The c-myb transcription factor is highly expressed in immature hematopoietic cells and down-regulated during differentiation. To define its role during the hematopoietic lineage commitment, we silenced c-myb in human CD34+ hematopoietic stem/progenitor cells. Noteworthy, c-myb silencing increased the commitment capacity toward the macrophage and megakaryocyte lineages, whereas erythroid differentiation was impaired, as demonstrated by clonogenic assay, morphologic and immunophenotypic data. Gene expression profiling and computational analysis of promoter regions of genes modulated in c-myb–silenced CD34+ cells identified the transcription factors Kruppel-Like Factor 1 (KLF1) and LIM Domain Only 2 (LMO2) as putative targets, which can account for c-myb knockdown effects. Indeed, chromatin immunoprecipitation and luciferase reporter assay demonstrated that c-myb binds to KLF1 and LMO2 promoters and transactivates their expression. Consistently, the retroviral vector-mediated overexpression of either KLF1 or LMO2 partially rescued the defect in erythropoiesis caused by c-myb silencing, whereas only KLF1 was also able to repress the megakaryocyte differentiation enhanced in Myb-silenced CD34+ cells. Our data collectively demonstrate that c-myb plays a pivotal role in human primary hematopoietic stem/progenitor cells lineage commitment, by enhancing erythropoiesis at the expense of megakaryocyte diffentiation. Indeed, we identified KLF1 and LMO2 transactivation as the molecular mechanism underlying Myb-driven erythroid versus megakaryocyte cell fate decision.
Introduction
Hematopoiesis is a tightly regulated process involving the ordered regulation of gene expression throughout development, from the hematopoietic stem cells (HSCs) to mature and fully differentiated cells. The c-myb gene encodes for a basic helic turn helix transcription factor composed of 3 functional domains: a DNA binding domain at the N terminus, a central transactivation domain and a C-terminal negative regulatory domain.1
Most of what is known about c-myb involves its role in hematopoiesis, during which it is highly expressed in immature, proliferating cells of all hematopoietic lineages and down-regulated during terminal differentiation. The critical role of c-myb in regulating normal human hematopoiesis was first related to cell-cycle control, because its down-regulation by antisense oligonucleotides determines a G1 phase cell-cycle arrest.2,,–5 However, several works indicate multiple cellular roles for this transcription factor during normal hematopoiesis, showing that c-myb targets some differentiation-related genes, such as CD34,6 c-kit,7 c-myc,8 GATA1,9 neutrophil elastase,10 and myeloperoxidase.11
c-myb is essential for the hematopoietic system development, because c-myb−/− mice die at E15 due to failure of fetal hepatic erythropoiesis.12 c-myb knockout and knockdown models collectively demonstrate that c-myb plays a pivotal role at multiple stages of hematopoiesis,12,13 but the c-myb target genes responsible for these effects remain largely unknown. Moreover, little is known about the role of c-myb in human adult hematopoietic differentiation and in particular on the molecular mechanisms governing c-myb–mediated differentiation.
To investigate the role of c-myb in the human hematopoietic commitment and to get new insights into the molecular mechanisms underlying c-myb–driven lineage specification, we silenced c-myb in human CD34+ stem/progenitor cells and primary myeloblasts in vitro. Our data showed that c-myb silencing in CD34+ cells forces their commitment toward the mono-macrophage and megakaryocyte lineages whereas the erythroid and granulocyte ones are strongly impaired. Moreover, microarrays data, together with chromatin immunoprecipitation (ChIP) and Luciferase assays and functional rescue experiments, allowed us to identify the transactivation of Kruppel-Like Factor 1 (KLF1) and LIM Domain Only 2 (LMO2) expression as a new molecular mechanism through which c-myb enhances erythopoiesis and represses megakaryocytopoiesis.
Methods
Human CD34+ stem/progenitor cells purification
Human CD34+ cells were purified from umbilical cord blood (CB) samples, collected after normal deliveries, according to the institutional guidelines for discarded material. Mononuclear cells were isolated by Ficoll-Hypaque (Lymphoprep; Nycomed Pharma) gradient separation, washed twice with phosphate-buffered saline, and then CD34+ cells were purified by immunomagnetic sorting (EasySep Human CD34 Positive Selection kit, StemCell Technologies Inc.). The purity of CD34+ cells, assessed by flow cytometry, was always > 95% (data not shown). After immunomagnetic separation, CD34+ cells were seeded in 24-well plates at 5 × 105/mL in Iscove modified Dulbecco medium (IMDM; GIBCO) containing 20% Human Serum (Bio-Whittaker), stem cell factor (SCF; 50 ng/mL), Fms-like tyrosine kinase 3 ligand (Flt3L; 50 ng/mL), TPO (thrombopoietin; 20 ng/mL), interleukin-6 (IL-6; 10 ng/mL), and IL-3 (10 ng/mL; all from R&D Systems), and electroporated 24 hours later.
Human CD14− myeloblasts purification
Human CD14− myeloblasts were obtained by CB CD34+ stem/progenitor cells liquid culture according to Montanari M et al14 and purified by immunomagnetic sorting (EasySep Human CD14 Positive Selection kit; StemCell Technologies Inc). The detailed procedure of CD14− cells purification is reported in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The purity of CD14− cells, assessed by flow cytometry, was always > 95% (data not shown).
After immunomagnetic separation, CD14− cells were seeded in 6-well plates at 106/mL in IMDM, added with 20% fetal calf serum (Bio-Whittaker), in the presence of human hematopoietic cytokines, namely SCF (10 ng/mL), Flt3L (10 ng/mL), IL-6 (10 ng/mL), and IL-3 (10 ng/mL; all from R&D Systems) and electroporated 24 hours later.
siRNA transfection
The electroporation of CD34+ stem/progenitor cells and CD14− myeloblasts was performed using the Amaxa Nucleofector® Technology as previously described.15
A mix of 3 Silencer Pre-designed siRNAs targeting human c-myb (supplemental Table 1) was used (Ambion).
Each sample was electroporated 3 times, once every 24 hours. For each electroporation, 5 × 105 CD34+ cells or 107 CD14− cells were resuspended in 100 μL of Human CD34+ Nucleofection Solution (Amaxa Biosystem), containing 5 μg of siRNA and pulsed with the program U-01 (CD34+ cells) or X-01 (CD14− cells). Transfection efficiency was evaluated by the nucleofection of a nontargeting Alexa Fluor 488–conjugated siRNA (QIAGEN) followed by flow cytometric analysis at 6 hours after transfection. To exclude nonspecific effects of siRNA nucleofection, for each experiment, one sample electroporated without siRNAs (MOCK) and one transfected with a nontargeting siRNA (NegCTR; siCONTROL Non-Targeting Pool, Dharmacon) were performed.
CD34+ cell-culture conditions
After each transfection, CD34+ cells were transferred into prewarmed fresh medium in 24-well plates and maintained in the same culture conditions as before (see “Human CD34+ stem/progenitor cells purification”). Cells were analyzed 24 and 48 hours after the last nucleofection (also reported as “postnucleofection,” meaning 4 and 5 days after cell purification, respectively) for both cell viability and c-myb expression. For liquid culture differentiation assays, the 24-hour postnucleofection CD34+ cells were plated (5 × 105/mL) in IMDM added with 20% BIT 9500 serum substitute (bovine serum albumin, insulin, and transferrin; StemCell Technologies), in order to set up erythroid (erythropoietin, EPO, 0.4 U/mL, SCF 50 ng/mL), megakaryocyte (TPO, 100 ng/mL),16 granulocyte colony stimulating factor (GCSF, 25 ng/mL), and monocyte (macrophage colony-stimulating factor, 100 ng/mL; SCF, 20 ng/mL; IL6, 20 ng/mL; and Flt3L, 50 ng/mL)17 unilineage cultures in addition to multilineage cell cultures (SCF, 50 ng/mL; Flt3L, 50 ng/mL; TPO, 20 ng/mL; IL-3, 10 ng/mL; IL-6, 10 ng/mL; all cytokines from R&D Systems). The medium was replaced every 3 days, whereas for granulocyte unilineage culture GCSF 25 ng/mL was added everyday. Differentiation was assessed by morphological analysis of May-Grunwald-Giemsa–stained cytospins and by flow cytometric analysis of CD14, CD15, CD163, Glycophorin A (GPA), and CD41 antigen expression at 5, 7, 9, 11, and 13 days after nucleofection (ie, 8, 10, 12, 14, and 16 days after CD34+ cell purification), respectively.
CD14− myeloblasts culture conditions
After each transfection, myeloblasts were seeded in 6-well plates (106/mL) into prewarmed IMDM, added with 20% fetal calf serum and human cytokines, namely SCF (10 ng/mL), Flt3L (10 ng/mL), IL-6 (10 ng/mL), and IL-3 (10 ng/mL). Cells were analyzed 24 and 48 hours after nucleofection for both cell viability and c-myb expression. Twenty-four hours after nucleofection, myeloblasts were seeded in the same culture conditions as described above and treated with all-trans retinoic acid (ATRA) 10−6M (Sigma-Aldrich) or GCSF 25 ng/mL (added everyday) for 3 and 10 days, respectively (ie, up to days 4 and 11 after nucleofection).
Differentiation was assessed by flow cytometric analysis of CD14 antigen expression 4 days after nucleofection and by morphological analysis of May-Grunwald-Giemsa–stained cytospins at day 4 after nucleofection for both untreated and ATRA-treated cells and at day 11 after nucleofection for GCSF-treated cells.
Microarray analysis
For both CD34+ cells and CD14− myeloblasts, total cellular RNA pools (100 ng) of MOCK, NegCTR and siRNA-treated (MYBsiRNA) cells, obtained from 3 independent experiments, were converted in biotinilated cRNA according to the 2-cycle target labeling protocol advised by Affymetrix. Similarly, the Affymetrix Human HG-U133A GeneChip arrays hybridization, staining, and scanning were performed using Affymetrix standard protocols as previously described.15,18
The GeneChip Operating Software (GCOS) absolute analysis algorithm was used to determine the amount of a transcript mRNA (signal), whereas the GCOS comparison analysis algorithm was used to compare gene expression levels between 2 samples.
Present genes were selected as the sequences showing the Detection call “P” and Signal ≥ 100 at least in one sample. Differentially expressed genes were selected as the sequences showing a Change call “I” or “D” and Signal Log Ratio (SLR) ≥ +1 or ≤ − 1 (Fold Change = 2SLR) in both the pairwise comparisons between MYBsiRNA and MOCK or NegCTR samples.
DAVID TOOL 2008 (http://david.abcc.ncifcrf.gov/) software was used to examine selected lists of genes to identify overrepresentation of functional classes accordingly with gene ontology (GO) classification. All of the data have been deposited in the Gene Expression Omnibus MIAME-compliant public database (National Center for Biotechnology Information) at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE13110 for CD34+ cells and http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE21943 for CD14− myeloblasts.
The methods for CD14− myeloblasts purification, Western blot detection of c-myb, KLF1, and LMO2 protein levels, methylcellulose and collagen clonogenic assays, morphological and immunophenotypic analysis, RNA extraction; quantitative real-time polymerase chain reaction (qRT-PCR), Myb kinetics during erythroid differentiation, ChIP; luciferase reporter assay; retroviral vectors construction and packaging, and CD34+ cells transduction, purification, and nucleofection are described in supplemental Methods.
Statistical analysis
The statistics used for data analysis was based on the 2-tail Student t test for averages comparison in paired samples. In silencing experiments, MOCK vs NegCTR, MOCK vs. MYBsiRNA, and NegCTR vs MYBsiRNA were compared. Data were analyzed by Microsoft Excel Software (Version 2007), and P < .05 was considered significant.
Results
c-myb silencing in CD34+ stem/progenitor cells
To get further insights into the role of c-myb in regulating proliferation and lineage commitment, we silenced its expression in human primary CB CD34+ hematopoietic stem/progenitor cells using a siRNA approach. We applied the Nucleofector technology (Amaxa) by optimizing CD34+ cells nucleofection protocol as previously detailed.15 Transfection efficiency, monitored by the nucleofection of a nontargeting Alexa Fluor 488–conjugated siRNA, ranged between 92% and 98% (supplemental Figure 1A-B).
In a set of 8 independent experiments, CD34+ cells were transfected with a mixture of 3 siRNAs targeting MYB mRNA (supplemental Table 1) and with a nontargeting siRNA as a negative control (NegCTR). The expression level of c-myb in control samples (MOCK and NegCTR) and MYBsiRNA cells was assessed by Western blot analysis 24 hours after each nucleofection (supplemental Figure 1C and Figure 1A). Western blot analysis demonstrated that the best down-regulation of c-myb protein levels in MYBsiRNA CD34+ cells was achieved 24 hours after the last nucleofection (hereafter reported as “postnucleofection” or “after nucleofection”; Figure 1A and supplemental Figure 1C).
Effects of c-myb silencing on cell-cycle distribution and clonogenic activity of CD34+ cells. (A) Western blot analysis of c-myb expression 24 hours after nucleofection. (B) Results of statistical analysis on the percentage of cells in the different cell-cycle phases performed by PI staining 24 hours after nucleofection (mean ± 2 SEM; n = 8). (C) Methylcellulose clonogenic assay results (mean ± 2 SEM; n = 8). Values are reported as percentages. (D-E) Megakaryocyte clonogenic assay results (mean ± 2 SEM; n = 4) in terms of megakaryocyte colony number (D) and size (E). Values are reported as number of megakaryocyte colonies for 1000 plated cells. Error bars in the graphs represent SD. *P ≤ .05, **P ≤ .01, and ***P ≤ .001 vs both MOCK and NegCTR samples. n = number of experiments.
Effects of c-myb silencing on cell-cycle distribution and clonogenic activity of CD34+ cells. (A) Western blot analysis of c-myb expression 24 hours after nucleofection. (B) Results of statistical analysis on the percentage of cells in the different cell-cycle phases performed by PI staining 24 hours after nucleofection (mean ± 2 SEM; n = 8). (C) Methylcellulose clonogenic assay results (mean ± 2 SEM; n = 8). Values are reported as percentages. (D-E) Megakaryocyte clonogenic assay results (mean ± 2 SEM; n = 4) in terms of megakaryocyte colony number (D) and size (E). Values are reported as number of megakaryocyte colonies for 1000 plated cells. Error bars in the graphs represent SD. *P ≤ .05, **P ≤ .01, and ***P ≤ .001 vs both MOCK and NegCTR samples. n = number of experiments.
Biological effects of c-Myb silencing on proliferation and commitment of CD34+ stem/progenitor cells
To examine the effects of c-myb silencing on CD34+ cells proliferation, cell-cycle analysis by propidium iodide (PI) staining was performed at 24 and 48 hours after nucleofection (see experimental flowchart in supplemental Figure 2). Cell-cycle analysis at 24 hours revealed that siRNA-mediated c-myb down-regulation determined a strong G1-phase cell-cycle arrest, whereas S and G2/M phases nearly halve respective to control samples (Figure 1B and supplemental Figure 3Ai-iii). These differences were mantained at 48 hours (supplemental Figure 3Aiv-vi,B).
The cell-cycle arrest was paralleled by a significant decrease in the absolute cell number in MYBsiRNA sample versus controls, as determined by Trypan Blue exclusion assay (supplemental Figure 3C).
To better characterize the role of c-myb during the hematopoietic stem/progenitor cells commitment, MYBsiRNA CD34+ cells clonogenic activity was evaluated by methylcellulose- and collagen-based clonogenic assays. Methylcellulose assay highlighted a 5-fold reduction in the clonogenic efficiency of MYBsiRNA CD34+ cells versus MOCK and NegCTR samples (supplemental Figure 4A). Furthermore, c-myb silencing induced a remarkable increase of the percentage of monocyte (CFU-M) and granulo-monocyte (CFU-GM) colonies and a decrease of the erythroid ones (BFU-E), whereas granulocyte progenitors (CFU-G) were not significantly affected (Figure 1C). These results indicated that c-myb silencing during CD34+ hematopoietic stem/progenitor cells commitment: (1) determines a cell-cycle arrest that strongly impairs CD34+ cells clonogenic efficiency and (2) enhances monocyte-macrophage commitment while negatively interfering with the erythroid one.
Next we examined the effect of c-myb silencing on the megakaryocyte commitment by plating MOCK, NegCTR, and MYBsiRNA CD34+ cells in a collagen-based serum-free semisolid culture medium that supports the growth of megakaryocyte progenitors in vitro. The results, reported in Figure 1D, demonstrated that c-myb silencing strongly enhances the megakaryocyte commitment of CD34+ stem/progenitor cells. Moreover, CFU-MKs scoring based on the size of the colonies showed that, although small (3-21 cells) colonies were significantly affected too, the most remarkable differences between c-myb–silenced CD34+ cells and controls were present in medium (21-49 cells) and large (> 50 cells) CFU-MKs (Figure 1E). Because large and medium CFU-MKs arise from more primitive megakaryocyte progenitors, whereas the smaller CFU-MKs are derived from more mature megakaryocyte progenitors, our data strongly suggest that c-myb silencing is able to affect the earliest phases of megakaryocyte differentiation.
The effects of c-myb silencing on CD34+ cells commitment were also evaluated by qRT-PCR and flow cytometric analysis of lineage differentiation markers expression and by morphological evaluation of May-Grunwald-Giemsa–stained cytospins.
Quantitative RT-PCR analysis of differentiation-related genes, performed 48 hours after nucleofection, showed the up-regulation of the mono-macrophage marker CD14 and the monocytopoietic transcription factor MafB,19 and the down-regulation of the granulocyte marker myeloperoxidase (MPO) in c-myb–silenced CD34+ cells (supplemental Figure 4B).
Flow cytometric analysis at day 7 (for GPA and CD41) and day 11 (for CD14 and CD163) after nucleofection confirmed the higher expression of CD14 together with the macrophage CD163 and the megakaryocyte CD41 markers in MYBsiRNA CD34+ cells versus MOCK and NegCTR samples, whereas the erythroid-specific marker GPA showed a significant decrease (Figure 2A).
c-myb silencing effects on CD34+ stem/progenitor cells differentiation. (A) Flow cytometric analysis of CD14 and CD163 (day 11 after nucleofection), GPA and CD41 (day 7 after nucleofection) markers after liquid multilineage culture (mean ± 2 SEM; n = 8). Error bars in the graphs represent SD. ***P ≤ .001 in MYBsiRNA versus MOCK and NegCTR. (B) Flow cytometric analysis of (i) GPA, CD41, (ii) CD15 and CD14 markers after liquid unilineage (i) erythroid, megakaryocyte, (ii) granulocyte and mono-macrophage culture, respectively (n = 3). For each marker, flow cytometry data at 5, 9, and 13 days after nucleofection are reported. Error bars represent SD. *P ≤ .05, **P ≤ .01, and ***P ≤ .001 in MYBsiRNA versus MOCK and NegCTR. (C) Table summarizing flow cytometry data for unilineage cultures (mean ± 2 SEM; n = 3). (D-E) Morphological analysis of May-Grunwald-Giemsa–stained cytospins after both liquid multilineage culture (day 11; D) and liquid unilineage (E) erythroid (day 7, i-iii), megakaryocyte (day 7, iv-vi), granulocyte (day 11, vii-ix) and mono-macrophage (day 11, x-xii) culture after nucleofection. The images were captured by Axioskop 40 microscope, by means of AxioCam HRc Digital Camera and Axiovision software 3.1 (all Carl Zeiss MicroImaging Inc). The images were then processed with Adobe Photoshop 7.0 software. Original magnification ×630. n = number of experiments.
c-myb silencing effects on CD34+ stem/progenitor cells differentiation. (A) Flow cytometric analysis of CD14 and CD163 (day 11 after nucleofection), GPA and CD41 (day 7 after nucleofection) markers after liquid multilineage culture (mean ± 2 SEM; n = 8). Error bars in the graphs represent SD. ***P ≤ .001 in MYBsiRNA versus MOCK and NegCTR. (B) Flow cytometric analysis of (i) GPA, CD41, (ii) CD15 and CD14 markers after liquid unilineage (i) erythroid, megakaryocyte, (ii) granulocyte and mono-macrophage culture, respectively (n = 3). For each marker, flow cytometry data at 5, 9, and 13 days after nucleofection are reported. Error bars represent SD. *P ≤ .05, **P ≤ .01, and ***P ≤ .001 in MYBsiRNA versus MOCK and NegCTR. (C) Table summarizing flow cytometry data for unilineage cultures (mean ± 2 SEM; n = 3). (D-E) Morphological analysis of May-Grunwald-Giemsa–stained cytospins after both liquid multilineage culture (day 11; D) and liquid unilineage (E) erythroid (day 7, i-iii), megakaryocyte (day 7, iv-vi), granulocyte (day 11, vii-ix) and mono-macrophage (day 11, x-xii) culture after nucleofection. The images were captured by Axioskop 40 microscope, by means of AxioCam HRc Digital Camera and Axiovision software 3.1 (all Carl Zeiss MicroImaging Inc). The images were then processed with Adobe Photoshop 7.0 software. Original magnification ×630. n = number of experiments.
Moreover, morphological evaluation of May-Grunwald-Giemsa–stained cytospins at day 11 after nucleofection clearly showed a uniform macrophage morphology in MYBsiRNA sample, whereas controls still showed a heterogeneous mixture of cells belonging to the myeloid lineages at different maturation stages (eg, promyelocytes, granulocytes and erythroblasts) (Figure 2D; statistical analysis of morphological assay is reported in supplemental Figure 4C).
Indeed, unilineage differentiation culture experiments further supported our results (Figure 2B-C). In particular, morphological analysis of TPO treated cells highlighted a significant increase of megakaryocyte differentiation in MYBsiRNA sample (Figure 2Evi) respect to controls (Figure 2Eiv-v). Similarly, after macrophage colony-stimulating factor-driven mono-macrophage unilineage differentiation, MYBsiRNA sample displayed a uniform macrophage morphology (Figure 2Exii), whereas controls were mainly represented by a mixture of immature myeloid cells (Figure 2Ex-xi). On the contrary, GCSF was not able to induce complete granulocyte differentiation in MYB-silenced cells (Figure 2Eix) as it was in the control samples (Figure 2Evii-viii). In the same way, EPO-driven erythroid differentiation was similarly impaired by c-myb silencing. In particular, at day 7 after nucleofection, EPO-treated MYBsiRNA sample showed a uniform proerythroblasts population (Figure 2Eiii), whereas controls were represented by a mixture of proerythroblasts together with basophilic erythroblasts and smaller cells with pyknotic nuclei, representing later stages of erythropoiesis (Figure 2Ei-ii). Thus, EPO-driven culture data suggested that erythropoiesis could be delayed by Myb silencing. This effect was not simply due to an acceleration of the physiological Myb down-regulation during erythropoiesis, because c-myb was only transiently knocked-down after siRNA Nucleofection and retrieved later on in MYBsiRNA cells, as demonstrated by the kinetics of c-myb expression during erythropoiesis (supplemental Figure 5).
GEP of c-myb silenced CD34+ cells
Next, we investigated the changes in gene expression induced by c-myb silencing in CD34+ cells, using Affymetrix HGU133A GeneChip array. Microarrays analysis was performed at 24 hours postnucleofection based on the observation that this timing disclosed the most silencing effect in terms of c-myb protein expression (see Figure 1A and supplemental Figure 1C).
As already highlighted by GO Biological Process classification (supplemental Table 2), detailed analysis of microarray data showed the down-regulation of several genes involved in cell-cycle progression and proliferation, such as v-myc myelocytomatosis viral oncogene homolog (MYC), Cyclin A1 (CCNA1), and minichromosome maintenance complex component 4 (MCM4), already reported as c-myb primary response genes,8,20,21 whereas proliferation inhibitors GAS1 and p57 (CDKN1C) resulted increased in MYBsiRNA CD34+ cells (Figure 3A). Among down-regulated genes were also detected erythroid transcription factors (KLF1 and LMO222 ), globin genes (HBA2 and HBB), erythroid cytoskeleton proteins (ANK1, ADD2, ERAF, and ERMAP) and surface antigens (GYPC, RHCE, RHD) typically associated with the erythroid differentiation (Figure 3B). Some granulocyte-specific genes coding for primary azurophilic granule proteins, such as myeloperoxidase (MPO) and elastase 2 (ELA2), which have been already reported as c-myb target genes,10,11 were similarly down-regulated in c-myb–silenced CD34+ cells (Figure 3B).
c-myb silencing effects on CD34+ cells gene expression profile. Microarrays data of (A) cell-cycle–related, (B) erythroid, granulocyte, (C) mono-macrophage, and (D) megakaryocyte genes. * indicates genes already reported as c-myb targets. (E) Microarrays data validation by qRT-PCR of a subset of transcripts (mean ± 2 SEM; n = 3). Error bars represent SD. *P ≤ .05 and **P ≤ .01 in MYBsiRNA versus MOCK and NegCTR. n = number of experiments. (F-G) Western blot detection of KLF1 (F) and LMO2 (G) expression levels 24 hours after nucleofection.
c-myb silencing effects on CD34+ cells gene expression profile. Microarrays data of (A) cell-cycle–related, (B) erythroid, granulocyte, (C) mono-macrophage, and (D) megakaryocyte genes. * indicates genes already reported as c-myb targets. (E) Microarrays data validation by qRT-PCR of a subset of transcripts (mean ± 2 SEM; n = 3). Error bars represent SD. *P ≤ .05 and **P ≤ .01 in MYBsiRNA versus MOCK and NegCTR. n = number of experiments. (F-G) Western blot detection of KLF1 (F) and LMO2 (G) expression levels 24 hours after nucleofection.
Moreover, c-myb silencing resulted in a striking induction of mono-macrophage–specific markers (CD14 and CD163) and receptors (MRC1 and TLR2), typical monocyte granule proteins (CTSB and RNASE1) and metalloproteinases (MMP2 and MMP12), and chemokines and chemokine receptors (CCL4; CXCL1, CXCL2, CXCL5, and CCR5) (Figure 3C). Together with these mono-macrophage markers, MafB (a master regulator of monocytopoiesis19 ) and c-Maf (highly expressed during macrophage differentiation23,24 ) transcription factors were overexpressed in c-myb–silenced CD34+ cells (Figure 3C). Similarly MYBsiRNA CD34+ cells showed increased expression of transcription factors involved in megakaryocyte commitment, such as Friend leukemia virus integration 1 (FLI1),25 myeloid ecotropic integration site 1 (MEIS1),26 and pre-B-cell leukemia transcription factor 1 (PBX1),27 genes coding for platelet-specific surface antigens, like ITGA2B (CD41) and ITGB3 (CD61) or secreted molecules, like platelet factor 4 (PF4), whose expression is directly regulated by MEIS1 and pre–B-cell leukemia transcription factor 128 (Figure 3D).
To confirm the microarray data, we carried out a TaqMan qRT-PCR analysis on a validation set selected among the differentially expressed genes between MYBsiRNA versus MOCK/NegCTR samples. All of the assessed genes showed in qRT-PCR the same expression pattern obtained by microarray analysis (Figure 3E). In addition, the down-regulation of the erythroid transcription factors KLF1 and LMO2 after Myb silencing was further validated by Western blot (Figure 3F-G).
As a whole, microarray data are in agreement with our functional data on the role of c-myb in hematopoietic stem/progenitor cells, further supporting the idea that c-myb silencing in CD34+ cells forces their commitment to the mono-macrophage and megakaryocyte lineages, while impairing the erythroid and granulocyte ones.
Identification of c-myb target genes by ChIP and luciferase reporter assay
To identify potential c-myb target genes, which may account for the biological effects of its silencing on CD34+ cells, we focused our attention on the differentially expressed genes highlighted by GEP analysis. We searched for potential cis-elements in KLF1, LMO2, FLI1, c-MAF, and MAFB promoter regions by matching the genomic sequences upstream and immediately downstream the transcription starting site to the transcription factor database TRANSFAC (http://motif.genome.jp; supplemental Table 3).
We next moved to study by Chromatin Immunoprecipitation (ChIP) the direct binding of c-myb to the promoter regions previously screened for putative c-myb binding sites. ChIP was performed as described by Lang et al.7 Cathepsin G (CTSG) promoter-specific PCR was included as a positive control for c-myb antibody-mediated chromatin immunoprecipitation, since CTSG has already been demonstrated to be a c-myb target gene.29 Immunoprecipitation with c-myb antibody in c-myb–expressing CB CD34+ cells resulted in amplification of KLF1 (Figure 4A) and LMO2 (Figure 4C) promoter regions, whereas immunoprecipitation without antibody (No Antibody) or with an isotype-matched control antibody (IgG Isotype Control) resulted in virtually undetectable amplification. These results demonstrated the direct binding of c-myb to KLF1 and LMO2 promoters in vivo. Conversely, ChIP data failed to reveal the binding of c-myb to the promoter region of FLI1, c-MAF and MAFB, suggesting that the expression of these genes is not directly regulated by c-myb but it could be rather an indirect effect of c-myb silencing, probably involving other c-myb transcriptional targets (data not shown).
Validation of KLF1 and LMO2 as c-myb direct target genes. (A) KLF1 promoter-specific PCR performed in a representative ChIP experiment. CTSG promoter-specific PCR is shown as a positive control for immunoprecipitation. ChIP and PCRs were performed in triplicate. (B) c-myb transactivation of KLF1 promoter-driven luciferase expression. The amounts (in micrograms) of cotransfected plasmids are reported. In the panel below, protein extracts from the transfected cells were tested for c-myb expression levels by Western blot. Luciferase levels (mean ± 2 SEM;n = 3) are normalized by setting the empty pXP1/pCMV6XL5 vectors-transfected sample as = 1. Error bars represent SD. *P ≤ .05 versus pXP1KLF1-685+empty pCMV6XL5-transfected sample. (C) LMO2 promoter-specific PCR performed in a representative ChIP experiment. CTSG promoter-specific PCR is reported as a positive control for immunoprecipitation. ChIP and PCRs were performed in triplicate. (D) c-myb transactivation of LMO2 promoter-driven luciferase expression. The amounts (in micrograms) of cotransfected plasmids are reported. In the panel below, c-myb expression levels were detected by Western blot in protein extracts from the transfected cells. Luciferase levels (mean ± 2 SEM; n = 3) are normalized by setting the empty pXP1/pCMV6XL5 vectors-transfected sample as = 1. Error bars represent SD. *P ≤ .05 and **P ≤ .01 versus pXP1LMO2-175+empty pCMV6XL5-transfected sample. n = number of experiments.
Validation of KLF1 and LMO2 as c-myb direct target genes. (A) KLF1 promoter-specific PCR performed in a representative ChIP experiment. CTSG promoter-specific PCR is shown as a positive control for immunoprecipitation. ChIP and PCRs were performed in triplicate. (B) c-myb transactivation of KLF1 promoter-driven luciferase expression. The amounts (in micrograms) of cotransfected plasmids are reported. In the panel below, protein extracts from the transfected cells were tested for c-myb expression levels by Western blot. Luciferase levels (mean ± 2 SEM;n = 3) are normalized by setting the empty pXP1/pCMV6XL5 vectors-transfected sample as = 1. Error bars represent SD. *P ≤ .05 versus pXP1KLF1-685+empty pCMV6XL5-transfected sample. (C) LMO2 promoter-specific PCR performed in a representative ChIP experiment. CTSG promoter-specific PCR is reported as a positive control for immunoprecipitation. ChIP and PCRs were performed in triplicate. (D) c-myb transactivation of LMO2 promoter-driven luciferase expression. The amounts (in micrograms) of cotransfected plasmids are reported. In the panel below, c-myb expression levels were detected by Western blot in protein extracts from the transfected cells. Luciferase levels (mean ± 2 SEM; n = 3) are normalized by setting the empty pXP1/pCMV6XL5 vectors-transfected sample as = 1. Error bars represent SD. *P ≤ .05 and **P ≤ .01 versus pXP1LMO2-175+empty pCMV6XL5-transfected sample. n = number of experiments.
To get further insights into the role of c-myb in the regulation of KLF1 and LMO2 expression, we cloned the genomic sequences upstream the transcription starting site into the promoterless vector pXP1 containing firefly luciferase reporter gene (see supplemental Methods). Our data demonstrated that increasing levels of c-myb protein are able to transactivate KLF1 promoter-driven luciferase expression in HEK293T cells. As shown in Figure 4B, c-myb determined a dose-dependent increase in KLF1 (−685/+19) promoter-driven luciferase activity. On the contrary, c-myb did not affect luciferase expression levels for pXP1KLF1 (−548/+19) plasmid, suggesting that c-myb transactivating activity depends on the binding to a region of human KLF1 promoter between 548 and 685 bps upstream from the transcription starting site. Similarly, as reported in Figure 4D, increasing levels of c-myb caused a dose-dependent increase in LMO2 (−175/+439) promoter-driven luciferase expression while not affecting the reporter gene expression for pXP1LMO2 (−110/+439). Thus, c-myb–driven transactivation of LMO2 expression depends on the binding of c-myb to a human LMO2 promoter site localized between 175 and 110 bps upstream from the transcription starting site.
In conclusion, ChIP and luciferase assay data collectively demonstrated that c-myb is able to bind to KLF1 and LMO2 promoters in vivo and to transactivate their expression.
c-myb silencing in primary myeloblasts
To get further insights into the role of c-myb during the terminal differentiation of hematopoietic precursors, we silenced its expression in human primary CD14− myeloblasts, which maintain a granulo-monocyte differentiation bipotentiality.14
c-myb siRNAs were transfected at days 1-3 after the CD14− myeloblasts purification (see timing flowchart in supplemental Figure 6A) based on the observation that this timing represented a phase of phisiological increase in c-myb expression in myeloblasts (supplemental Figure 6B-C).
In a set of 5 independent experiments, CD14− cells were transfected with the same mixture of 3 siRNAs targeting MYB mRNA already described for CD34+ cells (supplemental Table 1).
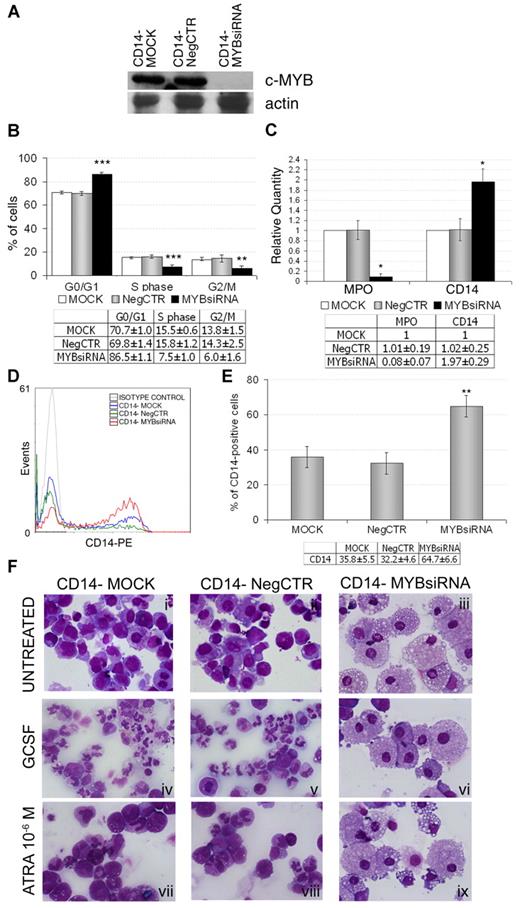
Our data showed that c-myb–silenced myeloblasts (Figure 5A) were blocked in the G1 phase of the cell cycle at 24 and 48 hours after nucleofection (Figure 5B and supplemental Figure 7) and subsequently were forced toward macrophage differentiation, as demonstrated by the strong down-regulation of MPO mRNA and the up-regulation of CD14 expression (Figure 5C). Flow cytometric analysis at day 4 after nucleofection confirmed the CD14 protein expression pattern (Figure 5D-E). Moreover, morphological evaluation of May-Grunwald-Giemsa–stained cytospins showed a significant increase of the macrophage fraction compared with control samples (Figure 5Fi-iii). Indeed, c-myb–silenced CD14− cells differentiate to macrophage even after treatment with GCSF 25 ng/mL (Figure 5Fiv-vi) or with ATRA 10−6M (Figure 5Fvii-ix) for 10 and 3 days, respectively, starting 24 hours after nucleofection, demonstrating that c-myb knockdown strongly impairs the ability of myeloblasts to differentiate to granulocytes.
c-myb silencing effects on CD14− myeloblasts differentiation. (A) Western blot analysis of c-myb expression 24 hours after the last nucleofection. (B) Results of statistical analysis on the percentage of cells in the different cell-cycle phases performed by PI staining 24 hours after nucleofection (mean ± 2 SEM; n = 5). (C) qRT-PCR data (mean ± 2 SEM; n = 3). (D-E) Flow cytometry analysis of CD14 at day 4 after nucleofection: (D) histogram showing a representative experiment and (E) statistical analysis (mean ± 2 SEM; n = 5). *P ≤ .05, **P ≤ .01, and ***P ≤ .001 vs MOCK and NegCTR. Error bars represent SD. (F) Morphological analysis of May-Grunwald-Giemsa–stained cytospins at day 4 after nucleofection (ie, 3 days after ATRA treatment) for both untreated (i-iii) and ATRA-treated (vii-ix) myeloblasts and at day 11 (ie, after 10 days of GCSF treatment) for GCSF-treated cells (iv-vi). The images were captured by Axioskop 40 microscope, by means of AxioCam HRc Digital Camera and Axiovision software 3.1 (all Carl Zeiss MicroImaging Inc). The images were then processed with Adobe Photoshop 7.0 software. Original magnification ×630. n = number of experiments.
c-myb silencing effects on CD14− myeloblasts differentiation. (A) Western blot analysis of c-myb expression 24 hours after the last nucleofection. (B) Results of statistical analysis on the percentage of cells in the different cell-cycle phases performed by PI staining 24 hours after nucleofection (mean ± 2 SEM; n = 5). (C) qRT-PCR data (mean ± 2 SEM; n = 3). (D-E) Flow cytometry analysis of CD14 at day 4 after nucleofection: (D) histogram showing a representative experiment and (E) statistical analysis (mean ± 2 SEM; n = 5). *P ≤ .05, **P ≤ .01, and ***P ≤ .001 vs MOCK and NegCTR. Error bars represent SD. (F) Morphological analysis of May-Grunwald-Giemsa–stained cytospins at day 4 after nucleofection (ie, 3 days after ATRA treatment) for both untreated (i-iii) and ATRA-treated (vii-ix) myeloblasts and at day 11 (ie, after 10 days of GCSF treatment) for GCSF-treated cells (iv-vi). The images were captured by Axioskop 40 microscope, by means of AxioCam HRc Digital Camera and Axiovision software 3.1 (all Carl Zeiss MicroImaging Inc). The images were then processed with Adobe Photoshop 7.0 software. Original magnification ×630. n = number of experiments.
GEP of c-myb silenced myeloblasts and identification of granulocyte c-myb target genes by ChIP and luciferase reporter assay
Because GEP of Myb-silenced CD34+ stem/progenitor cells did not highlight any master regulators of granulocyte commitment, to identify new putative Myb targets involved in granulocyte differentiation, we profiled the gene expression changes in Myb-silenced myeloblasts by Affymetrix HGU133A GeneChip microarray. GEP was performed 24 hours after nucleofection, because this timing showed the strongest down-regulation of c-myb protein expression (Figure 5A). Both MOCK and NegCTR CD14− samples were used as controls to select genes increased and decreased in MYBsiRNA CD14− cells.
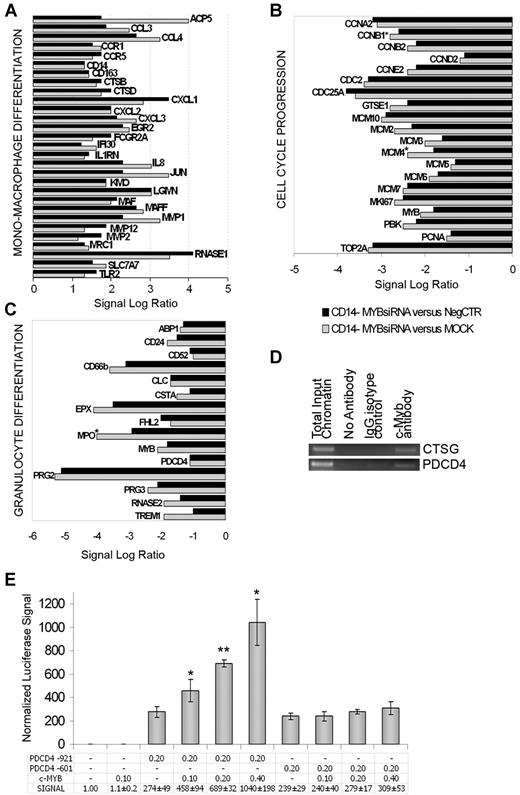
As shown in Figure 6A, c-myb silencing in myeloblasts caused a strong induction of mono-macrophage-specific markers (CD14 and CD163) and receptors (MRC1 and TLR2), typical monocyte granule proteins (CTSB and RNASE1) and metalloproteinases (MMP2 and MMP12), together with the c-MAF23,24 transcription factor, as already highlighted by Myb-silenced CD34+ cells GEP data (Figure 3C).
c-myb silencing effects on CD14− myeloblasts gene expression profile and validation of PDCD4 as c-myb direct target gene. Microarrays data of (A) mono-macrophage, (B) cell-cycle–related, and (C) granulocyte genes. * indicates genes already reported as c-myb targets. (D) PDCD4 promoter-specific PCR performed in a representative ChIP experiment. CTSG promoter-specific PCR is shown as a positive control for immunoprecipitation. ChIP and PCRs were performed in triplicate. (E) c-myb transactivation of PDCD4 promoter-driven luciferase expression. The amounts (in micrograms) of cotransfected plasmids are reported. Luciferase levels (mean ± 2 SEM;n = 3) are normalized by setting the empty pXP1/pCMV6XL5 vectors-transfected sample as = 1. Error bars represent SD. *P ≤ .05 and **P ≤ .01 vs pXP1PDCD4-921+empty pCMV6XL5-transfected sample. n = number of experiments.
c-myb silencing effects on CD14− myeloblasts gene expression profile and validation of PDCD4 as c-myb direct target gene. Microarrays data of (A) mono-macrophage, (B) cell-cycle–related, and (C) granulocyte genes. * indicates genes already reported as c-myb targets. (D) PDCD4 promoter-specific PCR performed in a representative ChIP experiment. CTSG promoter-specific PCR is shown as a positive control for immunoprecipitation. ChIP and PCRs were performed in triplicate. (E) c-myb transactivation of PDCD4 promoter-driven luciferase expression. The amounts (in micrograms) of cotransfected plasmids are reported. Luciferase levels (mean ± 2 SEM;n = 3) are normalized by setting the empty pXP1/pCMV6XL5 vectors-transfected sample as = 1. Error bars represent SD. *P ≤ .05 and **P ≤ .01 vs pXP1PDCD4-921+empty pCMV6XL5-transfected sample. n = number of experiments.
Microarray data also showed the down-regulation of genes involved in cell-cycle progression (cyclins A2, B1, B2, D2 and E2, and CDC2) and DNA replication (minichromosome maintenance [MCM] family members; Figure 6B). Similarly, several granulocyte-specific genes coding for neutrophils granule proteins (MPO) or markers (CD24, CD52 and CD66b) were decreased in c-myb–silenced myeloblasts (Figure 6C). Among down-regulated genes, Four and a Half LIM domain protein 2 (FHL2), whose overexpression in bone marrow cells enhances granulocyte differentiation,30 and programmed cell death 4 (PDCD4), which is up-regulated by several granulocyte differentiation inducers in both CD34+ cells and human promyelocytic leukemia cell lines,31 were also detected (Figure 6C).
Next, to identify new Myb target genes involved in granulocyte differentiation, we selected from microarray data FHL2 and PDCD4, down-regulated in Myb-silenced myeloblasts (Figure 6C), and ZFP36L2, down-regulated in Myb-silenced CD34+ cells (data not shown), whose knockout has been recently reported to impair granulocyte differentiation.32
Next, as already reported for the other putative Myb target genes, we identified potential cis-elements in FHL2, PDCD4, and ZFP36L2 promoter regions (supplemental Table 3) and screened them by ChIP (see supplemental Methods).
ChIP in highly proliferating c-myb–expressing myeloblasts demonstrated the binding of endogenous c-myb to PDCD4, because an enrichment of the PDCD4 promoter region in c-myb antibody-immunoprecipitated sample respect to controls was detected by PCR (Figure 6D). Conversely, the binding of c-myb to the promoter regions of FHL2 in myeloblasts and ZFP36L2 in CD34+ cells was not detected by ChIP (data not shown).
Afterward, we assayed c-myb ability to transactivate the PDCD4 promoter-driven expression of the firefly luciferase reporter gene (see supplemental Methods). Our data demonstrated that increasing levels of c-myb protein are able to transactivate PDCD4 promoter-driven luciferase expression in HEK293T cells. In particular, c-myb transactivating activity is due to the binding to the portion of PDCD4 promoter between 921 and 601 bps upstream the transcription starting site, because c-myb did not affect luciferase expression levels for pXP1PDCD4 (−601/+7) plasmid (Figure 6E).
Collectively, ChIP and Luciferase assay data demonstrated that c-myb binds PDCD4 promoter in vivo and transactivates its expression.
Rescue of KLF1 and LMO2 expression in Myb-silenced CD34+ cells
Finally, we focused our attention on the role of KLF1 and LMO2 transcription factors, which we identified as new c-myb targets, in mediating the effects of c-myb on CD34+ stem/progenitor cells differentiation. Thus, given the well-known role of these transcription factors in erythroid differentiation,22 we determined whether constitutive KLF1 or LMO2 expression could prevent the impairment of erythropoiesis in Myb-silenced CD34+ cells. For this purpose, human CD34+ cells were transduced with a retroviral vector directing the expression of either KLF1 (LKLF1IDN) or LMO2 (LLMO2IDN) and purified by immunomagnetic procedure (see timing flowchart reported in Figure 7A and supplemental Methods). Cells were then electroporated to silence c-myb. Forty-eight hours after the last of 3 nucleofection cycles, cells were assayed by qRT-PCR for c-myb, KLF1, and LMO2 expression (Figure 7B), and seeded in serum-free liquid culture.
Rescue of KLF1 and LMO2 expression in Myb-silenced CD34+ cells. (A) Flowchart reporting the experiment timing (expressed in days) after CB-derived CD34+ stem/progenitor cells purification (in the upper part) and after the last of 3 nucleofection cycles (reported as postnucleofection on the lower part). (B) qRT-PCR analysis of (i) c-myb, (ii) KLF1, and (iii) LMO2 expression 48 hours after nucleofection. For each transcript, data are reported as Relative Quantity (RQ) of the mRNA in LXIDN MYBsiRNA (transduced with the empty retroviral vector and transfected with the c-myb siRNA), LKLF1IDN NegCTR, LLMO2IDN NegCTR (transduced with the retroviral vector for the overexpression of KLF1 and LMO2, respectively, and transfected with the nontargeting siRNA), LKLF1IDN MYBsiRNA and LLMO2IDN MYBsiRNA (transduced with the retroviral vector for the overexpression of KLF1 and LMO2, respectively, and transfected with the c-myb siRNA) respect to the LXIDN NegCTR sample (transduced with the empty retroviral vector and transfected with the nontargeting siRNA), which was set as calibrator. (iv) Table summarizing c-myb, KLF1, and LMO2 expression data (mean ± 2 SEM; n = 3). Error bars in the graphs represent SD. *P ≤ .05 and **P ≤ .01 vs LXIDN NegCTR sample. (C) Flow cytometric analysis of GPA and CD41 markers at day 7 after nucleofection after liquid culture with a multilineage cocktail of cytokines (i,ii) or with a cocktail of EPO and SCF, supporting the erythroid differentiation (iii-iv). Flow cytometry data (expressed as percentages of GPA- and CD41-positive cells) are reported in the graphs (i) and (iii) as mean ± SD and are summarized in the tables in panels ii and iv, respectively, as mean ± 2SEM. Results come from 3 independent experiments. *P ≤ .05 vs LXIDN MYBsiRNA sample. (D) Morphological analysis of May-Grunwald-Giemsa–stained cytospins after both liquid multilineage (day 18, i-iv) (C) and liquid unilineage erythroid (day 13, vi-x) culture post-CD34+ cells purification. The images were captured by the microscope Axioskop 40 microscope by means of AxioCam HRc Digital Camera and Axiovision software 3.1 (all Carl Zeiss MicroImaging Inc). The images were then processed with Adobe Photoshop 7.0 software. Original magnification ×630. n = number of experiments.
Rescue of KLF1 and LMO2 expression in Myb-silenced CD34+ cells. (A) Flowchart reporting the experiment timing (expressed in days) after CB-derived CD34+ stem/progenitor cells purification (in the upper part) and after the last of 3 nucleofection cycles (reported as postnucleofection on the lower part). (B) qRT-PCR analysis of (i) c-myb, (ii) KLF1, and (iii) LMO2 expression 48 hours after nucleofection. For each transcript, data are reported as Relative Quantity (RQ) of the mRNA in LXIDN MYBsiRNA (transduced with the empty retroviral vector and transfected with the c-myb siRNA), LKLF1IDN NegCTR, LLMO2IDN NegCTR (transduced with the retroviral vector for the overexpression of KLF1 and LMO2, respectively, and transfected with the nontargeting siRNA), LKLF1IDN MYBsiRNA and LLMO2IDN MYBsiRNA (transduced with the retroviral vector for the overexpression of KLF1 and LMO2, respectively, and transfected with the c-myb siRNA) respect to the LXIDN NegCTR sample (transduced with the empty retroviral vector and transfected with the nontargeting siRNA), which was set as calibrator. (iv) Table summarizing c-myb, KLF1, and LMO2 expression data (mean ± 2 SEM; n = 3). Error bars in the graphs represent SD. *P ≤ .05 and **P ≤ .01 vs LXIDN NegCTR sample. (C) Flow cytometric analysis of GPA and CD41 markers at day 7 after nucleofection after liquid culture with a multilineage cocktail of cytokines (i,ii) or with a cocktail of EPO and SCF, supporting the erythroid differentiation (iii-iv). Flow cytometry data (expressed as percentages of GPA- and CD41-positive cells) are reported in the graphs (i) and (iii) as mean ± SD and are summarized in the tables in panels ii and iv, respectively, as mean ± 2SEM. Results come from 3 independent experiments. *P ≤ .05 vs LXIDN MYBsiRNA sample. (D) Morphological analysis of May-Grunwald-Giemsa–stained cytospins after both liquid multilineage (day 18, i-iv) (C) and liquid unilineage erythroid (day 13, vi-x) culture post-CD34+ cells purification. The images were captured by the microscope Axioskop 40 microscope by means of AxioCam HRc Digital Camera and Axiovision software 3.1 (all Carl Zeiss MicroImaging Inc). The images were then processed with Adobe Photoshop 7.0 software. Original magnification ×630. n = number of experiments.
Flow cytometric analysis of lineage differentiation markers performed on liquid multilineage culture at day 13 post-CD34+ cells purification (ie, 7 days after nucleofection, Figure 7A) showed an increase in the erythroid (GPA-positive) fraction at the expense of the megakaryocyte (CD41-positive) population in KLF1-overexpressing Myb-silenced (LKLF1IDN MYBsiRNA, 13.8% ± 1.9% GPA-positive cells, 45.8% ± 5.9% CD41-positive cells) versus Myb-silenced cells (LXIDN MYBsiRNA, 8.4% ± 1.8% GPA-positive cells, 57.1% ± 4.8% CD41-positive; Figure 7Ci-ii). In agreement with flow cytometry, morphological analysis at day 18 highlighted a significant rescue of erythropoiesis, at the expense of megakaryocytopoiesis, in LKLF1IDN MYBsiRNA versus LXIDN MYBsiRNA sample (Figure 7Di-iv). Indeed, the rescue of erythropoiesis was even more evident after 5 days of erythroid unilineage culture (ie, at day 13 post-CD34+ cells purification, Figure 7Ciii-iv,Dv-viii: GPA-positive cells: 60.9% ± 11.7% in LKLF1IDN MYBsiRNA versus 41.1% ± 3.6% in LXIDN MYBsiRNA). Similarly, flow cytometric analysis showed a partial rescue of erythropoiesis by LMO2 overexpression in MYB-silenced cells (GPA-positive cells: 58.3% ± 2.1% in LLMO2IDN MYBsiRNA, 41.1% ± 3.6% in LXIDN MYBsiRNA, Figure 7Ciii-iv) after 5 days of erythroid unilineage culture. Morphological analysis further supported flow cytometric data, showing an increase in the erythroid cell fraction after LMO2 overexpression in Myb-silenced cells (LLMO2IDN MYBsiRNA versus LXIDN MYBsiRNA, Figure 7Dv-vi,ix-x).
Collectively, our data demonstrated that both KLF1 and LMO2 overexpression were able to retrieve, at least in part, the erythroid differentiation impaired by Myb silencing. Moreover, KLF1 overexpression was able to partially rescue the erythroid versus megakaryocyte differentiation unbalance in Myb-silenced cells.
Discussion
In this paper, we investigated the role of c-myb in human primary CD34+ stem/progenitor cells commitment by using the siRNA nucleofection system to transiently knockdown c-myb expression.
Our data collectively demonstrate that c-myb is essential for the erythroid and granulocyte differentiation of human primary hematopoietic CD34+ stem/progenitor cells. In fact, c-myb silencing in CB-derived CD34+ cells: (1) causes a cell-cycle arrest that strongly impairs their clonogenic ability (Figure 1B and supplemental Figure 4A) and (2) forces their commitment toward the mono-macrophage and megakaryocyte lineages, whereas the erythroid and granulocyte ones are strongly impaired (Figures 1C-E,2).
Similarly, in human primary myeloblasts, which are precursors endowed with mono/granulocyte differentiation capacity,14 c-myb silencing leads to a massive cell-cycle arrest and an induction of mono-macrophage differentiation together with a strong impairment of granulocyte differentiation, even after GCSF or ATRA treatment (Figure 5).
Gene expression profiling of Myb-silenced CD34+ cells evidenced the modulation of key regulators of cell cycle and erythroid, megakaryocyte and mono-macrophage differentiation, providing a further molecular support to our functional data (Figure 3). Moreover, computational prediction of c-myb binding sites in the promoter regions of differentially expressed genes identified new potential c-myb targets, such as the erythroid transcription factors KLF1 and LMO222 (supplemental Table 3). Indeed, ChIP and luciferase assay demonstrated that c-myb is able to bind KLF1 and LMO2 promoters and to transactivate their expression (Figure 4).
LMO2 (RBTN2) encodes a LIM domain-containing protein that is essential for erythropoiesis as LMO2−/− mice die in utero due to failure of embryonic yolk sac erythropoiesis.33 Similarly, KLF1 (EKLF) transcription factor plays a key role in definitive erythropoiesis, since KLF1−/− mice die because of severe anemia at the time of switch to adult β-globin expression.22 Moreover, recent works demonstrated that KLF1 also negatively interferes with megakaryocytopoiesis34 by repressing FLI1 expression.35
Thus, the role of c-myb in erythropoiesis could be explained by the transactivation of KLF1 and LMO2, which are master regulators of erythroid differentiation,22 together with GATA1, already reported as a c-myb target.9 Consistently, the retroviral vector-mediated overexpression of either KLF1 or LMO2 in Myb-silenced CD34+ cells partially rescued the erythroid differentiation. Noteworthy, KLF1 overexpression was also able to negatively interfere with the megakaryocyte differentiation, enhanced by c-myb silencing (Figure 7), demonstrating that the induction of KLF1 expression is a relevant mechanism in Myb-mediated repression of megakaryocytopoiesis.
In agreement with our data, different c-myb knockdown mouse models display anemia and megakaryocytosis.13,36 Indeed, inactivating mutations in both c-myb37,38 or the transcriptional coregulator p30039 genes result in TPO-independent expansion of megakaryocytopoiesis in Mpl−/− thrombocytopenic mice, suggesting that the c-myb/p300 transcriptional complex plays a repressive role in megakaryocyte differentiation. However, till now c-myb target genes responsible for these effects have remained largely unknown. In this paper for the first time we shed some light on the molecular mechanisms responsible for c-myb dependent repression of megakaryocytopoiesis by identifying KLF1 as a c-myb transcriptional target.
It is noteworthy that, although c-myb silencing modulates the expression of transcription factors involved in erythroid (LMO2, KLF122 ), mono-macrophage (c-Maf,23,24 MafB19 ), and megakaryocyte (FLI125 ) differentiation, master regulators of granulocyte differentiation, that is, CCAAT/enhancer binding protein α (C/EPBα; Figure 3E), β, ϵ, and PU.1 (data not shown), are not affected, suggesting that, even if usually referred as a master regulator of the granulocytopoiesis,40 c-myb does not prime the granulocyte commitment, but plays a pivotal role in later stages of differentiation by transactivating the expression of PDCD4 (Figure 6C-E) or genes coding for granules proteins like MPO,11 ELA210 and CTSG.29
Collectively, our data demonstrated that c-myb is a key regulator of human primary hematopoietic stem/progenitor cells lineage commitment, by enhancing erythropoiesis at the expense of megakaryocytopoiesis. In particular, we identified KLF1 and LMO2 transactivation as the molecular mechanism through which c-myb regulates erythroid versus megakaryocyte lineage fate decision. Indeed, c-myb–driven transactivation of KLF1 and LMO2 expression enhances erythropoiesis, while in turn KLF1 is able to repress megakaryocytopoiesis.35
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This paper is dedicated to the memory of Stefano Ferrari, Professor of Biochemistry at University of Modena and Reggio Emilia.
This work was supported by the Italian Ministry of University and Research (Progetti di Ricerca di Interesse Nazionale, PRIN) 2006 (contract no. 2006057308), Regione Emilia Romagna (area 1b, “Medicina Rigenerativa”) and by Associazione Italiana per la Ricerca sul Cancro (AIRC) 2007. E.B. is the recipient of a fellowship from AIL (Italian Association Against Leukemia). S.S. is the recipient of a fellowship from FIRC (Italian Foundation for Cancer Research). E.T. is a BioPharmaNet fellow.
Authorship
Contribution: E.B. performed silencing and rescue experiments and biological effects characterization; R.Z. performed microarray analysis and luciferase assays; S.S. performed chromatin immunoprecipitation; E. Tenedini performed microarray analysis; R.N. performed luciferase assays; E. Tagliafico performed computational analysis of putative c-myb targets; and R.M. and S.F. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rossella Manfredini, Department of Biomedical Sciences, Biological Chemistry Section, University of Modena and Reggio Emilia, via Campi 287, 41100 Modena, Italy; e-mail: manfredini.rossella@unimore.it