Abstract
Existing anticoagulants effectively inhibit the activity of coagulation factors of the extrinsic and common pathway but have substantial limitations and can cause severe bleeding complications. Here we describe a novel therapeutic approach to thrombosis treatment. We have developed and characterized the efficacy and safety of selective second-generation antisense oligonucleotides (ASOs) targeting coagulation factor XI (FXI), a member of the intrinsic coagulation pathway. Systemic treatment of mice with FXI ASO led to a potent, specific, and dose-dependent reduction of FXI mRNA levels in the liver with corresponding reductions in plasma levels of FXI protein and activity. FXIASO treatment produced potent, dose-dependent antithrombotic activity in various venous and arterial thrombosis models, comparable with warfarin or enoxaparin. However, unlike warfarin or enoxaparin, FXI inhibition did not cause bleeding. Coadministration of FXI ASO with enoxaparin or the antiplatelet drug clopidogrel produced improved antithrombotic activity without increased bleeding. Finally, plasma-derived FXI concentrate was shown to effectively and rapidly reverse the anticoagulant effect of FXI antisense therapy. These results support the concept that inhibition of FXI through antisense therapy might serve as a new and effective strategy for the treatment and prevention of venous thromboembolism with improved specificity and safety.
Introduction
Precise regulation of blood coagulation is critical for normal hemostasis. Vessel wall injury-induced exposure of extravascular tissue factor to circulating coagulation factor VIIa (FVIIa) activates the extrinsic coagulation cascade, which, through subsequent activation of coagulation FIX, FX, and FII (prothrombin), ultimately produces a fibrin and platelet cross-linked clot at the site of injury.1,2 However, in pathogenic conditions, such as thrombosis, additional coagulation pathways are activated that mediate both the propagation and the maintenance of the growing thrombi.3
Several lines of evidence suggest that the intrinsic coagulation pathway, and in particular FXI, contributes to the pathogenesis of thrombosis. In humans, increased plasma levels of FXI have been associated with deep vein thrombosis,4 ischemic stroke,5 and myocardial infarction.6 In addition, the incidence of ischemic stroke seems to be significantly lower in FXI deficiency compared with the general population.7 Furthermore, FXI deficiency has been shown to protect mice from experimentally induced thrombosis or stroke and to reduce occlusive thrombus formation in venous and arterial models of thrombosis.8–11 In addition, pharmacologic inhibition of FXI has also been shown to promote antithrombotic activity in various animal models and under different conditions.12–14
In contrast to its important role in the pathogenesis of thrombosis, FXI appears to play a relatively minor role in normal hemostasis. FXI activation is thought to play a principal function in the propagation and stabilization of a growing thrombus by virtue of a positive feedback amplification loop between thrombin and FXI itself and by activation of thrombin-activatable fibrinolysis inhibitor, which protects the fibrin clot against fibrinolysis.3,15,16 Consistent with this notion, only a minority of subjects with severe FXI deficiency present with bleeding tendencies, which are generally mild to moderate and typically only occur under extreme circumstances.17–19 In contrast, deficiencies in components of either the extrinsic or common pathways are consistently associated with severe bleeding or even incompatible with life.20–23 Currently available anticoagulant agents, such as vitamin K antagonists (including warfarin or coumadin), (low-molecular-weight) heparins (eg, enoxaparin or Lovenox) and small-molecule FXa or FIIa inhibitors, all target components of the extrinsic or common pathways and can be associated with serious bleeding complications.24–27 Taken together, these findings suggest that targeting intrinsic coagulation FXI may be an attractive therapeutic approach for identifying novel antithrombotic agents with greater safety.
To further define the role of FXI in thrombosis and hemostasis, we evaluated a previously unexplored therapeutic modality for coagulation factor inhibition. Second-generation antisense oligonucleotides (ASOs) selectively depleting FXI expression in the liver were developed and the pharmacologic profile of FXI ASO treatment in mice, both alone and in combination with existing standard of care antithrombotic agents, was characterized.
Antisense therapy is based on basepair hybridization through which ASOs selectively bind to their complementary mRNA target.28 This binding typically results in the selective and catalytic degradation of the targeted mRNA by a mechanism involving the nuclease RNAse H28 and leads to a corresponding reduction in target protein levels. Antisense technology is a potentially attractive therapeutic modality for the development of FXI inhibitors for several reasons. First, FXI is synthesized primarily in the liver, which is one of most sensitive tissues for ASO therapy.28,29 Several second-generation ASOs designed against hepatic targets are currently in clinical development and have demonstrated significant clinical activity and safety, for example in hyperlipidemia.28,30 Furthermore, second-generation ASOs exhibit dose-linear and predictable pharmacokinetics in humans,30 which can simplify dose selection and help to ensure patient safety. Third, because of prolonged tissue elimination half-lives, second-generation ASOs can be administered relatively infrequently (once weekly or even less frequent), which should provide acceptable patient convenience.30 Fourth, compared with other anticoagulant modalities, including small-molecule inhibitors and the natural product anticoagulants, such as warfarin, antisense inhibitors offer a high degree of target selectivity, which should provide greater patient safety. Finally, because second-generation ASOs are not substrates for cytochrome P450 enzymes, which are involved in the metabolism of several drug classes in humans and because of their high specificity, antisense drugs avoid common drug-drug interactions, which frequently limit therapeutic applications, thereby making ASO drugs particularly well suited for combination therapy. The results of the current study support the conclusion that FXI antisense therapy could serve as an effective, convenient, and safe alternative for the treatment and prevention of venous and arterial thrombosis in humans.
Methods
Oligonucleotides
All oligonucleotides were 20 nucleotides in length and chemically modified with phosphorothioate in the backbone and 2′-O-methoxyethyl on the wings with a central deoxy gap (“5-10-5” design). Oligonucleotides were synthesized using an Applied Biosystems 380B automated DNA synthesizer (PerkinElmer Life and Analytical Sciences-Applied Biosystems) and purified as previously described.31 To identify a potent FXI ASO for testing in mice, ASOs were designed and tested in primary mouse hepatocytes for their relative ability to suppress FXI mRNA levels. From these experiments, an optimized FXI ASO was selected for evaluation in mice.
Animals and oligonucleotide dosing
Eight-week-old male BALB/c and C57BL/6 mice (Charles River Laboratories) were treated according to the indicated treatment schedules. The animals were housed in micro-isolator cages on a constant 12-hour light-dark cycle with controlled temperature and humidity and were given access to food and water ad libitum. All animal husbandry and procedures performed at Isis and Academic Medical Center (Amsterdam, The Netherlands) were approved by the Institutional Animal Care and Use Committee and the Dutch Animal Experiments Committee, respectively.
Preparation of platelet-poor plasma
Blood samples were collected under anesthesia by cardiac puncture. Blood was quickly withdrawn from the heart using a 1-mL plastic syringe with a 27-G needle and collected into a final ratio of 9 parts of whole blood to one part of 3.2% sodium citrate. Mice were killed by cervical dislocation. Blood samples were immediately mixed by tapping and inverting the tube 5 times to ensure proper anticoagulation and then centrifuged for 10 minutes at 600g at room temperature. After removal of the platelet-rich plasma, the samples were centrifuged a second time for 5 minutes. Platelet poor plasma was stored at −80°C until assays were performed.
FXI-deficient plasma
The FXI-deficient plasma is a commercial preparation from a human patient with a congenital deficiency supplied by George King Bio-Medical.
Measurement of hepatic FXI mRNA
Mouse liver was homogenized in RLT buffer. Total RNA was prepared according to the RNAeasy mini kit (QIAGEN). The quantitative real-time (RT-PCR) was done by ABI Prism 7700 sequence detector (Applied Biosystems). The sequences of primers and probe used for mouse FXI were: ACATGACAGGCGCGATCTCT (forward), TCTAGGTTCACGTACACATCTTTGC (reverse), and TTCCTTCAAGCAATGCCCTCAGCAATX (probe) (Integrated DNA Technologies). The amount of each FXI mRNA was normalized to the amount of total RNA determined by Ribogreen. RNase protection assay (RPA) was performed using the RPA kit (BD Biosciences PharMingen) according to the manufacturer's instructions.
aPTT and PT
Activated partial thromboplastin time (aPTT) and prothrombin time (PT) were measured using the ACL 1000 coagulation analyzer (IL Instrumentation; Beckman Coulter). aPTT test of mouse platelet-poor plasma was initiated using ellagic acid mixture (APTT-XL, Pacific Hemostasis; Fisher Diagnostics) and calcium chloride, whereas initiation of the PT assay in mouse platelet-poor plasma was performed by adding thromboplastin. aPTT and PT measurements of saline-treated mice were pooled and served as baseline reference. aPTT and PT ratios were calculated by dividing measured values by these baseline values.
FXI and FXII activity
To measure plasma FXI and FXII activity, mouse plasma was 40 times diluted in human FXI- or FXII-deficient plasma followed by aPTT measurement, using pooled values from normal mice as reference.
FeCl3-induced IVC thrombosis
Antithrombotic activity was studied using a well-established ferric chloride (FeCl3) induced inferior vena cava (IVC) thrombosis model, previously described by others.10,32 Total mRNA was purified from liver and vena cava tissue samples, and analyzed by RT-PCR for FXI and platelet factor 4 (PF4) mRNA levels, respectively. PF4 mRNA levels were used to determine the effect of treatment on platelet deposition as a measure of thrombus formation.33 PF4 mRNA levels in the IVC tissue exposed to FeCl3 were normalized to nonexposed vena cava tissue.
FeCl3-induced mesenteric vein thrombosis
Male BALB/c mice were maintained under isoflurane (2.5% inhalant). Fluorescently labeled mouse platelets were injected intravenously followed by a 3-minute exposure of the mesenteric vein to a 10% FeCl3 solution. Using intravital microscopy, thrombus formation in the mesenteric vein was continuously recorded on video for a maximum of 40 minutes or until total occlusion. Time to formation of 30-μm thrombi and full occlusion was recorded.
Stenosis-induced IVC thrombosis
For stenosis-induced IVC thrombosis, a model, also known as the “St Tomas model,” which uses a combination of reduced blood flow and endothelial damage, was applied.34 In short, the IVC of male BALB/c mice, anesthetized with 2.5% inhalant isoflurane, was exposed and mobilized via a midline abdominal incision below the left renal vein and separated from the abdominal aorta by blunt dissection. A 6-0 silk tie (Ethicon) was placed behind the vessel just below the left renal vein, and 2 silk sutures (4-0; Ethicon) were placed longitudinally over the IVC and tied over the top. Finally, the 4-0 sutures were removed. Next, 2 neurovascular surgical clips (Braun Medical) were applied at 2 separate positions below the ligation for 20 seconds each. Finally, the bowel was placed back into the abdominal cavity and the laparotomy was closed. Twenty-four hours after thrombosis induction, thrombi that had formed in the IVC were collected and fixed in 10% formalin for more than 24 hours and then photographed and weighed. Liver total mRNA was prepared and analyzed for FXI mRNA levels by RT-PCR.
FeCl3-induced aortic thrombosis
Arterial antithrombotic activity of FXI ASO treatment in mice was studied using a method similar to the FeCl3-induced IVC model described in “FeCl3-induced IVC thrombosis” with this difference that, in this model not the IVC, the descending aorta was exposed to 10% FeCl3.Thirty minutes after initial FeCl3 exposure, 2 small hemostats were used to clamp both the descending aorta and IVC. The descending aorta was cut and peeled away from the IVC, and the extracted part was processed similar to the IVC tissue described previously.
Tail vein bleeding assay
A mouse tail bleeding assay was used as described.32 Bleeding was assessed by total blood loss or weight difference before and after bleeding within a 40-minute period, or by bleeding time, determined as time until the bleeding stopped with a maximum recording time of 10 minutes.
Hepatectomy surgical bleeding model
Bleeding risk of FXI antisense treatment after a surgical procedure was assessed using a previously described partial hepatectomy model.35 FXI or FII ASO was dosed subcutaneously at 20 mg/kg twice/week for 2.5 weeks, and low-molecular-weight heparin (Lovenox) was dosed subcutaneously at 50 mg/kg once/day on 3 consecutive days. Two days after ASO treatment or 2 hours after Lovenox treatment, male BALB/c mice were anesthetized with ketamine (100 mg/kg)/xylazine (10 mg/kg) intraperitoneally and the liver was exposed via a midline abdominal incision. After ligation and resection of the lower left lobe of the liver, the body cavity and incision were closed by suture and wound clips, respectively. Four hours later, the health of the mice was evaluated by recording survival and cage conditions, and blood was drawn for red blood cell count, hemoglobin, and hematocrit analysis using a clinical analyzer.
FXI ASO reversal studies
Plasma-derived FXI (Hemoleven; LFB) was tested for its ability to reverse FXI ASO anticoagulant activity in mice. Male BALB/c mice were treated with saline or FXI ASO (40 mg/kg twice weekly for 3 weeks). Two days after final dosing, half of the FXI ASO-treated mice received Hemoleven (10 μg) intravenously once daily for 2 consecutive days. aPTT was measured 24 hours after the final dose of Hemoleven.
Anticoagulant coadministration studies
Male BALB/c mice were treated with different doses of Lovenox or the antiplatelet drug clopidogrel either alone or in combination with FXI ASO. Lovenox was injected subcutaneously once daily for 3 consecutive days, whereas clopidogrel was administered orally, with 2 loading doses on day 1 and one single dose on day 2. Measurements were performed 2 hours after dose. In both treatment groups, half of the mice additionally received FXI ASO treatment (20 mg/kg) subcutaneously twice weekly for 3 weeks. Antithrombotic activity and bleeding tendency were determined using the FeCl3-induced IVC thrombosis model (described in “FeCl3-induced IVC thrombosis”) and the tail vein bleeding assay described in the tail vein bleeding assay section. In addition, bleeding potential for clopidogrel was also determined in combination with a small-molecule FXa inhibitor36 (0.5 mg/kg daily on 2 consecutive days) 4 hours after the final dose.
Statistical analysis
Statistical comparisons were made by analysis of variance. Student t test was used for comparison of single pairs. Correction for multiple comparisons between individual groups was made using Fisher LSD post-hoc test. Data are mean plus or minus SEM. P values less than .05 were considered significant. Data were analyzed using SPSS software package for Windows, Version 14.0 (SPSS). Graphics were constructed using GraphPad Prism, Version 5 for Windows (GraphPad Software).
Results
ASO suppression of FXI levels in mice
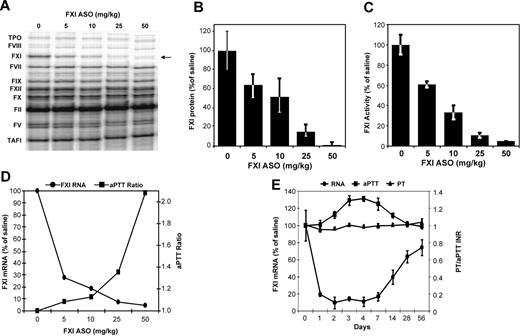
For initial characterization of FXI ASO activity in mice, we measured the effect of FXI ASO treatment on both hepatic FXI mRNA levels and nontargeted coagulation factor mRNA levels (Figure 1A), protein levels (Figure 1B), and levels of plasma FXI activity (Figure 1C). FXI ASO treatment resulted in a dose-dependent reduction of FXI mRNA levels in liver with a maximal reduction of approximately 98% observed at the highest dose tested (Figure 1D-E). FXI ASO was highly specific for FXI as all other measured coagulation factor mRNAs remained unchanged, as did the activity of another intrinsic pathway factor, FXII (not shown). Furthermore, reduction in FXI mRNA levels correlated well with a reduction in circulating FXI plasma protein and activity levels (Figure 1B-C). Next, we determined the anticoagulant effect of FXI ASO treatment in mice by measuring aPTT and PT ratios. FXI antisense therapy resulted in a dose-dependent prolongation of aPTT (Figure 1D). To characterize the kinetics of FXI ASO activity, the onset and duration of action in mice after a single subcutaneous injection (50 mg/kg) were determined by monitoring both hepatic FXI mRNA levels, aPTT and PT. FXI ASO treatment resulted in a time-dependent reduction in FXI mRNA levels with an onset of action of approximately 1 day after treatment. This activity was maintained at maximal inhibition (∼ 90%) for days 2 to 7, followed by a gradual return to basal levels between days 14 and 28 (Figure 1E). aPTT prolongation with no effect on PT correlated well with the decrease in hepatic FXI mRNA levels. aPTT started to increase by day 2 after treatment. Maximal aPTT prolongation was achieved at day 3 and was maintained until approximately day 7 when levels gradually normalized again returning to baseline by day 28 (Figure 1E). These data are consistent with other reports on the duration of action of second-generation ASOs in liver29,30 and indicate that an approximate 60% reduction in FXI mRNA levels is needed to promote anticoagulant activity in mice, as measured by aPTT prolongation.
Effect of FXI ASO treatment on hepatic FXI mRNA levels, FXI enzymatic activity, and aPTT ratio in mice. FXI ASO activity was initially characterized in normal mice by measuring the effect of FXI ASO treatment on hepatic FXI mRNA levels and plasma FXI activity levels. Because FXI has a relatively long half-life (∼ 52 hours in human plasma), a multidose regimen with doses ranging from 5 to 50 mg/kg was initially used. Male BALB/c mice were treated subcutaneously with FXI ASO twice weekly for 3 weeks at indicated doses (n = 4 per dosing group). Three days after final dosing, we measured (A) hepatic mRNA levels of FXI as well as nontargeted coagulation factors by RPA, (B) FXI plasma protein levels, (C) FXI enzymatic activity levels, and (D-E) aPTT and PT ratios. Onset and duration of hepatic FXI mRNA reduction and corresponding anticoagulation in mice in response to a single injection of FXI ASO (50 mg/kg). *P ≤ .05 compared with untreated control.
Effect of FXI ASO treatment on hepatic FXI mRNA levels, FXI enzymatic activity, and aPTT ratio in mice. FXI ASO activity was initially characterized in normal mice by measuring the effect of FXI ASO treatment on hepatic FXI mRNA levels and plasma FXI activity levels. Because FXI has a relatively long half-life (∼ 52 hours in human plasma), a multidose regimen with doses ranging from 5 to 50 mg/kg was initially used. Male BALB/c mice were treated subcutaneously with FXI ASO twice weekly for 3 weeks at indicated doses (n = 4 per dosing group). Three days after final dosing, we measured (A) hepatic mRNA levels of FXI as well as nontargeted coagulation factors by RPA, (B) FXI plasma protein levels, (C) FXI enzymatic activity levels, and (D-E) aPTT and PT ratios. Onset and duration of hepatic FXI mRNA reduction and corresponding anticoagulation in mice in response to a single injection of FXI ASO (50 mg/kg). *P ≤ .05 compared with untreated control.
Effect of FXI ASO treatment on thrombogenesis and hemostasis in mice
To study the effect of FXI inhibition on thrombus formation and hemostasis, FXI ASO treatment was evaluated in several mouse models of thrombosis and bleeding, respectively.
FeCl3-induced IVC thrombosis.
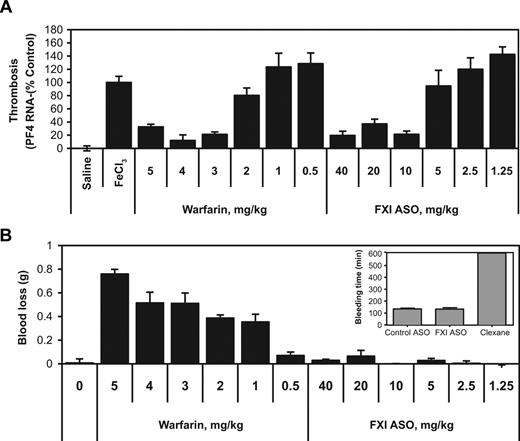
The antithrombotic activity of FXI ASO therapy was first evaluated in a FeCl3-induced IVC thrombosis model and compared with the effect of warfarin treatment. Within 2 days after the final FXI ASO injection, thrombosis was induced and PF4 mRNA levels were determined at the lesion site as a measure of thrombosis.33 Similar to the dose-dependent effects of FXI ASO treatment on hepatic FXI mRNA protein and FXI activity levels (Figure 1A-B), FXI ASO therapy also produced a dose-dependent reduction in thrombus formation with maximal effects observed at doses between 5 and 10 mg/kg (Figure 2A). Although the observed maximal antithrombotic activity of FXI ASO treatment was similar to that observed with warfarin at doses of 3 mg/kg and higher, the effects of these 2 treatment strategies on bleeding were remarkably different. As expected, warfarin treatment resulted in marked blood loss in a tail bleeding assay (Figure 2B). Moreover, the increase in bleeding caused by warfarin was observed at subefficacious doses, starting as low as 1 mg/kg. In contrast, FXI ASO treatment did not affect bleeding in mice, even at the highest doses tested. The effect of FXI ASO treatment on bleeding evaluated in the tail vein bleeding assay was also compared with that of low-molecular-weight heparin Lovenox (Figure 2B inset). Compared with control ASO treatment, FXI ASO treatment had no effect on bleeding, whereas Lovenox treatment resulted in markedly prolonged bleeding times (P < .001) and exceeded the maximally recorded time of 10 minutes in 50% of the cases (Figure 2B inset).
Effect of FXI ASO, warfarin, or Levonox therapy on thrombosis and bleeding in mice. (A) Male BALB/c mice were treated with either FXI ASO at doses ranging from 1.25 to 40 mg/kg administered subcutaneously twice weekly for 3 weeks, or warfarin, which was injected intraperitoneally once daily for a period of 6 days with the final dose injected approximately 4 hours before endpoint measurements (n = 10). Thrombosis was induced by 3-minute exposure of the IVC to a 10% FeCl3 solution and assessed by RT-PCR measurement of PF4 mRNA levels at the site of injury. (B) Effect of FXI antisense and warfarin treatment at indicated doses on tail bleeding in male BALB/c mice (n = 10). (Inset) Male C57BL/6 mice were treated with FXI ASO, control ASOs (25 mg/kg, subcutaneously injected twice/week for 3 weeks), or Lovenox (single dose of 25 mg/kg injected subcutaneously 2.5 hours before bleeding measurements), and the effects on tail bleeding were evaluated (n = 8, 8 and 6 mice per treatment group, respectively).
Effect of FXI ASO, warfarin, or Levonox therapy on thrombosis and bleeding in mice. (A) Male BALB/c mice were treated with either FXI ASO at doses ranging from 1.25 to 40 mg/kg administered subcutaneously twice weekly for 3 weeks, or warfarin, which was injected intraperitoneally once daily for a period of 6 days with the final dose injected approximately 4 hours before endpoint measurements (n = 10). Thrombosis was induced by 3-minute exposure of the IVC to a 10% FeCl3 solution and assessed by RT-PCR measurement of PF4 mRNA levels at the site of injury. (B) Effect of FXI antisense and warfarin treatment at indicated doses on tail bleeding in male BALB/c mice (n = 10). (Inset) Male C57BL/6 mice were treated with FXI ASO, control ASOs (25 mg/kg, subcutaneously injected twice/week for 3 weeks), or Lovenox (single dose of 25 mg/kg injected subcutaneously 2.5 hours before bleeding measurements), and the effects on tail bleeding were evaluated (n = 8, 8 and 6 mice per treatment group, respectively).
FeCl3-induced mesenteric vein thrombosis.
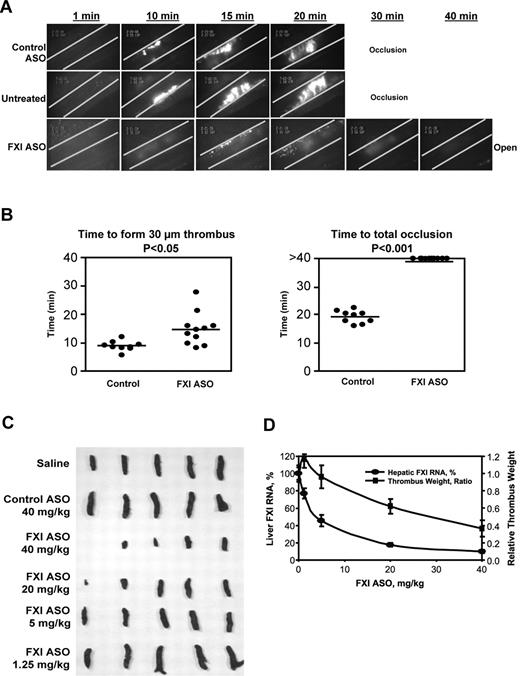
To examine the mechanisms underlying FXI ASO antithrombotic activity, we evaluated the effects of FXI ASO treatment by intravital microscopy in a FeCl3-induced mesenteric vein thrombosis model as described in “FeCl3-induced mesenteric vein thrombosis” (supplemental Video 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). As shown in Figure 3A, mice treated with saline (“untreated”) or control ASO exhibited complete mesenteric vein occlusion within 30 minutes after FeCl3 injury. In contrast, FXI ASO treatment completely prevented occlusion of the mesenteric vein up to the end of the observation period (40 minutes after FeCl3 exposure). Although small thrombi (∼ 30 μm in diameter) did initially form near the injured vessel wall in the FXI ASO treatment group (Figure 3B), this partial thrombus formation was markedly delayed in time, reduced in size, density, and stability, and eventually cleared (Figure 3A-B). Continuous video recording using the intravital microscopy system demonstrated that impairment of thrombus growth in the mesenteric vein of FXI ASO-treated mice was the result of platelet shedding with a continuous breakdown of small platelet aggregates from the growing thrombi. The small thrombi that initially had formed appeared to detach easily from the vessel wall, leading to complete clearance of the thrombotic lesion at the end of observation (supplemental Video 1).
Antithrombotic effect of FXI ASO treatment in mouse models of FeCl3-induced mesenteric vein thrombosis and stenosis-induced IVC thrombosis. Thrombus formation in male BALB/c mice treated with FXI ASO or control ASO (50 mg/kg, subcutaneously) twice weekly for 3 weeks or without treatment (“Untreated”). For testing in the mesenteric vein model, thrombosis was induced by a 3-minute exposure of the mesenteric vein to a 10% FeCl3 solution after intravenous injection of fluorescein-labeled mouse platelets. (A) Continuous recording of venous thrombus formation by intravital microscopy for a total period of 40 minutes or until total occlusion (supplemental Video 1). (B) Time to formation of a 30-μm thrombus or to total occlusion. *P ≤ .05 for FXI ASO-treated group compared with control group. For testing in the stenosis IVC model, 30 male BALB/c mice were treated with FXI ASO (dose ranging from 1.25 mg/kg to 40 mg/kg), control ASO (40 mg/kg), or saline by a twice-weekly subcutaneous injection for a period of 3 weeks. Vena cava thrombosis was induced using a model that combines reduced blood flow with endothelial damage. Twenty-four hours after thrombosis induction, thrombi that had formed in the IVC were collected, fixed in formalin, photographed, and weighed. Thrombus weight relative to saline control mice was calculated. Liver total mRNA was prepared and analyzed for FXI mRNA levels by RT-PCR. (C) The appearance of formed thrombi in the vena cava in different treatment and dosing groups. Thrombus formation in the first mouse of the FXI ASO 40-mg/kg treatment group was completely prevented. (D) Correlation of hepatic FXI mRNA levels and relative thrombus weight in mice treated with FXI ASO at indicated doses.
Antithrombotic effect of FXI ASO treatment in mouse models of FeCl3-induced mesenteric vein thrombosis and stenosis-induced IVC thrombosis. Thrombus formation in male BALB/c mice treated with FXI ASO or control ASO (50 mg/kg, subcutaneously) twice weekly for 3 weeks or without treatment (“Untreated”). For testing in the mesenteric vein model, thrombosis was induced by a 3-minute exposure of the mesenteric vein to a 10% FeCl3 solution after intravenous injection of fluorescein-labeled mouse platelets. (A) Continuous recording of venous thrombus formation by intravital microscopy for a total period of 40 minutes or until total occlusion (supplemental Video 1). (B) Time to formation of a 30-μm thrombus or to total occlusion. *P ≤ .05 for FXI ASO-treated group compared with control group. For testing in the stenosis IVC model, 30 male BALB/c mice were treated with FXI ASO (dose ranging from 1.25 mg/kg to 40 mg/kg), control ASO (40 mg/kg), or saline by a twice-weekly subcutaneous injection for a period of 3 weeks. Vena cava thrombosis was induced using a model that combines reduced blood flow with endothelial damage. Twenty-four hours after thrombosis induction, thrombi that had formed in the IVC were collected, fixed in formalin, photographed, and weighed. Thrombus weight relative to saline control mice was calculated. Liver total mRNA was prepared and analyzed for FXI mRNA levels by RT-PCR. (C) The appearance of formed thrombi in the vena cava in different treatment and dosing groups. Thrombus formation in the first mouse of the FXI ASO 40-mg/kg treatment group was completely prevented. (D) Correlation of hepatic FXI mRNA levels and relative thrombus weight in mice treated with FXI ASO at indicated doses.
Stenosis-induced IVC thrombosis
A third model that was used to further profile the antithrombotic activity of FXI ASO treatment in mice was a stenosis-induced IVC thrombosis model (“St Tomas model”), which uses a combination of reduced blood flow and endothelial damage to induce thrombosis34 (“Stenosis-induced IVC thrombosis”). FXI ASO treatment resulted in a dose-dependent reduction in thrombus formation, as was shown by a decrease in thrombus size and weight (Figure 3C-D). Furthermore, dose-dependent reduction in thrombus weight correlated well with a dose-dependent reduction in hepatic FXI mRNA levels in treated animals (Figure 3D). Finally, the antithrombotic activity of FXI ASO treatment appeared to be specific as no antithrombotic activity was observed in animals treated with control ASO (Figure 3C-D).
Partial hepatectomy surgical bleeding model.
To further assess the risk for bleeding after FXI ASO treatment, a surgical hepatectomy bleeding model was used in mice pretreated with either vehicle, FXI ASO, a prothrombin (FII) ASO, or Lovenox, as described in “Hepatectomy surgical bleeding model.” Treatment with FXI and FII ASO resulted in reductions of more than 90% of hepatic FXI and FII mRNA levels, respectively (data not shown). All animals in the various treatment groups survived the surgical procedure, except for the Lovenox and FII ASO treatment groups, where 4 and 1 animals died, respectively. General cage inspection revealed obvious bloody cage conditions in the Lovenox and FII ASO treatment groups, whereas cage conditions appeared normal in animals treated with vehicle or FXI ASO. Hematologic analysis demonstrated significantly reduced levels of red blood cell count, hemoglobin, and hematocrit levels in surviving mice treated with either Lovenox or FII ASO compared with control animals (Figure 4). In contrast, no differences were observed in any of these parameters between FXI ASO and vehicle-treated animals.
Effects of anticoagulant treatment on bleeding in a mouse hepatectomy surgical bleeding model. Mice were treated with vehicle (saline), FXI or FII ASO (20 mg/kg twice/week for 3 weeks), or Lovenox (50 mg/kg once/day on 3 consecutive days). Two days after ASO treatment or 2 hours after Lovenox treatment, animals underwent a surgical hepatectomy procedure. Four hours after incision closure, relative bleeding was determined by measuring red blood cell count, hemoglobin levels, and hematocrit values. *P ≤ .05 compared with vehicle group with surgery.
Effects of anticoagulant treatment on bleeding in a mouse hepatectomy surgical bleeding model. Mice were treated with vehicle (saline), FXI or FII ASO (20 mg/kg twice/week for 3 weeks), or Lovenox (50 mg/kg once/day on 3 consecutive days). Two days after ASO treatment or 2 hours after Lovenox treatment, animals underwent a surgical hepatectomy procedure. Four hours after incision closure, relative bleeding was determined by measuring red blood cell count, hemoglobin levels, and hematocrit values. *P ≤ .05 compared with vehicle group with surgery.
Reversal of FXI ASO activity by plasma-derived FXI concentrate
Despite the absence of bleeding associated with FXI ASO treatment, the availability of an effective and convenient antidote strategy is nonetheless desirable. To address this, we evaluated the ability of plasma-derived FXI concentrate (Hemoleven) to reverse FXI antisense-mediated effects on aPTT in mice. As expected, FXI ASO treatment produced a significant prolongation of aPTT compared with control treated animals (1.28 ± 0.01 international normalized ratio [INR] vs 1.00 ± 0.01 INR). Treatment with FXI protein concentrate, however, rapidly normalized the FXI ASO-mediated aPTT prolongation (1.04 ± 0.02; data not shown).
Antithrombotic effect and safety of FXI ASO in combination with enoxaparin (Lovenox) or clopidogrel (Plavix)
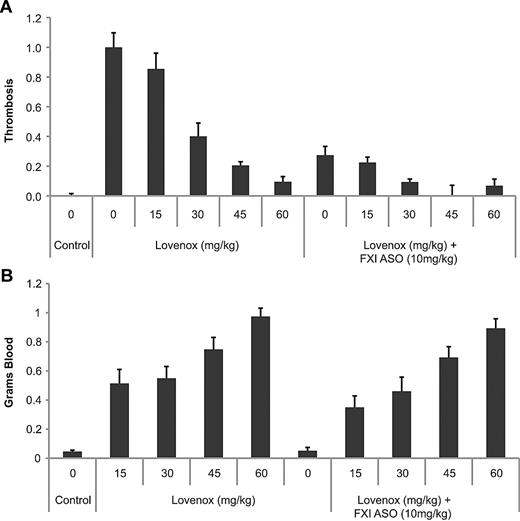
Because combination therapy is a relatively common approach to maximize antithrombotic activity in patients but may have undesirable effects, particularly relating to exacerbation of bleeding complications, we evaluated the effects of FXI ASO in combination with either Lovenox or clopidogrel treatment on thrombosis and bleeding in mice. The antithrombotic activity of Lovenox alone or in combination with FXI ASO treatment was evaluated in the IVC mouse model of venous thrombosis. Lovenox treatment alone resulted in a dose-dependent reduction in thrombus formation, which was significantly improved when combined with FXI ASO, as was demonstrated by a lower maximal effective dose of Lovenox (60 mg/kg vs 30 mg/kg; Figure 5A). As expected, treatment with Lovenox resulted in a dose-dependent increase in bleeding, as demonstrated in the tail vein bleeding assay. However, combining Lovenox with FXI ASO therapy did not exacerbate the increase in bleeding produced by Lovenox treatment alone (Figure 5B).
Effect of FXI antisense treatment alone or in combination with Lovenox on thrombosis and bleeding in mice. Mice (n = 12/group) were treated with indicated doses of Lovenox alone (once daily for 3 consecutive days) or in combination with FXI ASO treatment (20 mg/kg twice/week for 3 weeks). Effects on FeCl3-induced IVC thrombosis (A) or tail vein bleeding (B) were determined 2 hours after the final dose of Lovenox, as described in “Anticoagulant coadministration studies.” *P < .05.
Effect of FXI antisense treatment alone or in combination with Lovenox on thrombosis and bleeding in mice. Mice (n = 12/group) were treated with indicated doses of Lovenox alone (once daily for 3 consecutive days) or in combination with FXI ASO treatment (20 mg/kg twice/week for 3 weeks). Effects on FeCl3-induced IVC thrombosis (A) or tail vein bleeding (B) were determined 2 hours after the final dose of Lovenox, as described in “Anticoagulant coadministration studies.” *P < .05.
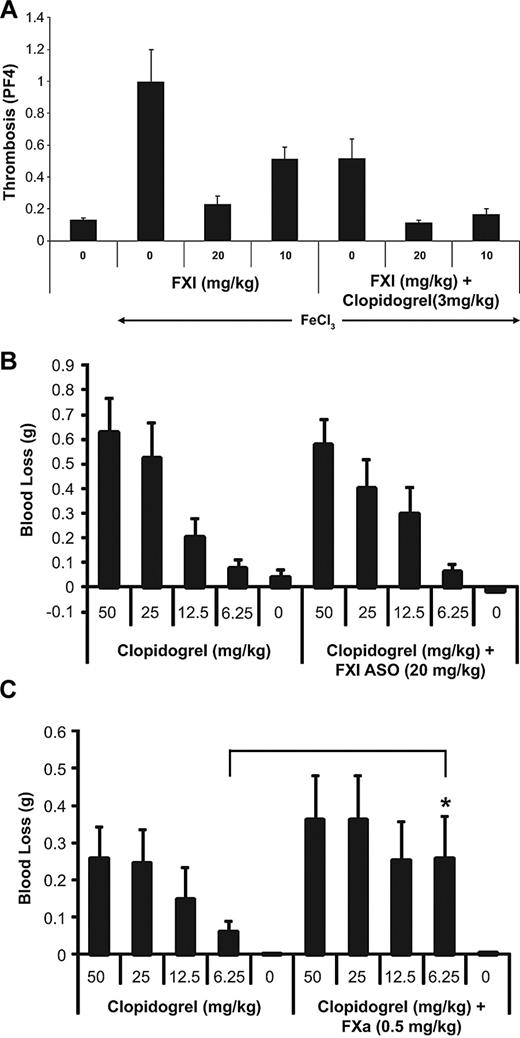
To determine the effects of FXI ASO treatment in combination with clopidogrel, a FeCl3-induced descending aorta model of arterial thrombosis was used. Treatment with clopidogrel alone resulted in a reduction in thrombus formation, which was markedly improved when combined with FXI ASO treatment, as was demonstrated by enhanced antithrombotic efficacy at both the lower dose and the higher dose of FXI ASO treatment, compared with FXI or clopidogrel treatment alone (Figure 6A).
Antithrombotic activity and bleeding evaluation of FXI ASO alone or in combination with clopidogrel in an aortic arterial thrombosis model. As described in “FeCl3-induced aortic thrombosis,” mice were treated with saline (“0”) or FXI ASO (dose 10 mg/kg or 20 mg/kg twice weekly for 3 weeks), either alone or in combination with clopidogrel (3 mg/kg), and thrombosis was induced 4 hours after the final dose of clopidogrel by exposing the descending aorta to a FeCl3 solution (A). Effects of FXI ASO or FXa small-molecule inhibitor in combination with clopidogrel on bleeding in mice. As described in “Anticoagulant coadministration studies,” mice were treated with increasing dose levels of clopidogrel as indicated, either alone or in combination with FXI ASO (20 mg/kg) (B) or in combination with a FXa small-molecule inhibitor (0.5 mg/kg) (C), and blood loss was determined using a mouse tail vein bleeding assay.
Antithrombotic activity and bleeding evaluation of FXI ASO alone or in combination with clopidogrel in an aortic arterial thrombosis model. As described in “FeCl3-induced aortic thrombosis,” mice were treated with saline (“0”) or FXI ASO (dose 10 mg/kg or 20 mg/kg twice weekly for 3 weeks), either alone or in combination with clopidogrel (3 mg/kg), and thrombosis was induced 4 hours after the final dose of clopidogrel by exposing the descending aorta to a FeCl3 solution (A). Effects of FXI ASO or FXa small-molecule inhibitor in combination with clopidogrel on bleeding in mice. As described in “Anticoagulant coadministration studies,” mice were treated with increasing dose levels of clopidogrel as indicated, either alone or in combination with FXI ASO (20 mg/kg) (B) or in combination with a FXa small-molecule inhibitor (0.5 mg/kg) (C), and blood loss was determined using a mouse tail vein bleeding assay.
To determine the effects of clopidogrel on bleeding when administered in combination with FXI ASO or a small-molecule FXa inhibitor,36 mice were treated with increasing doses of clopidogrel, either alone or in combination with FXI ASO (20 mg/kg) or a small-molecule FXa inhibitor (0.5 mg/kg) and the tail vein bleeding assay was used. The dose chosen for the FXa small-molecule inhibitor was the lowest dose that produced maximum antithrombotic activity in the mouse FeCl3 IVC model (data not shown). As expected, clopidogrel alone produced a dose-dependent increase in bleeding time (Figure 6B). Moreover, the effect of clopidogrel on bleeding was significantly increased when combined with a small-molecule FXa inhibitor (Figure 6C). However, the combination of clopidogrel with FXI ASO therapy did not significantly exacerbate the increase in bleeding produced by treatment with clopidogrel alone (Figure 6B).
Discussion
In this report, we are the first to describe the use of antisense technology to target hepatic-derived coagulation FXI as a novel treatment strategy for thromboembolic diseases. FXI antisense treatment in mice produced a dose-dependent and highly specific suppression of hepatic FXI mRNA levels. Even at maximal dose, which resulted in a reduction in FXI mRNA levels of approximately 98%, the expression of other, nontargeted liver-derived coagulation factors remained unaffected. Kinetic studies and dose-response analysis demonstrated that suppression of FXI mRNA levels correlated well with reduced plasma FXI activity levels and aPTT prolongation without affecting PT values. After cessation of FXI ASO treatment, FXI mRNA levels, FXI protein and activity levels, and aPTT values returned to pretreatment levels without a “rebound effect,” which is probably the result of the slow, steady, and gradual loss of activity after treatment termination. The absence of this rebound effect after cessation of FXI ASO dosing is an attractive property for anticoagulant treatment as it avoids potential exposure to a prothrombotic condition.
FXI ASO antithrombotic activity was demonstrated in several mouse models of thrombosis, with maximal antithrombotic effects observed with reductions in circulating FXI activity of approximately 70%. Using a FeCl3-induced IVC thrombosis model, FXI ASO treatment was shown to have an antithrombotic effect similar to that of warfarin. However, unlike warfarin, FXI ASO treatment did not result in increased bleeding. In a FeCl3-induced mesenteric vein thrombosis model, intravital microscopy analysis demonstrated that FXI ASO therapy prevented vessel occlusion in contrast to mice treated with control ASO or saline, in which complete occlusion occurred within 20 minutes after FeCl3 challenge. Although small thrombi did initially form at the site of injury in FXI ASO-treated mice, this partial thrombus formation was substantially delayed in time, reduced in size, density, and stability, and eventually cleared. This observation suggests that FXI is not involved in the initiation of thrombus formation, but instead, is mainly involved in the propagation and/or stabilization of thrombus growth. This may also explain why inhibition of FXI is not associated with increased bleeding, as this may not affect the initial formation of small thrombi at the site of injury helping to achieve hemostasis but may limit the propagation and stabilization of a growing thrombus, thereby preventing vessel occlusion.
FXI ASO treatment also produced marked antithrombotic activity in a stenosis-induced venous thrombosis model and in a arterial thrombosis model. The demonstration of antithrombotic activity in both arterial and venous models of thrombosis, and under conditions of either local vascular wall injury (FeCl3) or reduced blood flow (stenosis), demonstrates that targeting FXI has potential utility for the treatment and prevention of a broad range of thrombotic conditions.
Vitamin K antagonists, such as warfarin, have several features that limit their therapeutic use, including a narrow therapeutic window and high interpatient and intrapatient pharmacokinetic variability, resulting in high rates of serious bleeding complications.37–39 Low-molecular-weight heparins have more predictable pharmacokinetic and pharmacologic profiles but require multiple subcutaneous injections every week, and complications, such as bleeding events and heparin-induced thrombocytopenia, remain an issue.38 In contrast, recent studies suggest that the intrinsic coagulation pathway, and FXI in particular, plays a relatively minor role in normal hemostasis, whereas it plays a significant role in thrombosis,3–6,10,12–14,32 which is further supported by our data. Despite the strong antithrombotic activity of FXI ASO treatment in mice, FXI ASO treatment had no detectable effect on bleeding as demonstrated in both a tail vein bleeding assay and a surgical hepatectomy model. In contrast, treatment with warfarin or the low-molecular-weight heparin Lovenox resulted in marked increases in bleeding, which was observed at subefficacious doses.
The long tissue half-life of ASOs potentially represents an important advantage for antithrombotic therapy because it can permit reasonable patient convenience based on once-weekly or less frequent subcutaneous injection. However, the extended duration of action of ASO treatment further emphasizes the importance of an antidote strategy that effectively and rapidly reverses ASO activity during a bleeding crisis or during acute surgery. Because antisense treatment produces a reduction in the circulating levels of the targeted coagulation factor, simple protein replacement therapy would be expected to produce immediate reversal of anticoagulant activity. Indeed, replacement therapy by plasma-derived FXI concentrate was shown to rapidly and completely reverse the anticoagulant effect of FXI antisense treatment, as demonstrated by normalization of aPTT. The use of plasma or plasma-derived coagulation factors as antidotes may also be more straightforward in the case of antisense therapy as only small increases in coagulation factor levels may be needed to normalize activity.
Combination therapy is a relatively common approach to maximize antithrombotic activity in patients who are at risk for thromboembolic events but may also have undesirable effects, particularly relating to exacerbation of bleeding complications. Lovenox is often used for treatment and prevention of venous thrombosis. The antiplatelet drug clopidogrel (Plavix) effectively inhibits platelet aggregation and is commonly used to prevent arterial thrombosis, including atherothrombotic complications after myocardial infarction, ischemic cerebrovascular events, or in patients with acute coronary syndrome. Because both Lovenox and clopidogrel can cause significant bleeding complications, either alone or in combination with other anticoagulant agents, it is important to understand whether FXI inhibition has the potential to exacerbate bleeding complications when used in combination with one of these important antithrombotic modalities. We evaluated the safety and efficacy of FXI ASO therapy in combination with Lovenox or with clopidogrel, which was compared with the combination of clopidogrel with a small-molecule FXa inhibitor. Although coadministration of FXI ASO with either Lovenox or clopidogrel resulted in an increase in antithrombotic activity compared with either drug alone, this combined treatment did not result in any increase in bleeding beyond that produced by Lovenox or clopidogrel alone. In contrast, combination of clopidogrel with a small-molecule FXa inhibitor resulted in marked increases in bleeding beyond that of clopidogrel therapy alone.
A positive feedback amplification loop involving thrombin and FXI is generally thought to be responsible for the role of FXI in thrombus formation and stabilization.3,15,16 Furthermore, because polyphosphate release from activated platelets can promote thrombus formation40 and because thrombin induces platelet activation and polyphosphate release, the mechanism underlying the role of FXI in thrombus formation may involve the promotion of polyphosphate release by activated platelets as a result of the positive thrombin-FXI positive feedback loop. Studies addressing the role of platelet polyphosphates in the antithrombotic mechanism of FXI depletion will be the subject of future studies.
In conclusion, we have described the use of antisense technology to target the hepatic-derived, intrinsic pathway coagulation factor XI in mice as a potentially novel strategy for the treatment or prevention of thromboembolic disease. We conclude that antisense therapy against FXI is effective and safe, both alone and in combination with standard antithrombotic agents, in preclinical models. Further evaluation is warranted to confirm its potential as a novel, effective, and possibly safer alternative for the treatment and prevention of thrombosis in humans.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Andrew Watt and Sue Freier for their research support and Pam Black for manuscript preparation.
Authorship
Contribution: B.P.M., M.L., E.S.S., and J.C.M.M. conceived and implemented the project strategy; E.C.L., H.Z., J.R.C., A.R.M., C.Z., D.G., A.S.R., and C.B. designed and performed in vitro studies and animal studies; E.C.L. and H.Z. analyzed the data and wrote the first draft of the manuscript; M.L., B.P.M., and J.C.M.M. supervised the research and edited and finalized the manuscript.
Conflict-of-interest disclosure: J.R.C., A.R.M., C.Z., D.G., C.B., and B.P.M. are employees of Isis Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Brett P. Monia, Isis Pharmaceuticals Inc, Department of Antisense Drug Discovery, 1896 Rutherford Rd, Carlsbad, CA 92008; e-mail: bmonia@isisph.com.
References
Author notes
H.Z. and E.C.L. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal