p40phox, p67phox, and p47phox are cytosolic and GDP-Rac1/2 is partitioned between a cytosolic complex with RhoGDI and neutrophil membranes.7-9 Activation of neutrophil cell-surface receptors triggers a signaling network that coordinates assembly of soluble phox proteins around cytochrome b558 and GTP-Rac1/2 in a specified membrane location, to form an active enzyme complex.3,4,10,11 This active complex catalyses the electrogenic transfer of electrons from NADPH on one side of the membrane, to molecular oxygen on the other side, to generate the superoxide anion, which is then converted to other ROS depending on the location and context of activation.
A substantial body of work has begun to define important components of the regulatory network governing oxidase assembly and activation but several features are still unclear. Most work indicates that the core contacts that stimulate NADPH oxidase activity are between cytochrome b558, p67phox, and GTP-Rac1/2.4,12,13 The delivery of GTP-Rac1/2 to sites of oxidase assembly is catalyzed by guanine nucleotide exchange factors (GEFs), the precise family member being dependent on the type of receptor involved.14 The delivery of p67phox is thought to be driven by the combined actions of GTP-Rac1/2, p40phox and p47phox.4 Recombinant p40phox and p67phox bind tightly to one another, and these 2 proteins co-exist as a complex in neutrophil cytosol.9,15 The phospholipid PtdIns3P is generated in phagosomal membranes16,17 and binds with high specificity and affinity to the PX domain of p40phox,15,18,19 and this interaction is important for p67phox activity on both mouse and human phagosomes.20,21 p40phox/p67phox also readily binds recombinant p47phox to form a heterotrimer,4 but the extent to which p47phox exists in a soluble complex with p40phox/p67phox in unstimulated neutrophils, or the extent to which this trimer only forms on stimulation is debated.4,9,15 It is generally agreed, however, that protein kinase C-mediated phosphorylation of p47phox C terminus relieves an autoinhibitory constraint that allows binding of p47phox to p22phox and that, through promoting association of p67phox with cytochrome b558, this is an important trigger in oxidase assembly.4 Several further phosphorylation events on phox proteins have also been identified, but their precise roles in the activation process are still uncertain.11
Many different receptors and signal transduction systems activate the neutrophil NADPH oxidase, but there are still substantial gaps in our knowledge of how they connect to the GEF, protein kinase, and lipid messenger generating pathways coordinating oxidase activation described in the previous two paragraphs. Such receptors include protein tyrosine kinase-coupled receptors for opsonins, which support phagocytosis of particulate stimuli (eg, β2-integrin receptors for the complement fragment iC3b or low affinity Fc-receptors for immunoglobulin G (IgG) [FcγRs]).22 In this context, the oxidase is generally perceived to deliver ROS into the phagosome lumen, where it participates in microbe destruction. However, β2-integrins and FcγRs can also stimulate extracellular ROS formation in response to neutrophil adhesion and spreading on extracellular matrix23 or immobilized immune complexes,24 respectively. Extracellular ROS formation is also stimulated by Gi-coupled receptors for chemoattractants (eg, bacterial peptide fMLP).25 Further, in most physiological settings, many of these different receptors operate in the context of costimulation by each other and other receptors for chemokines, cytokines, inflammatory lipids, and complex pathogen ligands that are also found at the inflammatory site. In particular, several cytokines are poor activators of the oxidase in isolation but can dramatically potentiate activation by other ligands (eg, granulocyte macrophage colony-stimulating factor or tumor necrosis factorα).26
Among the major obstacles to achieving a more detailed understanding of the pathways linking oxidase activation to different receptor systems in neutrophils are their short lifespan in vitro and refractory nature to standard protein expression protocols. We have tried to overcome some of these obstacles using viral transduction of mouse bone marrow progenitors and repopulation of the hematopoietic cell compartment in radiation chimeras to express several different fluorescent protein reporters of oxidase assembly in fully differentiated neutrophils. We have then used live and fixed cell fluorescence imaging in the context of different genetically engineered backgrounds to investigate the mechanism and spatiotemporal kinetics of oxidase assembly in response to several different agonists, with a particular focus on the role of Rac1/2 and PtdIns3P in directing the localization and function of p40phox/p67phox.
Methods
Mouse strains
p40phox−/−,27 p40phoxR58A/R58A,28 Rab27a−/− mice,29 and mice permitting conditional Rac1 knockdown on a wild-type (WT) and Rac2−/− background,30 were described previously. Full details can be found in the supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Neutrophil isolation and measurement of ROS production
Human and mouse neutrophils were isolated as described previously.26 Nitroblue tetrazolium (NBT) assays were essentially as previously described,31 with full details described in supplemental Methods. All peripheral blood was drawn from healthy volunteers with written informed consent in accordance with the Declaration of Helsinki and Cambridge Research Ethics Committee approval.
For chemiluminescence ROS assays, neutrophils were preincubated with wortmannin or dimethyl sulfoxide vehicle (0.1%) for 10 minutes before stimulation. Rate kinetics of intracellular and extracellular32 and total ROS28 production were measured using a luminol-based assay in 96-well plates (Berthold Technologies) essentially as described previously.28,32 Briefly, 5 × 105 cells were incubated with either: luminol (150μM)/superoxide dismutase (SOD; 375 U/mL; intracellular); isoluminol (150μM)/horseradish peroxidase (HRP; 18.75 U/mL; extracellular) or luminol/HRP (total) for 10 minutes at 37°C. Cells were then added manually to immobilized immune complexes or particles (final ratio 1:20 Staphylococcus aureus, 1:5 zymosan, 1:4 beads, or 20 μL of IgG-opsonized–sheep red blood cells [SRBCs]) prepared as described in the supplemental Methods, and measurement started immediately. Light emission was recorded by a Berthold Mircolumat Plus luminomter (Berthold Technologies). Data output is relative light units per second (RLU/s) or total RLU integrated over 20 minutes.
Phagocytosis assays
Where indicated, neutrophils were incubated with 80μM dynasore (for 10 minutes at 37°C) before addition of phagocytic stimuli. Neutrophils (5 × 104) were incubated in suspension at 37°C with either 1 × 106 serum-opsonized S aureus (for 7 minutes), 2.5 × 105 zymosan (for 15 minutes), 8 μL of IgG-SRBCs (for 12 minutes), or 5 × 104 beads (for 20 minutes), prepared as described in the supplemental Methods. For S aureus assays, samples were cytospun onto glass coverslips. For all other conditions, samples were allowed to adhere onto glass coverslips for 3 minutes at 37°C, with nonphagocytosed SRBCs hypotonically lysed beforehand. Cells were fixed in 4% paraformaldehyde and processed as previously described.33 Fixed cells were stained with antibodies against p67phox (Millipore); and CD14, EEA1, neutrophil elastase, or lactoferrin (Santa Cruz Biotechnology), as indicated for 1 hour at room temperature, followed by appropriate AlexaFluor488/568 secondary antibodies (Invitrogen). Coverslips were mounted on glass microslides with Aqua-Polymount antifading solution (Poly-Science), and fluorescence visualized by a Zeiss LSM510 META point-scanning confocal microscope with integrated camera, using a Plan/Apochromat 63×/1.4 oil objective. Cytosolic p67phox levels and phagosomal accumulation was quantitated using LSM 510 Image browser software Version 4.2.
Heterologous protein expression in primary neutrophils
Fluorescent fusion protein reporters were expressed in fully differentiated mouse neutrophils using viral transduction of mouse bone marrow progenitors and repopulation of the hematopoietic cell compartment in irradiated recipient mice. Fetal liver (FL) cells were harvested from E14.5-day-old embryos, cultured, and transduced in the presence of cell-specific cytokine cocktails as described in the supplemental Methods. Seventeen hours after transduction, cells were washed in Hanks balanced salt solution, resuspended in phosphate-buffered saline/10% fetal bovine serum (FBS) and 1-2 × 106 FL cells injected (300 μL) into 8-week-old recipient mice, which had been lethally irradiated (using 2 doses of 5 Gy from a 137Cs source, separated by 3 hours) the day before injection. A small proportion of cells were kept in culture for flow cytometric analysis of green fluorescent protein (GFP) expression 72 hours after transduction.
Live imaging
Neutrophils were isolated and primed (or left unprimed) as described and kept on ice until use. Neutrophils and IgG-SRBCs (∼ 1:20 ratio) were briefly centrifuged (for 1 minute at 200 × g at 4°C), gently mixed, and immediately added to imaging chambers containing prewarmed phosphate-buffered saline ++ on FBS-blocked coverslips. Serum-opsonized S aureus were added at a similar ratio; however, neutrophils were added to either FBS-blocked or noncoated borosilicate glass coverslips in imaging chambers in a volume of 1.5 mL, allowed to settle for 5 minutes at 37°C and S aureus added in a volume of 500 μL. Detailed live imaging protocols can be found in the supplemental Methods. For widefield imaging, GFP- and differential interference contrast (DIC)–images were recorded at an exposure time of 750 milliseconds, and 4-sesond intervals. The length of each video was 15 minutes. Images were recorded using an Olympus CellR epifluorescence microscope equipped with an Olympus 1X2-UCB camera, using a Plan/Apochromat 60×/1.4 oil objective. Quantification of mean fluorescence intensity (MFI) around the cup and/or phagosome was performed using ImageJ software Version 1.38x, as described in the supplemental Methods.
For 3-dimensional (3D) live imaging, a series of z-stacks were recorded at 0.5 μm intervals. To encompass the whole cell, a set of 20-22 z-stacks was acquired for each time frame. Typically, exposure was 50-100 milliseconds depending on intensity of intracellular fluorescence. The time-interval between each frame was minimum 2.78 seconds. Images were acquired on a spinning Nipkow disk Nikon-Eclipse confocal microscope equipped with an ultrasensitive EM-CCD camera (Andor iXon-897) allowing very fast image aquisition, using a Plan/Apo 100× oil objective (NA1.4). 3D reconstruction was performed using Volocity software Version 5.4.1. AVI videos of 3D-reconstructed, unprocessed z-stacks were created using Volocity and ImageJ software.
Results
Formazan-deposition assays reveal extra-phagosomal sites of oxidase activity in response to IgG-opsonized targets
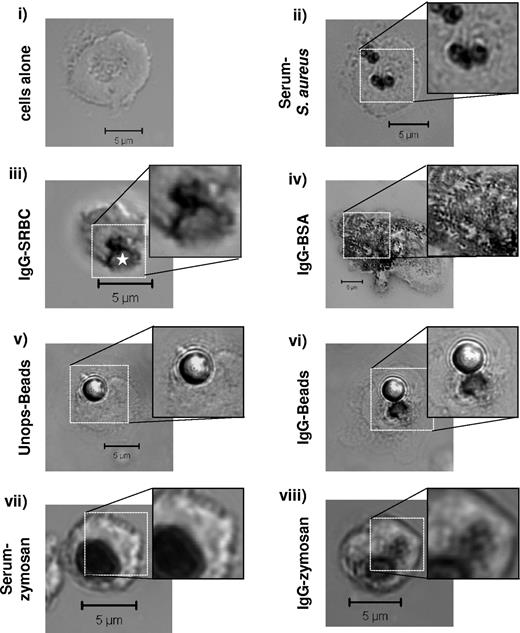
We first visualized subcellular sites of oxidase activity in primary mouse neutrophils using an assay based on O2−-mediated conversion of NBT to insoluble formazan salts. Phagocytosis of serum-opsonized S aureus induced substantial formazan deposition in both cytokine-primed (Figure 1ii) and unprimed (supplemental Figure 1) cells. These formazan deposits were always tightly localized to S aureus-containing phagosomes with no significant deposition seen in other subcellular locations. In contrast, phagocytosis of IgG-SRBCs elicited formazan deposition in both the phagosome itself and in adjacent puncta (primed cells are shown in Figure 1iii; no significant oxidase responses were seen in response to IgG-targets in unprimed cells; supplemental Figure 1). A similar pattern of formazan deposition was seen in response to phagocytosis of IgG-latex beads but not unopsonized beads (Figure 1v-vi). Further, extra-phagosomal formazan deposits were seen during phagocytosis of IgG-zymosan but not serum-opsonized–zymosan, despite similar overall levels of oxidase activation (Figure 1vii-viii). A large number of small, formazan deposits were also induced in response to immobilized IgG-bovine serum albumin (BSA) immune complexes (ie, in the absence of phagocytosis; Figure 1iv).
IgG-dependent extra-phagosomal ROS production. Primed WT mouse bone marrow–derived neutrophils (BMNs) were preincubated with NBT, left untreated (i), or incubated with serum-opsonized S aureus (ii); IgG-opsonized SRBCs (iii); immobilized immune complex IgG-BSA (iv); unopsonized (v) or IgG-opsonized (vi) latex beads; serum-opsonized (vii) or IgG-opsonized (viii) zymosan, as described in “Phagocytosis assays,” and supplemental Methods. Non-phagocytosed S aureus or SRBCs were lysed before processing. Samples were cytospun (i-ii) or allowed to adhere (iii-viii) onto glass coverslips, fixed in 4% paraformaldehyde, washed, and mounted as described in “Phagocytosis assays.” Dark formazan deposition was detected by DIC imaging on a Zeiss LSM 510 META point-scanning confocal microscope. Shown are representative DIC images, including an enlarged section of formazan deposition under each condition. The IgG-SRBC phagosome is indicated by a white star. Scale bars represent 5 μm.
IgG-dependent extra-phagosomal ROS production. Primed WT mouse bone marrow–derived neutrophils (BMNs) were preincubated with NBT, left untreated (i), or incubated with serum-opsonized S aureus (ii); IgG-opsonized SRBCs (iii); immobilized immune complex IgG-BSA (iv); unopsonized (v) or IgG-opsonized (vi) latex beads; serum-opsonized (vii) or IgG-opsonized (viii) zymosan, as described in “Phagocytosis assays,” and supplemental Methods. Non-phagocytosed S aureus or SRBCs were lysed before processing. Samples were cytospun (i-ii) or allowed to adhere (iii-viii) onto glass coverslips, fixed in 4% paraformaldehyde, washed, and mounted as described in “Phagocytosis assays.” Dark formazan deposition was detected by DIC imaging on a Zeiss LSM 510 META point-scanning confocal microscope. Shown are representative DIC images, including an enlarged section of formazan deposition under each condition. The IgG-SRBC phagosome is indicated by a white star. Scale bars represent 5 μm.
ROS responses to serum-S aureus are mediated by β2-integrin recognition of complement fragments,28 those to non-IgG–opsonized zymosan are dependent on the Dectin-1 receptor34 and those to IgG-beads, IgG-SRBCs, and IgG-BSA are totally dependent on the Fcγ-chain (reference35 and data not shown; DNS). Therefore, these results suggest the oxidase can assemble on intracellular, non-phagosomal membranes specifically in response to IgG-FcγR stimulation.
Endogenous or GFP-tagged oxidase subunits accumulate on a pre-phagosomal membrane compartment during phagocytosis of IgG-targets
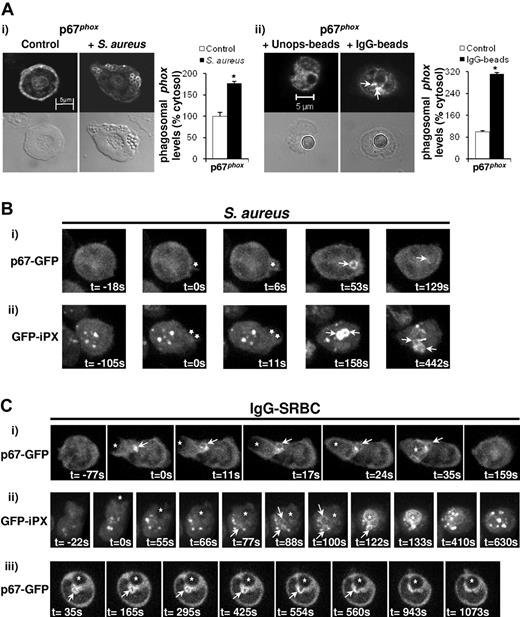
We initially visualized membrane accumulation of endogenous oxidase subunits using immunofluorescence in fixed mouse neutrophils. In response to phagocytosis of S aureus, p67phox (Figure 2Ai), p47phox (DNS), and p40phox (DNS) clearly accumulated around the perimeter of phagosomes. These accumulations were typically heterogeneous but no extra-phagosomal concentrations were observed. In response to phagocytosis of IgG-beads, endogenous p67phox accumulated on the phagosome itself and also on membrane structures in close juxtaposition to the phagosome, and these were similar in size, shape, and position to the equivalent formazan depostions described above (Figure 2Aii).
Endogenous and heterologously expressed p67phox or iPX are targeted to pre-phagosomal structures during phagocytosis of IgG-coated particles. (A) Primed WT neutrophils (5 × 104) were incubated without or with 1 × 106 serum-opsonized S aureus for 7 minutes at 37°C (i), or with 5 × 104 unopsonized or IgG-opsonized beads for 20 minutes at 37°C (ii), as described in “Phagocytosis assays.” Samples were cytospun (i) or allowed to adhere (ii) onto glass slides, fixed, and stained for p67phox as described in “Phagocytosis assays.” Mounted samples were visualized on a Zeiss LSM 510 META point-scanning microscope using fluorescence and DIC optics. Shown are representative fluorescence and DIC images for conditions tested. Phagosomal (i) and extra-phagosomal (ii; indicated by arrows) accumulation of p67phox was quantified for at least 50 events under each condition using LSM 510 Image browser software and expressed as mean ± SEM as a percentage of cytosolic levels, *P < .0001, Student t test. (B-C) BMNs expressing p67phox-GFP (Bi,Ci,Ciii) or GFP-iPX (Bii,Cii) in a WT genetic background were primed at a concentration of 2 × 107/mL, placed on ice and gently mixed at regular intervals. Aliquots of 5 × 105-1 × 106 cells were settled onto glass coverslips in imaging chambers that were previously blocked with 100% FBS. Cells were incubated with serum-opsonized S aureus (1-5:1 S.aureus:neutrophil; B), or IgG-SRBCs (20:1 IgG-SRBCs:neutrophils; C). z-stacks of neutrophils undergoing phagocytosis were captured over time on a Nikon-Eclipse spinning disk confocal microscope equipped with an ultrasensitive EM-CCD camera, as described in “Live imaging.” A total of 20-22 slices were captured for each time frame, at a distance of 0.5 μm between each slice, encompassing the whole neutrophil. The exposure time was 100 milliseconds, and the time interval between each frame was 5.896 seconds (p67phox-GFP), 10.52 seconds (GFP-iPX; Bii), or 11.046 seconds (GFP-iPX; Cii). 3D reconstruction was performed using Volocity software, and reconstructions at individual time frames representing different stages of phagocytosis are shown. The time of attachment of particle to neutrophil and/or the time of formation of the phagocytic cup was defined as t = 0. Stars indicated the position of particles during various stages of phagocytosis; white arrows indicate the position of bacterial phagosomes containing p67phox-GFP or GFP-iPX (B) or pre-phagosomal accumulation of p67phox-GFP or GFP-iPX (C). Imaging experiments were performed with neutrophils from individual mice, on at least 2 different days. (Ciii) Series of confocal images over time, showing fusion of a pre-phagosomal structure containing p67phox-GFP with an IgG-SRBC phagosome.
Endogenous and heterologously expressed p67phox or iPX are targeted to pre-phagosomal structures during phagocytosis of IgG-coated particles. (A) Primed WT neutrophils (5 × 104) were incubated without or with 1 × 106 serum-opsonized S aureus for 7 minutes at 37°C (i), or with 5 × 104 unopsonized or IgG-opsonized beads for 20 minutes at 37°C (ii), as described in “Phagocytosis assays.” Samples were cytospun (i) or allowed to adhere (ii) onto glass slides, fixed, and stained for p67phox as described in “Phagocytosis assays.” Mounted samples were visualized on a Zeiss LSM 510 META point-scanning microscope using fluorescence and DIC optics. Shown are representative fluorescence and DIC images for conditions tested. Phagosomal (i) and extra-phagosomal (ii; indicated by arrows) accumulation of p67phox was quantified for at least 50 events under each condition using LSM 510 Image browser software and expressed as mean ± SEM as a percentage of cytosolic levels, *P < .0001, Student t test. (B-C) BMNs expressing p67phox-GFP (Bi,Ci,Ciii) or GFP-iPX (Bii,Cii) in a WT genetic background were primed at a concentration of 2 × 107/mL, placed on ice and gently mixed at regular intervals. Aliquots of 5 × 105-1 × 106 cells were settled onto glass coverslips in imaging chambers that were previously blocked with 100% FBS. Cells were incubated with serum-opsonized S aureus (1-5:1 S.aureus:neutrophil; B), or IgG-SRBCs (20:1 IgG-SRBCs:neutrophils; C). z-stacks of neutrophils undergoing phagocytosis were captured over time on a Nikon-Eclipse spinning disk confocal microscope equipped with an ultrasensitive EM-CCD camera, as described in “Live imaging.” A total of 20-22 slices were captured for each time frame, at a distance of 0.5 μm between each slice, encompassing the whole neutrophil. The exposure time was 100 milliseconds, and the time interval between each frame was 5.896 seconds (p67phox-GFP), 10.52 seconds (GFP-iPX; Bii), or 11.046 seconds (GFP-iPX; Cii). 3D reconstruction was performed using Volocity software, and reconstructions at individual time frames representing different stages of phagocytosis are shown. The time of attachment of particle to neutrophil and/or the time of formation of the phagocytic cup was defined as t = 0. Stars indicated the position of particles during various stages of phagocytosis; white arrows indicate the position of bacterial phagosomes containing p67phox-GFP or GFP-iPX (B) or pre-phagosomal accumulation of p67phox-GFP or GFP-iPX (C). Imaging experiments were performed with neutrophils from individual mice, on at least 2 different days. (Ciii) Series of confocal images over time, showing fusion of a pre-phagosomal structure containing p67phox-GFP with an IgG-SRBC phagosome.
To measure assembly kinetics of oxidase subunits in live cells, we generated mouse bone marrow chimeras heterologously expressing GFP-tagged versions of p40phox (GFP-p40phox) in a p40phox−/− background, or p67phox (p67phox-GFP) in a WT background (no p67phox−/− mice are available; supplemental Figure 2). An N-terminal GFP-tag for p40phox and a C-terminal GFP-tag for p67phox were chosen on the basis that these constructs support significant oxidase activity in p40phox-deficient neutrophils (DNS) and p67phox-deficient K562 cells.36 GFP-p40phox was expressed at levels equivalent to endogenous p40phox in WT cells, and p67phox-GFP was expressed at levels similar to endogenous p67phox (supplemental Figure 2). Neutrophils were isolated from these mice, and movements of GFP-tagged proteins during phagocytosis of serum-S aureus and IgG-SRBCs analyzed by wide-field and confocal, fluorescence imaging. Examples of 3D video sequences assembled using a high speed, z-stacking confocal microscope describing the membrane accumulation of p67phox-GFP during phagocytosis of serum-S aureus or IgG-SRBCs are shown in supplemental Videos 1 and 2, respectively, with selected images from these videos shown in Figure 2B and C. Examples of wide-field imaging of p67phox-GFP during phagocytosis of serum-S aureus or IgG-SRBCs are also shown in Figures 3 and 4, respectively; examples of GFP-p40phox images are shown in supplemental Figure 3.
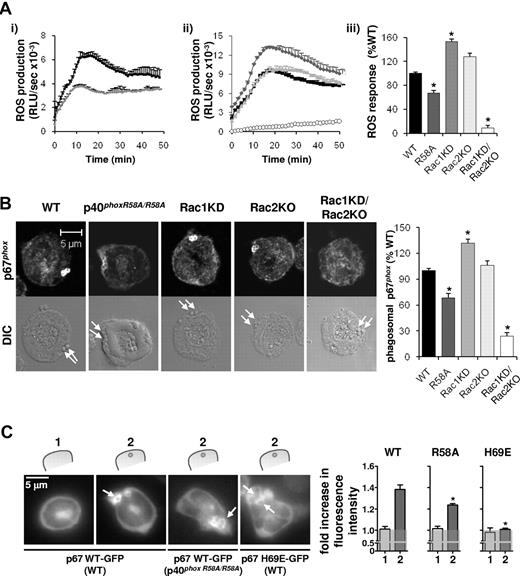
Assembly and activation of the NADPH oxidase in response to serum-opsonized S aureus is partially dependent on binding of p40phox to PtdIns3P, but fully dependent on Rac. (A) Primed neutrophils (1 × 106) isolated from WT (black squares/bars) or p40phoxR58A/R58A (R58A, dark gray diamonds/bars) genotypes (i,iii); or from WT (black squares/bars), Rac1 knockdown on a WT background (Rac1KD, gray diamonds/bars), Rac2−/− (Rac2KO, light gray triangle/bar), and Rac1 knockdown on a Rac2 KO background (Rac1KD/Rac2KO, open circles/bar; ii-iii) were preincubated with luminol/HRP, before addition to serum-S aureus as described in “Measurement of ROS production.” Total ROS responses were measured by chemiluminesence and recorded on a 96-well plate using a Berthold Microlumat Plus luminometer as described in “Measurement of ROS production.” All incubations were performed in at least duplicate. Shown are data (mean ± range) from 1 experiment representative of at least 3, expressed as RLU/s (i-ii), as well as accumulated light emission (ROS response) over 20 minutes for a combination of ≥ 3 experiments (mean ± SEM), expressed as a percentage of the response in WT mouse neutrophils (iii). *P < .0001, paired Student's t test (WT/R58A), 1-way analysis of variance (ANOVA) (WT, Rac1KD, Rac2KO, Rac1KD/Rac2KO), compared with WT response. (B) Primed BMNs from the indicated genetic backgrounds, after phagocytosis of serum-S aureus were fixed and stained and imaged for p67phox as described in Figure 2A. Phagosomal p67phox fluorescence was quantified as described in panel A and is expressed (mean ± SEM) as a percentage of WT levels, *P < .0001, 1-way ANOVA. Arrows indicate the position of S aureus bacteria in the DIC images. (C) For quantification of cytosolic (1) and phagosomal (2) fluorescence, mouse BMNs expressing p67phox WT-GFP on a WT or a p40phoxR58A/R58A genetic background or expressing p67phox H69E-GFP on a WT genetic background were primed and incubated with S aureus as described in Figure 2B. Images were recorded on an Olympus CellR epifluorescence microscope equipped with an Olympus 1 × 2-UCB camera and using a 60× oil objective, as further described in “Live imaging.” Images were recorded for 15 minutes in the GFP channel and DIC channel (not shown) at 4-second intervals and at an exposure time of 750 milliseconds. Representative images are shown. Fluorescence around the phagosomes was quantified using ImageJ software, as described in “Live imaging” and supplemental Figure 9. Data are presented as a ratio of MFI of phagosome:cytosol. The gray transparent area represents a ratio of phagosome:cytosol of 1.0 or lower, representing no phagosomal translocation. Data are mean ± SEM (n ≥ 19 phagosomes in at least 5 different neutrophils per construct/genetic background). *P < .0001 as determined by Student t test between WT and R58A, and between WT and H69E.
Assembly and activation of the NADPH oxidase in response to serum-opsonized S aureus is partially dependent on binding of p40phox to PtdIns3P, but fully dependent on Rac. (A) Primed neutrophils (1 × 106) isolated from WT (black squares/bars) or p40phoxR58A/R58A (R58A, dark gray diamonds/bars) genotypes (i,iii); or from WT (black squares/bars), Rac1 knockdown on a WT background (Rac1KD, gray diamonds/bars), Rac2−/− (Rac2KO, light gray triangle/bar), and Rac1 knockdown on a Rac2 KO background (Rac1KD/Rac2KO, open circles/bar; ii-iii) were preincubated with luminol/HRP, before addition to serum-S aureus as described in “Measurement of ROS production.” Total ROS responses were measured by chemiluminesence and recorded on a 96-well plate using a Berthold Microlumat Plus luminometer as described in “Measurement of ROS production.” All incubations were performed in at least duplicate. Shown are data (mean ± range) from 1 experiment representative of at least 3, expressed as RLU/s (i-ii), as well as accumulated light emission (ROS response) over 20 minutes for a combination of ≥ 3 experiments (mean ± SEM), expressed as a percentage of the response in WT mouse neutrophils (iii). *P < .0001, paired Student's t test (WT/R58A), 1-way analysis of variance (ANOVA) (WT, Rac1KD, Rac2KO, Rac1KD/Rac2KO), compared with WT response. (B) Primed BMNs from the indicated genetic backgrounds, after phagocytosis of serum-S aureus were fixed and stained and imaged for p67phox as described in Figure 2A. Phagosomal p67phox fluorescence was quantified as described in panel A and is expressed (mean ± SEM) as a percentage of WT levels, *P < .0001, 1-way ANOVA. Arrows indicate the position of S aureus bacteria in the DIC images. (C) For quantification of cytosolic (1) and phagosomal (2) fluorescence, mouse BMNs expressing p67phox WT-GFP on a WT or a p40phoxR58A/R58A genetic background or expressing p67phox H69E-GFP on a WT genetic background were primed and incubated with S aureus as described in Figure 2B. Images were recorded on an Olympus CellR epifluorescence microscope equipped with an Olympus 1 × 2-UCB camera and using a 60× oil objective, as further described in “Live imaging.” Images were recorded for 15 minutes in the GFP channel and DIC channel (not shown) at 4-second intervals and at an exposure time of 750 milliseconds. Representative images are shown. Fluorescence around the phagosomes was quantified using ImageJ software, as described in “Live imaging” and supplemental Figure 9. Data are presented as a ratio of MFI of phagosome:cytosol. The gray transparent area represents a ratio of phagosome:cytosol of 1.0 or lower, representing no phagosomal translocation. Data are mean ± SEM (n ≥ 19 phagosomes in at least 5 different neutrophils per construct/genetic background). *P < .0001 as determined by Student t test between WT and R58A, and between WT and H69E.
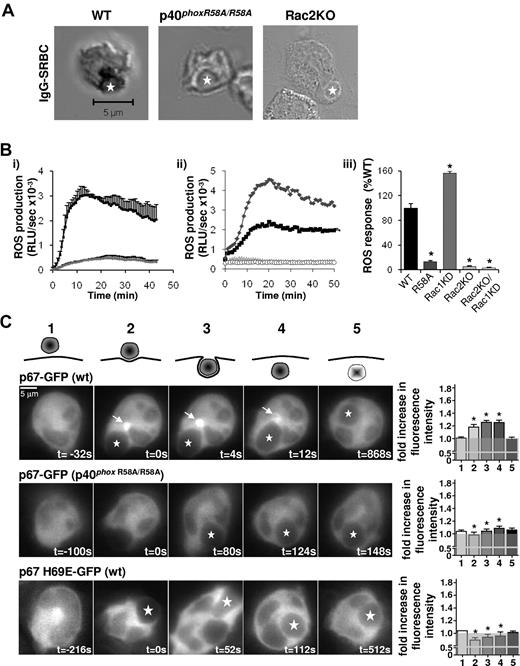
Assembly and activation of the NADPH oxidase in response to IgG-SRBCs is fully dependent on binding of p40phox to PtdIns3P and on Rac2. (A) BMNs from WT, p40phoxR58A/R58A, or Rac2KO genetic backgrounds were preincubated with NBT before incubation with IgG-SRBCs and processed as described in Figure 1, with the exception of Rac2KO samples, which were cytospun onto coverslips. Shown are representative images, with IgG-SRBC phagosomes indicated by stars. (B) Primed BMNs from WT (black squares/bar) or p40phoxR58A/R58A genotypes (dark gray diamonds/bar; i,iii); or from WT (black squares/bar), Rac1KD (gray diamonds/bars), Rac2KO (light gray triangles/bars), and Rac1KD/Rac2KO (open circles/bars) genotypes (ii-iii), were subjected to luminol-dependent chemiluminescence assays for total ROS generation as described in Figure 3A. Shown are data (mean ± range) from 1 experiment representative of at least 3, expressed as RLU/s (i-ii), as well as accumulated light emission (ROS response) over 20 minutes for a combination of ≥ 3 experiments (mean ± SEM), expressed as a percentage of the response in WT mouse neutrophils (iii). *P < .0001, paired Student t test (WT/R58A), 1-way ANOVA (WT, Rac1KD, Rac2KO, Rac1KD/Rac2KO) compared with WT response. (C) For quantification of cytosolic fluorescence (1), fluorescence at sites of attachment of IgG-SRBCs to neutrophil (2), around the phagocytic cup (3), the phagosome (4), and after phagocytosis (5), widefield epifluorescence microscopy was used. Mouse BMNs expressing p67phox WT-GFP on a WT or a p40phoxR58A/R58A genetic background or expressing p67phox H69E-GFP on a WT genetic background were primed and incubated with IgG-SRBCs as described in Figure 2C. Images were recorded and presented as described in Figure 3C. Quantification was performed using ImageJ software, as described in “Live imaging” and supplemental Figure 9. Data are presented as a ratio of MFI of phagosome:cytosol. The gray transparent area represents a ratio of phagosome:cytosol of 1.0 or lower, representing no phagosomal translocation. Data are mean ± SEM (n = 4-17 neutrophils). *P ≤ .01 as determined by Student t test between WT and R58A, and between WT and H69E.
Assembly and activation of the NADPH oxidase in response to IgG-SRBCs is fully dependent on binding of p40phox to PtdIns3P and on Rac2. (A) BMNs from WT, p40phoxR58A/R58A, or Rac2KO genetic backgrounds were preincubated with NBT before incubation with IgG-SRBCs and processed as described in Figure 1, with the exception of Rac2KO samples, which were cytospun onto coverslips. Shown are representative images, with IgG-SRBC phagosomes indicated by stars. (B) Primed BMNs from WT (black squares/bar) or p40phoxR58A/R58A genotypes (dark gray diamonds/bar; i,iii); or from WT (black squares/bar), Rac1KD (gray diamonds/bars), Rac2KO (light gray triangles/bars), and Rac1KD/Rac2KO (open circles/bars) genotypes (ii-iii), were subjected to luminol-dependent chemiluminescence assays for total ROS generation as described in Figure 3A. Shown are data (mean ± range) from 1 experiment representative of at least 3, expressed as RLU/s (i-ii), as well as accumulated light emission (ROS response) over 20 minutes for a combination of ≥ 3 experiments (mean ± SEM), expressed as a percentage of the response in WT mouse neutrophils (iii). *P < .0001, paired Student t test (WT/R58A), 1-way ANOVA (WT, Rac1KD, Rac2KO, Rac1KD/Rac2KO) compared with WT response. (C) For quantification of cytosolic fluorescence (1), fluorescence at sites of attachment of IgG-SRBCs to neutrophil (2), around the phagocytic cup (3), the phagosome (4), and after phagocytosis (5), widefield epifluorescence microscopy was used. Mouse BMNs expressing p67phox WT-GFP on a WT or a p40phoxR58A/R58A genetic background or expressing p67phox H69E-GFP on a WT genetic background were primed and incubated with IgG-SRBCs as described in Figure 2C. Images were recorded and presented as described in Figure 3C. Quantification was performed using ImageJ software, as described in “Live imaging” and supplemental Figure 9. Data are presented as a ratio of MFI of phagosome:cytosol. The gray transparent area represents a ratio of phagosome:cytosol of 1.0 or lower, representing no phagosomal translocation. Data are mean ± SEM (n = 4-17 neutrophils). *P ≤ .01 as determined by Student t test between WT and R58A, and between WT and H69E.
Phagocytosis of serum-S aureus in primed neutrophils was extremely rapid (t ≤ 6 seconds from first point of neutrophil contact), and hence phagocytic cups were poorly discerned, usually defined by only a single frame or missed entirely. Within these limitations, p67phox-GFP was found in the cytosol of resting neutrophils and typically accumulated on phagosomes within the first 3 frames that they were observed within the cell (4-16 seconds). Phagosomal accumulation of p67phox-GFP was unevenly distributed around the circumference of individual phagosomes, consistent with images acquired by immunofluorescence of endogenous phox proteins. Further, retention of p67phox-GFP on individual phagosomes varied substantially (78-150 seconds). Similar patterns of p67phox-GFP accumulation were seen in unprimed neutrophils (an example is shown in supplemental Video 3), although the time between particle engulfment and phagosomal accumulation of p67phox-GFP was highly variable (4-75 seconds) but on average slower than in primed cells (mean 26 seconds). We never observed any membrane accumulation of p67phox–GFP or GFP-p40phox, in primed or unprimed cells, that did not appear to be directly associated with the S aureus phagosome.
Phagocytosis of IgG-SRBCs was characteristically slower than for serum-S aureus (t ≥ 10 seconds) and clear phases of initial contact, emergence of a phagocytic cup, and phagosome closure could be defined. p67phox-GFP first accumulated on a large tubulovesicular structure at the base of the emerging phagocytic cup (marked by an arrow in Figure 2Ci). This structure appeared to grow in size and complexity during cup development and, in a number of examples, appeared to fuse with the developed phagosome (Figure 2Ciii). p67phox-GFP also accumulated on the phagosome itself but these accumulations were highly variable (lasting between 48-260 seconds) and were typically less intense than on the pre-phagosomal structure.
Previous studies have defined a role for PtdIns3P-p40phox interaction in phagocytosis-elicited oxidase activation.20,21 We sought to establish the relative kinetics of oxidase subunit assembly and PtdIns3P formation during neutrophil phagocytosis by generating bone marrow chimeras expressing GFP-iPX in a WT genetic background. GFP-iPX was found in both neutrophil cytosol and on vesicular compartments that colocalized with the endosomal marker EEA1 (DNS). During phagocytosis of S aureus, GFP-iPX accumulated on phagosomes soon after they separated from the plasma membrane (supplemental Video 4; Figure 2Bii), consistent with previous studies using cultured cells.16,17 We sought to more precisely define the relative kinetics of PtdIns3P and oxidase subunit accumulation during phagocytosis by co-expressing p67phox-GFP and mCherry-iPX in the same neutrophils. We obtained mouse bone marrow chimeras with good levels of co-expression of these reporters but systematically observed that cells with measurable levels of mCherry-iPX expression exhibited poor membrane accumulations of p67phox-GFP (supplemental Figure 4A) and poor formazan deposition in NBT assays (DNS), suggesting the iPX-reporter sequesters PtdIns3P in structures relevant to oxidase assembly. However, in a small number of examples we did manage to image both mCherry-iPX and p67phox-GFP accumulation during S aureus phagocytosis; in these cases p67phox-GFP accumulation slightly preceded that of mCherry-iPX (supplemental Video 5).
GFP-iPX also accumulated dramatically on IgG-SRBC phagosomes (supplemental Video 6; Figure 2Cii), but whether it also accumulated on the same pre-phagosomal structures as the oxidase was less clear. Pre-existing endosomal accumulations of this reporter made it very difficult to identify the appearance of relatively small structures and, to an even greater extent than was evident during S aureus phagocytosis, we were unable to localize both iPX and tagged oxidase subunits in the same cell (because expression of iPX severely blocked oxidase activity and membrane accumulation of p67phox-GFP; supplemental Figure 4B and DNS). However, the emergence of a GFP-iPX–decorated structure with the characteristic timing, position, and morphology of analogous accumulations of p67phox-GFP could usually be discerned, before extensive accumulation on the phagosome itself (marked by arrows in Figure 2Cii and supplemental Video 6).
p67phox accumulation on S aureus phagosomes is partially dependent on PtdIns3P binding to p40phox and totally dependent on p67phox binding to Rac1 or 2
We investigated the role of PtdIns3P binding to p40phox in regulating the assembly of an active oxidase during phagocytosis of S aureus using primed (Figure 3) and unprimed (supplemental Figure 1) neutrophils isolated from mice carrying a mutation in the PX domain of p40phox that prevents interaction with PtdIns3P (p40phoxR58A/R58A).28 Priming agents themselves did not stimulate ROS production, and the R58A mutation caused a similar, 30%-40% decrease in ROS formation in both primed (Figure 3A) and unprimed cells (supplemental Figure 1). Further, phagosomal accumulation of endogenous p67phox (Figure 3B) and of exogenously expressed p67phox-GFP (Figure 3C) were all similarly reduced by approximately 30% in primed p40phoxR58A/R58A neutrophils compared with WT cells.
We also investigated the role of Rac binding to p67phox during S aureus phagocytosis using neutrophils isolated from mice lacking Rac2 (Rac2KO) and/or supporting inducible deletion of Rac1 (Rac1KD). Loss of Rac2 or Rac1 alone did not reduce ROS formation or phagosomal accumulation of p67phox in response to S aureus and, indeed, loss of Rac1 characteristically generated increased responses (Figure 3A-B). Loss of both Rac2 and Rac1 however, prevented measurable ROS formation or p67phox translocation, indicating Rac1 and 2 play an essential but redundant role in oxidase activation in this context (phagocytosis of serum-S aureus was unaffected by loss of both Rac1 and 2; supplemental Figure 5). Over 80% deletion of Rac1 in a Rac2−/− background was required to see a significant reduction in S aureus oxidase responses (supplemental Figure 5), indicating only a small proportion of the neutrophil's total complement of Rac proteins is required to fully support this response. An absolute requirement for direct Rac binding to p67phox was also confirmed by the inability of p67phox-H69E-GFP, a mutant incapable of binding Rac1/2 (supplemental Figure 6), to translocate to S aureus phagosomes (Figure 3C; expression of p67phox-H69E-GFP was comparable to p67phox-GFP; supplemental Figure 2).
p67phox accumulation on both pre-phagosomal structures and the phagosomal membrane in response to IgG-SRBC phagocytosis is highly dependent on both PtdIns3P binding to p40phox and p67phox binding to Rac2
IgG-SRBC–stimulated ROS formation assessed by either NBT reduction (Figure 4A) or chemiluminescence (Figure 4B) was reduced by ≥ 85% in p40phoxR58A/R58A neutrophils. Membrane accumulation of p67phox-GFP in response to IgG-SRBCs was also severely reduced in p40phoxR58A/R58A neutrophils, with no discernable concentration on either pre-phagosomal compartments or the phagosome itself (Figure 4C).
ROS formation in response to IgG-SRBCs was significantly increased by loss of Rac1 (Figure 4B) but completely blocked by loss of Rac2 (Figure 4A-B). Further, p67phox-H69E-GFP was unable to translocate to any membrane structure during IgG-SRBC phagocytosis (Figure 4C), indicating Rac2 binding to p67phox is essential for oxidase assembly and activation under these conditions.
The pre-phagosomal compartment on which p67phox assemble during IgG-bead phagocytosis coincides with areas of granule delivery
FcγRs are known to undergo rapid endocytosis upon antibody engagement35 and therefore we sought to establish if receptor endocytosis was required for pre-phagosomal accumulation of the oxidase in response to IgG targets. The dynamin inhibitor dynasore37 blocked FcR-mediated endocytosis of transferrin (supplemental Figure 7) and soluble antibody (DNS), but did not significantly block intracellular formazan deposition in response to IgG-beads (supplemental Figure 7), IgG-SRBCs (supplemental Figure 7), or immobilized-IgG-BSA (DNS). Further, p67phox accumulation on extra-phagosomal structures was unaffected by dynasore in response to phagocytosis of IgG-beads (supplemental Figure 7). Together, these results indicate FcγR-endocytosis is unlikely to be a trigger for oxidase assembly under these conditions.
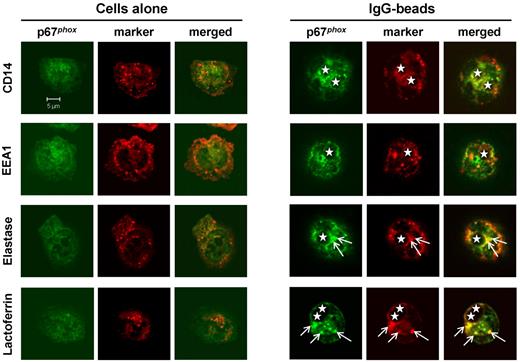
We attempted to define the source(s) of pre-phagosomal membrane on which the oxidase assembles during FcR-mediated phagocytosis by searching for colocalization with known markers of neutrophil membrane compartments. IgG-beads were chosen for most of these studies because, unlike rabbit IgG-opsonized SRBCs, they could be opsonized with mouse antibodies that were not recognized by the anti–rabbit secondary antibodies used for immunofluorescence localization of endogenous p67phox and granule markers.38 We did not observe good colocalization of p67phox-positive extra-phagosomal structures with CD14 (secretory vesicles) or EEA1 (early endosomes). We did, however, observe a small degree of colocalization of p67phox with lactoferrin (specific granules) and elastase (azurophil granules; Figure 5). Our interpretation of these results is that the membrane compartment on which p67phox assembles is probably derived, in part, from granule fusion but may contain a mixture of active ROS/proteases that reduce the sensitivity of epitope detection.
Extra-phagosomal structures containing endogenous p67phox are derived from azurophil and specific granules. Mouse neutrophils were incubated without (cells alone) or with IgG-opsonized 3-μm latex beads as described in “Phagocytosis assays” and were allowed to adhere on glass coverslips. Cells were fixed and permeabilized as described in “Phagocytosis assays,” stained with relevant antibodies, mounted, and visualized on a Zeiss LSM 510 META point-scanning confocal microscope. The position of the phagosome is indicated by a star. Arrows indicate the position of the p67phox-positive extra-phagosomal structure and of granule markers colocalizing with this structure.
Extra-phagosomal structures containing endogenous p67phox are derived from azurophil and specific granules. Mouse neutrophils were incubated without (cells alone) or with IgG-opsonized 3-μm latex beads as described in “Phagocytosis assays” and were allowed to adhere on glass coverslips. Cells were fixed and permeabilized as described in “Phagocytosis assays,” stained with relevant antibodies, mounted, and visualized on a Zeiss LSM 510 META point-scanning confocal microscope. The position of the phagosome is indicated by a star. Arrows indicate the position of the p67phox-positive extra-phagosomal structure and of granule markers colocalizing with this structure.
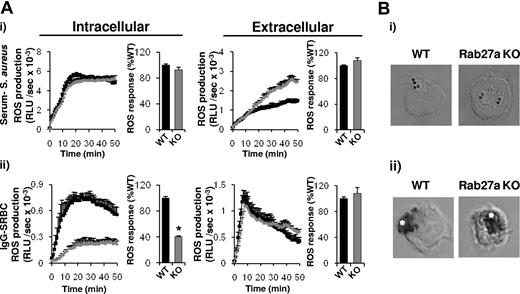
Oxidase delivery to IgG-SRBC phagosomes is partially dependent on Rab27a
Rab27a has previously been implicated in neutrophil granule secretion39,40 and in targeting the oxidase to phagosomes in dendritic cells.41 We therefore measured ROS responses to serum-S aureus and IgG-SRBCs in neutrophils lacking Rab27a (Fig 6). Using an enhanced chemiluminescence assay designed to distinguish between intra- and extracellular ROS formation, we found that loss of Rab27a caused significant decreases in intracellular but not extracellular ROS responses to IgG-SRBCs, while both intracellular and extracellular ROS responses to S aureus were unaffected (Figure 6A). Similarly, intracellular formazan deposition in response to S aureus was unaffected (Figure 6B). In contrast, formazan deposition in response to IgG-SRBCs, normally restricted to the base of the phagosome and the phagosome itself, was much more extensively distributed throughout the cell in Rab27a−/− neutrophils (Figure 6B). Therefore, we postulate that Rab27a directs the assembly of pre-phagosomal structures and/or their delivery to phagosomes containing IgG-opsonized targets.
Phagosomal delivery of the oxidase in response to IgG-SRBCs is dependent on Rab27a. Primed BMNs from WT (black squares/bars) or ashen Rab27a−/− (Rab27a KO, gray triangles/bars) animals were preincubated with luminol/SOD (intracellular) or isoluminol/HRP (extracellular; A) or NBT (B) as described in “Measurement of ROS production” and in the supplemental Methods. Cells (5 × 105) were added to either serum-S aureus (i) or IgG-SRBCs (ii), prepared as described in the supplemental Methods. ROS responses (A) and formazan deposition (B) were measured as described in Figures 3A and 1, respectively. Shown are (A) data (mean ± range) from 1 experiment representative of at least 3, expressed as RLU/s, as well as accumulated light emission (ROS production) over 20 minutes for a combination of ≥ 3 experiments (mean ± SEM), expressed as a percentage of the response in WT mouse neutrophils. *P < .0001, paired Student t test compared with WT response and (B) representative images.
Phagosomal delivery of the oxidase in response to IgG-SRBCs is dependent on Rab27a. Primed BMNs from WT (black squares/bars) or ashen Rab27a−/− (Rab27a KO, gray triangles/bars) animals were preincubated with luminol/SOD (intracellular) or isoluminol/HRP (extracellular; A) or NBT (B) as described in “Measurement of ROS production” and in the supplemental Methods. Cells (5 × 105) were added to either serum-S aureus (i) or IgG-SRBCs (ii), prepared as described in the supplemental Methods. ROS responses (A) and formazan deposition (B) were measured as described in Figures 3A and 1, respectively. Shown are (A) data (mean ± range) from 1 experiment representative of at least 3, expressed as RLU/s, as well as accumulated light emission (ROS production) over 20 minutes for a combination of ≥ 3 experiments (mean ± SEM), expressed as a percentage of the response in WT mouse neutrophils. *P < .0001, paired Student t test compared with WT response and (B) representative images.
The oxidase assembles on extra-phagosomal membranes during phagocytosis of IgG-targets in human neutrophils
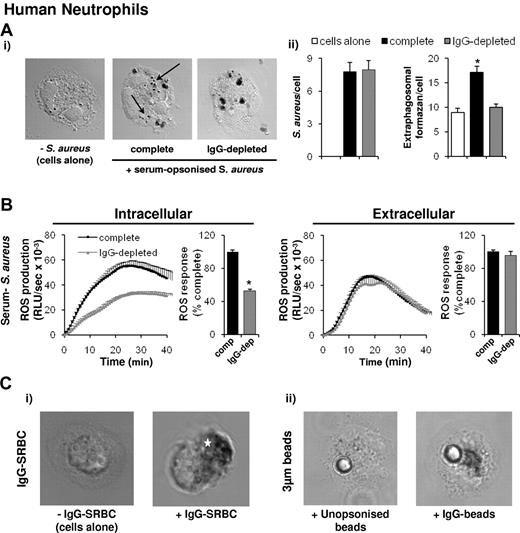
We also investigated whether the oxidase assembles on extra-phagosomal structures during serum-S aureus and IgG-target phagocytosis in human neutrophils. In contrast to our observations in mouse neutrophils, we observed a small number of formazan-positive intracellular structures in human cells that were not exposed to particles and a significant increase in extra-phagosomal formazan deposits on phagocytosis of both serum-opsonized S aureus (Figure 7A, marked by arrows) and zymosan (DNS). Intriguingly, these extra-phagosomal formazan deposits were abolished by depleting the human opsonizing serum of IgG (Figure 7A), and this coincided with a specific reduction in intracellular, but not extracellular, ROS formation (Figure 7B; zymosan DNS; IgG depletion did not significantly reduce levels of phagocytosis; Figure 7Aii). We have previously shown mouse neutrophil phagocytosis and ROS formation in response to mouse serum-opsonized S aureus are independent of FcγR-signaling28 and, consistent with this, we saw no effect of first depleting mouse serum of IgG before opsonizing S aureus or zymosan in these assays (DNS). We also observed substantial extra-phagosomal formazan deposits in human neutrophils phagocytosing IgG-SRBCs and IgG-beads (Figure 7Ci-ii), and endogenous p67phox also accumulated in extra-phagosomal compartments in response to IgG-beads (DNS). Taken together these results indicate human neutrophils also assemble active oxidase complexes in extra-phagosomal structures specifically in response to FcγR-stimulation.
IgG dependency of extra-phagosomal superoxide production in human neutrophils. (A) Primed human peripheral blood neutrophils were preincubated with NBT and left untreated (cells alone) or incubated with S aureus opsonized with either 10% normal serum (complete) or serum depleted of IgG (IgG-depleted) before opsonization, as described in the supplemental Methods. Nonphagocytosed S aureus were lysed by lysostaphin, and samples cytospun onto glass coverslips. Samples were prepared and visualized as described in Figure 1. Shown are representative images. Arrows mark extra-phagosomal formazan deposition in response to phagocytosis of S aureus opsonized with complete serum. (Aii) Number of S aureus phagocytosed under each condition (S aureus/cell), and extra-phagosomal formazan deposits (extra-phagosomal formazan/cell) in at least 40 cells from each condition were counted and expressed as mean ± SEM, P < .0001. (B) Human neutrophils were preincubated with luminol/SOD (intracellular) or isoluminol/HRP (extracellular) as described in “Measurement of ROS production,” before addition to S aureus opsonized with either 10% normal serum (complete, comp) or serum depleted of IgG (IgG-depleted, IgG-dep) before opsonization. ROS responses were measured and data are presented as described in Figure 6A. *P ≤ .0001, paired Student t test compared with complete serum-opsonized S aureus–induced responses. (C) Neutrophils were incubated with NBT, left untreated (cells alone) or incubated with IgG-SRBCs, or IgG-opsonized or unopsonized 3-μm beads, as described in the supplemental Methods. Cells were allowed to adhere to coverslips for 3 minutes at 37°C, and samples were prepared and visualized as described in Figure 1. Shown are representative images. Position of the IgG-SRBC phagosome is indicated by a star.
IgG dependency of extra-phagosomal superoxide production in human neutrophils. (A) Primed human peripheral blood neutrophils were preincubated with NBT and left untreated (cells alone) or incubated with S aureus opsonized with either 10% normal serum (complete) or serum depleted of IgG (IgG-depleted) before opsonization, as described in the supplemental Methods. Nonphagocytosed S aureus were lysed by lysostaphin, and samples cytospun onto glass coverslips. Samples were prepared and visualized as described in Figure 1. Shown are representative images. Arrows mark extra-phagosomal formazan deposition in response to phagocytosis of S aureus opsonized with complete serum. (Aii) Number of S aureus phagocytosed under each condition (S aureus/cell), and extra-phagosomal formazan deposits (extra-phagosomal formazan/cell) in at least 40 cells from each condition were counted and expressed as mean ± SEM, P < .0001. (B) Human neutrophils were preincubated with luminol/SOD (intracellular) or isoluminol/HRP (extracellular) as described in “Measurement of ROS production,” before addition to S aureus opsonized with either 10% normal serum (complete, comp) or serum depleted of IgG (IgG-depleted, IgG-dep) before opsonization. ROS responses were measured and data are presented as described in Figure 6A. *P ≤ .0001, paired Student t test compared with complete serum-opsonized S aureus–induced responses. (C) Neutrophils were incubated with NBT, left untreated (cells alone) or incubated with IgG-SRBCs, or IgG-opsonized or unopsonized 3-μm beads, as described in the supplemental Methods. Cells were allowed to adhere to coverslips for 3 minutes at 37°C, and samples were prepared and visualized as described in Figure 1. Shown are representative images. Position of the IgG-SRBC phagosome is indicated by a star.
Discussion
Formazan-deposition assays, immunofluorescence of endogenous oxidase subunits, and live fluorescent imaging of GFP-tagged subunits, all point to the rapid and direct assembly of the oxidase complex on S aureus-containing phagosomes in mature mouse neutrophils. This would be expected on the basis of the well-described function of the oxidase in bacterial phagosomes to participate in bacterial killing.2 Surprisingly however, the same approaches indicated the oxidase can assemble on non-phagosomal structures in response to IgG-targets and, in the case of FcγR-mediated phagocytosis, these structures develop at the base of the forming phagosome at sites of granule delivery, inviting questions concerning their mechanism of formation and function.
Using conditional deletion of Rac1 in a Rac2+/+ or Rac2−/− genetic background, we found that Rac2 and/or Rac1 were required for measurable ROS formation in response to both serum-S aureus and IgG-SRBCs. This is consistent with the view that GTP-Rac1/2 forms the critical complex with p67phox and cytochrome b558 that allows efficient electron transfer between NADPH and molecular oxygen. In vitro assays with recombinant proteins suggest both Rac1 and 2 isoforms can participate in active oxidase assembly but most previous work had suggested that Rac2 plays the major role in neutrophil oxidase activation.42,43 While our results confirm an absolute requirement for Rac2 in oxidase responses to IgG-SRBCs, they revealed a surprising redundancy between Rac1 and 2 in the ROS response to serum-S aureus, supporting previous findings that Rac2 is not essential for serum-zymosan ROS responses.44 Further, the effects of Rac 1 and/or Rac2 deletion on oxidase activation were paralleled by their effects on membrane accumulation of p67phox, suggesting the interaction between these proteins is also important in determining the overall affinity of p67phox for the relevant membrane/protein location. The molecular basis for this difference in isoform usage is unknown, but Rac2 has been shown to have a preference for less charged membrane surfaces.45
We also investigated the role of PtdIns3P binding to the PX domain of p40phox in the membrane recruitment and activation of the oxidase during phagocytosis of both serum-S aureus and IgG-SRBCs. We confirmed previous studies conducted in mouse neutrophils indicating a partial role for the PtdIns3P-p40phox interaction in determining levels of ROS formation in response to phagocytosis of S aureus.20 We have now extended these studies to show a more major role for this interaction in ROS responses to IgG-SRBCs. We also show a close correlation in both responses between the impacts of PtdIns3P-p40phox interaction on ROS formation and membrane accumulation of p67phox. These observations were also consistent with the effects of heterologous expression of a PtdIns3P-binding GFP-iPX domain, which severely reduced oxidase activation and p67phox membrane accumulation, particularly in response to IgG-targets. We also note that the relatively modest decreases in ROS responses to serum-S aureus seen in p40phoxR58A/R58A mouse neutrophils were much less than equivalent inhibitions reported in analogously mutated p40phoxR105Q/− human neutrophils,21 possibly reflecting intrinsic differences in the relative balance of pathways regulating oxidase assembly in mouse versus human neutrophils. However, we also found that, unlike the mouse, human neutrophil ROS responses to phagocytosis of serum-S aureus possessed a significant IgG-component and were associated with more numerous, IgG-dependent, extra-phagosomal formazan deposits. It is therefore possible that the greater dependence of human neutrophil ROS responses on PtdIns3P-interaction with p40phox is because of the greater involvement of FcγR-driven oxidase assembly in the assays conducted.
Previous studies in mouse neutrophils, together with work presented here, indicates that the PtdIns3P-p40phox interaction makes a differential contribution to ROS responses elicited by different agonists, having little impact on extracellular ROS responses to fMLP, phorbol 12-myristate 13-acetate (PMA),20 and either serum or IgG-opsonized zymosan (DNS), a partial impact on intracellular ROS responses to fMLP (DNS), PMA and S aureus20 , and a major impact on ROS responses to IgG-targets. Thus, it appears that PtdIns3P binding to p40phox is not a core requirement for neutrophil oxidase activity but a regulatory input guiding oxidase assembly in only certain contexts of activation. The simplest hypothesis is that among the balance of mutual interactions that govern the assembly of oxidase subunits, PtdIns3P binding to p40phox makes a significant contribution to driving increased local concentration of p67phox on some PtdIns3P-containing membranes, presumably dictated by the concentrations and affinities of all the relevant interactions. Consistent with this, we observed that loss of Rac2 results in ROS responses to S aureus becoming much more sensitive to the PI3K inhibitor wortmannin (supplemental Figure 8). Thus it is possible that when less Rac is available, p67phox translocation becomes more dependent on PtdIns3P-binding to p40phox. Of relevance to this idea are previous studies showing that phagocytic/endocytic structures accumulate PtdIns3P and lose Rac proteins on severance from the plasma membrane,46 thus sustained oxidase activity on these structures may increasingly depend on the PtdIns3P-p40phox interaction. Indeed a recent study concluded that in PLB-985 cells, PtdIns3P played a more important role in retaining p40phox/p67phox on the phagosome than initial recruitment to the cup and phagosome.32 This idea is also consistent with our observation that p67phox-GFP accumulates on S aureus phagosomes before GFP-iPX. However, Tian and colleagues also concluded that p40phox was more important in oxidase activation than recruitment.32 We cannot rule out a role for p40phox in oxidase activation beyond recruitment of p67phox, it is simply that our data do not demand it.
We attempted to identify the origin of the pre-phagosomal membranes on which the oxidase assembles in response to IgG-target phagocytosis. Due to their size, shape, granularity, and relative nuclear to cytoplasmic ratios, fully differentiated neutrophils present a challenge to accurately assess the colocalization of markers for different membrane compartments by standard immunofluorescence techniques. We were able to show significant colocalization between markers for specific and azurophil granules (lactoferrin and elastase) and p67phox on pre-phagosomal structures but a more extensive analysis is likely to require further techniques, such as imaging granule membrane and content markers by electron microscopy and live cell fluorescence. However, the simplest hypothesis that encompasses the morphology and kinetics of both formazan and phox protein accumulation during IgG-SRBC phagocytosis is that the oxidase first assembles on granule membranes, which then fuse with each other, further membranes, and finally the phagosome.
Several previous studies have suggested the oxidase may assemble on neutrophil intracellular membranes and granules in response to PMA.47-49 Recent work has also identified delivery of cytochrome b558-containing vesicles to dendritic cell phagosomes via a Rab27a-dependent mechanism.41 Further, Rab27a is important in the secretion of myeloperoxidase-enriched azurophil granules in neutrophils.40 Consistent with these findings, we observed a partial role for Rab27a in the delivery of ROS and peroxidases to common compartments during IgG-SRBCs, but not S aureus, phagocytosis. However, this effect is not simply due to a failure to deliver myeloperoxidase to IgG-SRBC phagosomes, because loss of Rab27a also created more numerous, extra-phagosomal formazan deposits, suggesting Rab27a is directly involved in oxidase trafficking to IgG-mediated phagosomes.
Clearly further work needs to be done to gain a clearer view of how the oxidase assembles on pre-phagosomal, internal membranes in both mouse and human neutrophils, in particular how relevant intracellular signals, including GTP-Rac and PtdIns3P are formed at these locations and how fusion events regulate their fate. We do not yet understand whether pre-phagosomal oxidase accumulation during IgG-SRBC phagocytosis merely reflects differences in extent and timing of granule fusion versus S aureus phagocytosis or reflects a more profound, functional difference. In this regard, recent studies in dendritic cells and macrophages have uncovered a role for the oxidase in resisting phagosomal acidification and allowing appropriate proteolysis and antigen presentation to occur.41,50 Antigen cross-presentation via dendritic cell phagosomes is dependent on the nature of the opsonin, and FcγR-directed delivery, but not complement-receptor directed delivery, is Rab27a-dependent.39 Given the IgG and partial Rab27a dependency of oxidase assembly on the extra-phagosomal structures described here, this could suggest a role for these structures in antigen presentation downstream of FcγR signaling. It will therefore be interesting to investigate whether sites of extra-phagosomal oxidase assembly in neutrophils represent compartments with distinct processing capabilities, such as extracting antigenic peptides from antibody-opsonized prey.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mary Dinauer and Chris Ellson for helpful discussions and for critical reading of the manuscript. We also thank Simon Andrews, John Coadwell, John Ferguson, Thomas Henley, Elena Vigorito, and Keith Boyle for technical assistance and helpful discussions. Finally, we thank the staff of the small animal unit (SAU) and the small animal barrier unit (SABU) at the Babraham Institute for assistance with care of animals.
This work is supported by the Biotechnology and Biological Sciences Research Council (BBSRC), Medical Research Council (MRC; G0600840), and Wellcome Trust (WT085889MA). T.C. is a UCB BBSRC-Collaborative Awards in Science and Engineering (CASE) award student.
Wellcome Trust
Authorship
Contribution: K.E.A. and T.A.M.C. designed and performed experiments, analyzed and interpreted data, and wrote the manuscript; K.D., R.B.H., and S.W. performed experiments; T.T., K.G., O.R., M.C.S., and V.L.J.T. provided valuable reagents; and L.R.S. and P.T.H. designed experiments, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Phillip Hawkins, Inositide Laboratory, Babraham Institute, Babraham Research Campus, Cambridge CB2 4AT, United Kingdom; e-mail: phillip.hawkins@bbsrc.ac.uk.
References
Author notes
K.E.A. and T.A.M.C. contributed equally to this study.
L.R.S. and P.T.H. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal