Laser-induced vessel wall injury leads to rapid thrombus formation in an animal thrombosis model. The target of laser injury is the endothelium. We monitored calcium mobilization to assess activation of the laser-targeted cells. Infusion of Fluo-4 AM, a calcium-sensitive fluorochrome, into the mouse circulation resulted in dye uptake in the endothelium and circulating hematopoietic cells. Laser injury in mice treated with eptifibatide to inhibit platelet accumulation resulted in rapid calcium mobilization within the endothelium. Calcium mobilization correlated with the secretion of lysosomal-associated membrane protein 1, a marker of endothelium activation. In the absence of eptifibatide, endothelium activation preceded platelet accumu-lation. Laser activation of human umbilical vein endothelial cells loaded with Fluo-4 resulted in a rapid increase in calcium mobilization associated cell fluorescence similar to that induced by adenosine diphosphate (10μM) or thrombin (1 U/mL). Laser activation of human umbilical vein endothelial cells in the presence of corn trypsin inhibitor treated human plasma devoid of platelets and cell microparticles led to fibrin for-mation that was inhibited by an inhibitory monoclonal anti–tissue factor antibody. Thus laser injury leads to rapid endothelial cell activation. The laser activated endothelial cells can support formation of tenase and prothrombinase and may be a source of activated tissue factor as well.
Introduction
The endothelium serves as a metabolically active interface between the blood and underlying tissues. It maintains vascular tone, regulates vessel permeability and inhibits thrombus formation. The resting endothelium secretes 3 inhibitors of platelet activation, nitric oxide,1 prostacyclin,2,3 and the ectonucleotidase CD39,4 which together form a defense against platelet thrombus formation. The resting endothelium also supports multiple anticoagulant pathways, most importantly that of activated protein C, which is both anticoagulant and cytoprotective.5 Hemostasis and thrombus formation are usually associated with exposure of the subendothelial matrix rich in collagen and tissue factor that lead to accumulation and activation of platelets and thrombin generation, respectively, at the site of injury. While some animal models of thrombosis mimic this exposure of the subendothelial matrix, in our laser-induced injury model the endothelium remains intact and the vessel wall is not denuded of endothelial cells.6 In our endothelial sparing model of laser-induced thrombus formation no collagen is detected at the site of injury but platelet thrombus formation and fibrin deposition both occur rapidly.7,8
We have examined thrombus formation after laser injury in Par4−/− mice whose platelets lack the protease activated receptor required for thrombin activation of mouse platelets.9 Fibrin formation after laser injury in these mice is normal despite formation of a very small platelet thrombus in which platelet activation is significantly delayed. Fibrin formation is thrombin-dependent and thrombin generation requires assembly of the tenase complex, activated factor VIII and activated factor IX, and the prothrombinase complex, activated factor V and activated factor X, on cell surfaces with exposed phosphatidylserine.10 While it has been generally accepted that activated platelets supply this critical surface our results in Par4−/− mice indicate that either minute quantities of activated platelets may be sufficient to support thrombin generation or that other cell surfaces, such as those of activated endothelial cells may provide the surface for enzyme assembly. Therefore we investigated the hypothesis that endothelial cells can be activated rapidly at a site of laser-induced injury and can participate in thrombus formation.
Methods
Cells
Primary human umbilical vein endothelial cells (HUVECs), Medium 200, and low serum growth supplement were obtained from Cascade Biologics. Human dermal microvascular endothelial cells (HDMECs), human aortic endothelial cells (HAECs), and corresponding endothelial cell medium were obtained from ScienCell Research Laboratories.
Mice
Wild-type C57BL/6J mice were obtained from The Jackson Laboratory. The Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee approved all animal care and experimental procedures.
Antibodies, dyes, and reagents
Rat anti–mouse CD41 antibody (clone MWReg30) was from Emfret and rat anti–mouse lysosomal-associated membrane protein 1 (LAMP-1) antibody (clone 1D4B; isotype immunoglobulin G [IgG]2a) was from eBioscience. Mouse anti–human fibrin monoclonal antibody (clone 59D8 kindly supplied by Professor Lawrence Brass, University of Pennsylvania School of Medicine) was purified by affinity chromatography using Protein A/G. Inhibitory tissue factor antibody cH36 was obtained from Altor Bioscience. Rat IgG2a isotype control was obtained from Pharmingen/BD Biosciences. Fab fragments of the anti-CD41 antibody were generated using the ImmunoPure Fab Preparation Kit from Pierce-ThermoScientific. Fab fragments of anti-CD41 antibody and mouse anti-fibrin antibody and anti–LAMP-1 antibody as well as rat IgG2a nonimmune IgG antibodies were labeled with Alexa Fluor 488 or Alexa Fluor 647 according to the manufacturer's instructions (Invitrogen). The molar ratio of Alexa Fluor to protein, determined spectrophotometrically, varied from 2.0 to 3.5.
Fluo-4 AM and DIOC6 (3,3′-dihexyloxacarbocyanine iodide) were obtained from Molecular Probes/Invitrogen, and prepared by solution at 3mM into dimethyl sulfoxide with 20% (wt/vol) Pluronic F-127 (Sigma-Aldrich) for in vitro experiments and by solution at 6mM into Cremophore EL (Sigma-Aldrich) for in vivo experiments.
The agonist adenosine diphosphate (ADP) was from Sigma-Aldrich, and thrombin was from Haematologic Technologies Inc. Eptifibatide (Integrilin) was purchased from Schering Plough.
Endothelial cell culture and stimulation
HUVECs were grown in Medium 200 containing low serum growth supplement and cells of passage 2-3 were seeded on gelatin-coated (Chemicon and Millipore) coverslips at a density of 1 × 105 per coverslip. The endothelial cells were cultured for 2-3 days under a 5% CO2/air atmosphere at 37°C until confluent. For calcium imaging using Fluo-4 AM, the cells were loaded as per the manufacturer's instruction at a final concentration of 3μM for 30 minutes.
Images of cells in the basal state were recorded for 1 minute prior to activation. For laser activation cells were subjected to a single pulse from a dye tuned (410-nm wavelength) nitrogen laser with continuous imaging to enable recording of early kinetic changes. For stimulation of endothelial cells with ADP (10μM), thrombin (1 U/mL) or histamine (10μM) agonists or vehicle control were added at a dilution of 1:10 to ensure rapid and complete mixing. Agonists were prepared in cell media and were prewarmed to 37°C. For in vitro fibrin generation experiments, blood was collected into 4% sodium citrate at a ratio of 1:9 and immediately centrifuged at 330g. Platelets were removed from the platelet rich plasma by further centrifugation at 2000 rpm. The platelet poor plasma was centrifuged at 106 000g for 1 hour at 4°C, aliquoted, and stored at −80°C. Fluo-4 AM–loaded cells cultured on photo-etched coverslips were incubated with prewarmed plasma in the presence of 0.1 mg/mL corn trypsin inhibitor and 10mM calcium. In addition, either the inhibitory tissue factor antibody cH36 or an isotype-matched control human IgG was added to the plasma. Selected cells were stimulated with the laser and cell activation was monitored in real time by fluorescence microscopy. A single cell within each of 3 noncontiguous fields of the grid on the photo-etched slide was activated and the x,y coordinates of the sites of activation were noted using the numbers on the underside of the slide. EDTA (ethylenediaminetetraacetic acid; 20mM) was added 15 minutes after addition of Ca2+ to inhibit further thrombin generation and cells on the photo-etched coverslips were fixed with 4% paraformaldehyde. The fixed cells were immunostained with Alexa 647–labeled fibrin specific antibody (clone 59D8) or Alexa 647–labeled isotype-matched control antibody. The areas at and contiguous to the sites of injury were examined by differential interference contrast and fluorescent microscopy using a 60× oil lens (1.47 numeric aperture). Cells and nuclei were visualized by actin staining with fluorescein isothiocyanate phalloidin (40nM) and DAPI (4′,6-diamidino-2-phenylindole; 300nM), respectively.
Intravital microscopy
Intravital videomicroscopy of the cremaster muscle microcirculation was performed as previously described.11,12 Mice were preanesthetized with intraperitoneal ketamine (125 mg/kg body weight; Abbott Laboratories), xylazine (12.5 mg/kg body weight; Phoenix Pharmaceuticals), and atropine (0.25 mg/kg body weight; American Pharmaceutical Partners). A tracheal tube was inserted, and the mouse was maintained at 37°C on a thermo-controlled rodent blanket. To maintain anesthesia, Nembutal (Abbott Laboratories) was administered through a cannulus placed in the jugular vein. After the scrotum was incised, the testicle and surrounding cremaster muscle were exteriorized onto an intravital microscopy tray. The cremaster preparation was superfused with thermo-controlled (36°C) and aerated (95% N2, 5% CO2) bicarbonate-buffered saline throughout the experiment. Microvessel data were obtained using an Olympus AX microscope with a 60× water-immersion objective (0.9 numeric aperture). The intravital fluorescence microscopy system has previously been described in detail.7 Digital images were captured with a Cooke Sensicam charge-coupled device camera in 640 × 480 format (2 × 2 binning).
The endothelium of the cremaster microcirculation was loaded with Fluo-4 AM by systemic infusion of Fluo-4 AM/Cremophore via the femoral artery. A period of 20 minutes was allowed after infusion for uptake and de-esterification of the dye within the endothelium. Concurrently aggregation was inhibited by infusion of the αIIbβ3 antagonist eptifibatide. Eptifibatide (10 μg/g mouse) was infused immediately prior to intiation of the first thrombus and was reinfused every 20 minutes for the duration of the experiment. Eptifibatide does not interfere with binding to platelets of the monoclonal anti-CD41 antibody, MWReg30, used for detection of platelets in these experiments. After laser-induced vessel wall injury, changes to endothelial Ca2+ levels were observed by excitation at 488 nm, and images were recorded over time.
Laser-induced injury
Vessel wall injury was induced with a Micropoint Laser System (Photonics Instruments) focused through the microscope objective, parfocal with the focal plane and aimed at the vessel wall.7 Typically, 1 or 2 pulses were required to induce vessel wall injury. Multiple thrombi were studied in a single mouse, with new thrombi formed upstream of earlier thrombi to avoid any contribution from thrombi generated earlier in the animal under study. There were no characteristic trends in thrombus size or thrombus composition in sequential thrombi generated in a single mouse during an experiment. Image analysis was performed using Slidebook Version 4.2 or higher (Intelligent Imaging Innovations). Fluorescence data were captured digitally at up to 50 frames/s and analyzed as previously described.12 Typically, widefield fluorescence images were captured at exposure times of 20 milliseconds, whereas brightfield images were captured with exposure times of 10 milliseconds. Data were collected for 3-5 minutes after vessel wall injury. The representative intravital color images are displayed either as binarized images with the threshold chosen as the mean of the maximum fluorescence values of a mask upstream of the thrombus taken throughout the capture, or as an intensity map in which the intensity of the fluorescence of each pixel is represented by a pseudocolor with blue being least intense and red being most intense. The complete data sets of the representative and multiple identical experiments are presented graphically, plotting the integrated fluorescence intensity of all pixels in the image as a function of time. Pixels considered are those above the previously defined threshold and corrected for background, with the background being the mean of the maximum fluorescence values in the upstream mask. The kinetics of thrombus formation were analyzed by determining median fluorescence values over time in approximately 20-30 thrombi.7
Statistical analysis
In vitro data are presented as means ± SEM and statistical significance was calculated with Student t test. For intravital experiments data were considered nonparametric and presented as medians. Welch correction was used for unpaired Student t test in the in vitro fibrin generation assay.
Results
Rapid activation of arteriolar endothelium in vivo
Endothelial responses to laser injury in vivo were investigated using intravital widefield microscopy and the cell permeant calcium sensitive dye Fluo-4 AM, the fluorescence intensity of which increases more than 100-fold after binding with calcium (Kd = 345nM). Fluo-4 AM was introduced into the mouse circulation via the femoral artery to maximize the delivery and uptake of dye in the cremaster muscle microcirculation. Fluo-4 AM is nonspecific in its uptake among cell types and therefore labels all hematopoietic cells as well as the endothelium. To focus on the endothelium we used the αIIbβ3 integrin antagonist eptifibatide to inhibit platelet accumulation at the site of injury. Inhibition of platelet accumulation was verified in an independent experiment using anti–mouse CD41 Fab fragments conjugated to Alexa 647 to label endogenous platelets prior to laser injury and imaging (data not shown).
Changes in endothelial cell Ca2+ levels in Fluo-4 AM– and eptifibatide–treated mice were recorded in one channel and a brightfield image in a second channel (Figure 1A). The response of the endothelium to laser injury was very rapid and showed an increase in Fluo-4 fluorescence, reflecting Ca2+ elevation within seconds of the laser pulse. Fluo-4 fluorescence remained elevated for several minutes. Analysis of the increase in integrated Fluo-4 fluorescence over time for multiple experiments yielded the median curve for Ca2+ flux shown in Figure 1B. We performed the same experiment using the nonspecific membrane stain DIOC6 in place of Fluo-4 to exclude the possibility that the increased Fluo-4 fluorescence after laser injury was a result of noncalcium related accumulation of Fluo-4 labeled species at the site of injury rather than a rise in Ca2+. DIOC6 has an excitation maximum at a similar wavelength to that of Fluo-4 and once infused labeled the membranes of endothelial cells and all hematopoietic cells at a comparable level to the baseline Fluo-4 fluorescence. After laser injury, DIOC6 fluorescence did not increase at the injury site, confirming that the increased Fluo-4 fluorescence observed at the site of laser-induced vessel injury was the result of Ca2+ mobilization in endothelial cells activated by the laser pulse (Figure 1B).
Activation of arteriolar endothelium in vivo by laser-induced injury. The endothelium of the cremaster microcirculation was loaded with either Fluo-4 AM or DIOC6 by systemic infusion of Fluo-4 AM/Cremophore or DIOC6 via the femoral artery. A period of 20 minutes was allowed after infusion for uptake and de-esterification of Fluo-4 AM or uptake of DIOC6 within the endothelium. Concurrently platelet accumulation was inhibited by infusion of eptifibatide. After laser-induced vessel wall injury, changes in endothelial Ca2+ levels were observed by excitation at 488 nm. (A) Representative composite fluorescence and brightfield images after vessel injury show Ca2+ elevation in the endothelium in the absence of platelet accumulation. The fluorescence signal is presented as a pseudocolor intensity map where blue represents the least intense and red represents most intense fluorescence signal. (B) Calcium elevation after vessel injury as determined by the median integrated fluorescence intensity (y-axis) from Fluo-4–loaded endothelium (top black curve, 27 thrombi from 3 mice) in comparison to the median integrated fluorescence of the inert dye DiOC6 (bottom gray curve, 15 thrombi from 3 mice). (C) Propagation of Ca2+ elevation and endothelial activation along the vessel after injury is presented as a pseudocolor intensity map as in panel A. Image is representative of peak endothelial activation, the site of injury is marked (X) and one line demarking the vessel wall on the same side as the injury (purple) and a second demarking the opposing side (gray). (D) Representative trace from a single experiment showing quantitation of the Fluo-4 fluorescence signal longitudinally along each vessel wall. A line was drawn along each wall and the intensity of the pixels at each step along this line was determined and plotted. The start of the line (0 pixels) is bottom right and the end (∼ 300 pixels) is top left.
Activation of arteriolar endothelium in vivo by laser-induced injury. The endothelium of the cremaster microcirculation was loaded with either Fluo-4 AM or DIOC6 by systemic infusion of Fluo-4 AM/Cremophore or DIOC6 via the femoral artery. A period of 20 minutes was allowed after infusion for uptake and de-esterification of Fluo-4 AM or uptake of DIOC6 within the endothelium. Concurrently platelet accumulation was inhibited by infusion of eptifibatide. After laser-induced vessel wall injury, changes in endothelial Ca2+ levels were observed by excitation at 488 nm. (A) Representative composite fluorescence and brightfield images after vessel injury show Ca2+ elevation in the endothelium in the absence of platelet accumulation. The fluorescence signal is presented as a pseudocolor intensity map where blue represents the least intense and red represents most intense fluorescence signal. (B) Calcium elevation after vessel injury as determined by the median integrated fluorescence intensity (y-axis) from Fluo-4–loaded endothelium (top black curve, 27 thrombi from 3 mice) in comparison to the median integrated fluorescence of the inert dye DiOC6 (bottom gray curve, 15 thrombi from 3 mice). (C) Propagation of Ca2+ elevation and endothelial activation along the vessel after injury is presented as a pseudocolor intensity map as in panel A. Image is representative of peak endothelial activation, the site of injury is marked (X) and one line demarking the vessel wall on the same side as the injury (purple) and a second demarking the opposing side (gray). (D) Representative trace from a single experiment showing quantitation of the Fluo-4 fluorescence signal longitudinally along each vessel wall. A line was drawn along each wall and the intensity of the pixels at each step along this line was determined and plotted. The start of the line (0 pixels) is bottom right and the end (∼ 300 pixels) is top left.
The area of endothelial activation visualized in the Fluo-4–treated animals was limited to the field of view indicating that activation was < 400 μm along the vessel after laser injury. The signal was apparent on both the side of the vessel targeted by the laser and the opposite side of the vessel indicating circumferential propagation of activation from the initial injury site. The extent of calcium elevation after injury was determined by measuring integrated fluorescence intensity along both sides of the vessel wall in the vicinity of the laser injury. Median values for integrated fluorescence intensity over time are presented in Figure 1C. The level of endothelial Ca2+ elevation on the targeted side of the vessel was approximately 3-fold higher than on the opposite side of the vessel. Maximal Ca2+ elevation was observed at the approximate site of laser injury and the parallel point on the opposite side of the vessel and decayed both proximally and distally from those points on both sides of the vessel (Figure 1D).
Endothelium activation precedes platelet accumulation and thrombus formation after injury
We established the temporal relationship between endothelial cell activation and platelet thrombus formation after laser-induced vessel wall injury. Platelets were labeled by infusion of anti–mouse CD41 Fab fragments coupled to Alexa 647, and Fluo-4 AM was infused as described in “Rapid activation of arteriolar endothelium in vivo.” Images monitoring thrombus formation indicated that the increase in fluorescence in the vessel wall at the site of injury occurred prior to the appearance of platelets at the site (Figure 2A). As platelets accumulated and became activated, they contributed to the observed increase in Fluo-4 fluorescence due to platelet Ca2+ mobilization. To determine the increase in integrated endothelial cell Fluo-4 fluorescence, we subtracted any Fluo-4 fluorescence that was colocalized with Alexa 647 fluorescence from the total Fluo-4 fluorescence. This method overestimated the platelet–Fluo-4 fluorescence contribution because there was no way of parsing among pixels that contained platelet- and endothelial-derived Fluo-4 fluorescence and those that contained only platelet-derived Fluo-4 fluorescence. However, the stringent nature of this analysis did not compromise the conclusion that endothelial cell activation occurs prior to platelet accumulation because a substantial Fluo-4 signal was observed prior to platelet appearance at the injury site. Median curves for the endothelial cell-derived increase in Fluo-4 fluorescence and the platelet-associated Alexa 647 fluorescence over time indicate that, after laser injury, there was rapid calcium mobilization in the endothelium that preceded platelet detection by up to 30 seconds. (Figure 2B).
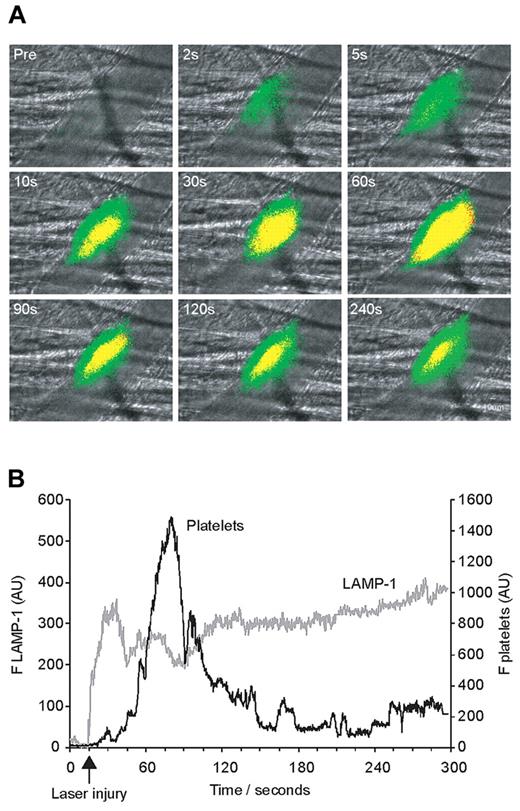
In vivo imaging of platelet accumulation concomitantly with endothelial calcium elevation after laser-induced injury. Platelets were labeled with anti–mouse CD41 Fab fragments conjugated to Alexa 647 infused via the jugular vein, and the endothelium of the cremaster microcirculation was loaded with Fluo-4 AM. After laser-induced vessel wall injury activation of the endothelium and thrombus formation were observed and recorded over time. (A) Composite fluorescence and brightfield images after vessel injury show Ca2+ elevation (green) in the endothelium in conjunction with and preceding platelet accumulation (red) or presence of both signals (yellow). The fluorescence signal is shown binarized for ease of visual interpretation. (B) Kinetic curves displaying the median integrated Fluo-4 fluorescence (gray curve on left y-axis) and median integrated platelet fluorescence (black curve on right y-axis) for 34 thrombi in 3 wild-type mice. Fluo-4 fluorescence originating from platelets and not the endothelium was eliminated by subtracting any Fluo-4 fluorescence in pixels where Alexa 647 fluorescence was observed.
In vivo imaging of platelet accumulation concomitantly with endothelial calcium elevation after laser-induced injury. Platelets were labeled with anti–mouse CD41 Fab fragments conjugated to Alexa 647 infused via the jugular vein, and the endothelium of the cremaster microcirculation was loaded with Fluo-4 AM. After laser-induced vessel wall injury activation of the endothelium and thrombus formation were observed and recorded over time. (A) Composite fluorescence and brightfield images after vessel injury show Ca2+ elevation (green) in the endothelium in conjunction with and preceding platelet accumulation (red) or presence of both signals (yellow). The fluorescence signal is shown binarized for ease of visual interpretation. (B) Kinetic curves displaying the median integrated Fluo-4 fluorescence (gray curve on left y-axis) and median integrated platelet fluorescence (black curve on right y-axis) for 34 thrombi in 3 wild-type mice. Fluo-4 fluorescence originating from platelets and not the endothelium was eliminated by subtracting any Fluo-4 fluorescence in pixels where Alexa 647 fluorescence was observed.
Calcium mobilization in the endothelium correlates with granule secretion
To confirm that the fluorescence intensity increase in the endothelium associated with calcium mobilization is an event associated with endothelial cell activation and to determine whether laser stimulation of endothelial cells leads to later stages of cell activation, we examined the surface expression of LAMP 113,14 after laser-induced injury. LAMP 1, a lysosomal membrane protein found in many cells including endothelial cells, is translocated to the cell membrane upon cell activation and granule secretion. Alexa 488–labeled anti–LAMP-1 and Alexa 647–labeled anti-CD41 Fab fragments were infused and laser-induced thrombus formation monitored. LAMP-1 was visible at the site of laser injury on the vessel wall at a time prior to the accumulation of platelets (Figure 3A). As platelets accumulated at the injury site and became activated, they contributed to LAMP-1 fluorescence because activated platelets also express LAMP-1 on their surface.15 These results indicate that endothelial cell activation can be monitored in vivo by both calcium mobilization and LAMP-1 secretion.
Exposure of activation and secretion marker LAMP-1 in comparison with platelet accumulation in vivo after laser injury. Anti–mouse CD41 Fab fragments conjugated to Alexa 647 and anti–mouse LAMP-1 antibodies conjugated to Alexa 488 were infused to label platelets and LAMP-1, respectively. Injuries were induced by laser pulses to cremaster arteriole vessel walls and subsequent LAMP-1 accumulation and thrombus formation recorded over time. (A) Images from a representative experiment showing fluorescence over time overlaying brightfield data before and after vessel injury. LAMP-1 accumulated rapidly at the vessel wall (green) followed by platelet accumulation (red) or presence of both signals (yellow). The fluorescence signal is shown binarized for ease of visual interpretation. (B) Kinetic curves displaying the median integrated platelet fluorescence (black curve on right y-axis) and median integrated LAMP-1 fluorescence (gray curve on left y-axis) for 18 thrombi in 3 wild-type mice. LAMP-1 fluorescence originating from platelets and not the endothelium was eliminated by subtracting any LAMP-1 fluorescence in pixels where Alexa 647 fluorescence was observed.
Exposure of activation and secretion marker LAMP-1 in comparison with platelet accumulation in vivo after laser injury. Anti–mouse CD41 Fab fragments conjugated to Alexa 647 and anti–mouse LAMP-1 antibodies conjugated to Alexa 488 were infused to label platelets and LAMP-1, respectively. Injuries were induced by laser pulses to cremaster arteriole vessel walls and subsequent LAMP-1 accumulation and thrombus formation recorded over time. (A) Images from a representative experiment showing fluorescence over time overlaying brightfield data before and after vessel injury. LAMP-1 accumulated rapidly at the vessel wall (green) followed by platelet accumulation (red) or presence of both signals (yellow). The fluorescence signal is shown binarized for ease of visual interpretation. (B) Kinetic curves displaying the median integrated platelet fluorescence (black curve on right y-axis) and median integrated LAMP-1 fluorescence (gray curve on left y-axis) for 18 thrombi in 3 wild-type mice. LAMP-1 fluorescence originating from platelets and not the endothelium was eliminated by subtracting any LAMP-1 fluorescence in pixels where Alexa 647 fluorescence was observed.
The increase in integrated endothelial cell–associated LAMP-1–associated fluorescence was measured after correcting for the LAMP-1 signal contributed by platelets. Laser-induced vessel injury led to rapid accumulation of LAMP-1 antigen on the surface of the surrounding endothelium and was detected before platelet accumulation (Figure 3B). Similar to observations of endothelial-associated Ca2+ elevation, endothelial cell-associated LAMP-1 accumulation after laser-induced injury was observed to spread from the site of injury around the vessel and, in some cases, to the opposite wall. Thus, calcium mobilization and granule secretion in the endothelium precede platelet accumulation in vivo during laser-induced vessel wall injury and thrombus formation.
Rapid activation of endothelial cells by laser injury in vitro
To determine that the endothelial cell activation and its sequelae observed after laser injury in vivo are a direct consequence of the laser pulse and not secondary factors we examined the calcium response of cultured HUVECs to a laser pulse using Fluo-4 AM. Fluo-4–loaded HUVECs were subjected to a single laser pulse of approximately 300 μJ, and subsequent changes in Fluo-4 fluorescence recorded (Figure 4A). The laser pulse was focused to a diffraction-limited spot of approximately 1.8-μm diameter, a target area well below the size of the targeted endothelial cell. The images show a representative resting endothelial cell prior to laser injury (0 seconds) and the increase in fluorescence as a result of increased intracellular Ca2+ after laser injury. At 0.5 seconds after the laser pulse Ca2+ elevation was observed within the cell body covering an area of approximately 13 μm in diameter centered at the laser target. Within several seconds an increased Ca2+ level was observed throughout the cell. Later time points showed a gradual reduction in fluorescence over approximately 3 minutes. No fluorescence was observed when the laser was aimed at a cell-free area of the cover-slip. The fluorescence signal from laser-activated cells was quantitated by defining the cell perimeter from the differential interference contrast image and calculating the mean pixel intensity from the 488-nm fluorescence excitation channel within the region of interest for each time point (Figure 4B). Plots of fluorescence versus time for 1 representative cell and the mean of 41 cells show peak Ca2+ elevation occurred in less than 10 seconds after the laser pulse and then declined. In similar experiments performed in Ca2+-free media approximately 25% of the calcium mobilization was preserved, indicating that the observed calcium flux was due to cell activation rather than disruption of the plasma membrane (not shown).
Rapid endothelial cell activation in vitro follows targeted laser pulse. HUVECs were loaded with Fluo-4 AM (3μM) and observed using fluorescence microscopy. (A) Representative images of a cell before and after a direct laser pulse to the point the indicated (X). An increase in Fluo-4 fluorescence (green) reflects a rise in intracellular Ca2+. (B) Quantification of this signal is plotted against time showing 1 representative trace (solid line) and the mean trace of laser-induced activation of 41 cells (dotted line). (C) Similarly prepared HUVECs loaded with Fluo-4 AM were stimulated with ADP (10μM), thrombin (1 U/mL), or histamine (10μM) as a comparison to the laser-induced activation. Agonists or vehicle were added after 10 seconds of image acquisition. The graph shows median curves as a comparison of the kinetics of these modes of activation. ADP, solid line; thrombin, – – –; histamine, -.-.; laser, ---; vehicle ⋯. (D) The peak cell activation was extracted from the kinetic data for each agonist and the laser. The mean ± SEM is plotted for each group; ADP stimulation, n = 82 cells from 7 experiments, histamine n = 45 cells from 3 experiments; thrombin n = 85 cells from 6 experiments; vehicle n = 10 cells from 2 experiments.
Rapid endothelial cell activation in vitro follows targeted laser pulse. HUVECs were loaded with Fluo-4 AM (3μM) and observed using fluorescence microscopy. (A) Representative images of a cell before and after a direct laser pulse to the point the indicated (X). An increase in Fluo-4 fluorescence (green) reflects a rise in intracellular Ca2+. (B) Quantification of this signal is plotted against time showing 1 representative trace (solid line) and the mean trace of laser-induced activation of 41 cells (dotted line). (C) Similarly prepared HUVECs loaded with Fluo-4 AM were stimulated with ADP (10μM), thrombin (1 U/mL), or histamine (10μM) as a comparison to the laser-induced activation. Agonists or vehicle were added after 10 seconds of image acquisition. The graph shows median curves as a comparison of the kinetics of these modes of activation. ADP, solid line; thrombin, – – –; histamine, -.-.; laser, ---; vehicle ⋯. (D) The peak cell activation was extracted from the kinetic data for each agonist and the laser. The mean ± SEM is plotted for each group; ADP stimulation, n = 82 cells from 7 experiments, histamine n = 45 cells from 3 experiments; thrombin n = 85 cells from 6 experiments; vehicle n = 10 cells from 2 experiments.
Activation of Fluo-4–loaded HUVECs by ADP (10μM), thrombin (1 U/mL), or histamine (10μM), physiologic cell agonists, was compared with laser activation. Calcium mobilization was monitored by fluorescence, and all 3 agonists induced Ca2+ elevation within seconds of their addition to the cells (Figure 4C). Thrombin induced the highest peak Ca2+ elevation followed by ADP and histamine. A comparison of these modes of activation revealed similar kinetics for both agonist and laser stimulation. Decay of the Ca2+ signal was most rapid in thrombin-activated cells and slowest in laser-activated cells. The peak Ca2+ responses elicited by thrombin, ADP, and laser pulse were significantly larger than control (P < .05) and the thrombin and laser responses were equivalent (P > .5). Similar results were obtained using cultured HAECs and HDMECs.
Propagation of endothelial cell activation to surrounding cells after laser stimulation
In vivo endothelial cells do not exist as independent units but rather as part of a confluent monolayer lining the lumen of the vasculature. We therefore investigated the effect of laser stimulation on a confluent cell population. A confluent monolayer of HUVECs was loaded with Fluo-4 and a single cell within the monolayer subjected to laser stimulation. Figure 5A shows representative images of the response of a cell monolayer to a single laser pulse fired at the point indicated (X). Calcium mobilization, as monitored by fluorescence, begins in the target cell 1 second after the laser pulse. Subsequent activation of the surrounding cells was visible, nearest first and spreading outward. Propagation of the calcium wave from one cell to another was not inhibited by either 18-α-glycyrrhetinic acid, a gap junction inhibitor, or the ADP scavenger apyrase. Similarly, after laser stimulation of a single cell we observed propagation of the calcium wave among cells seeded at a low density with no cytoplasmic bridges between them (data not shown).
Rapid propagation of endothelial cell activation within a confluent cell population follows laser-induced activation in vitro. HUVECs were loaded with Fluo-4 AM. Cells were observed for 1 minute prior to activation to confirm a stable baseline then subjected to a single pulse from a nitrogen laser and continuous imaging. (A) Images from different time points of a representative cell population. The first frame shows the cell monolayer in its basal state just prior to activation and the X marks the point upon which the laser is focused. The subsequent time points show activation of the target cell (within 1 second of laser firing), closely followed by activation of surrounding cells and then steady return toward a basal cytoplasmic Ca2+ concentration. (B) The mean pixel intensity of the 4 cells is plotted versus time to yield the corresponding 4 kinetic traces shown on the right. Cell 1, solid line; Cell 2, -.-.; Cell 3, - - -; Cell 4, ⋯.
Rapid propagation of endothelial cell activation within a confluent cell population follows laser-induced activation in vitro. HUVECs were loaded with Fluo-4 AM. Cells were observed for 1 minute prior to activation to confirm a stable baseline then subjected to a single pulse from a nitrogen laser and continuous imaging. (A) Images from different time points of a representative cell population. The first frame shows the cell monolayer in its basal state just prior to activation and the X marks the point upon which the laser is focused. The subsequent time points show activation of the target cell (within 1 second of laser firing), closely followed by activation of surrounding cells and then steady return toward a basal cytoplasmic Ca2+ concentration. (B) The mean pixel intensity of the 4 cells is plotted versus time to yield the corresponding 4 kinetic traces shown on the right. Cell 1, solid line; Cell 2, -.-.; Cell 3, - - -; Cell 4, ⋯.
We followed the spread of the wave of activation by quantifying the rise and peak in Ca2+ elevation in cells at varying distance from the point of laser injury (Figure 5B). The indicated cells showed a staggered rise in fluorescence indicating a time delayed propagation of cell-associated Ca2+ elevation and activation as the distance from the laser-injured cell increased. The spread of Ca2+ mobilization among the population diminished with distance from the point of injury and was not observed beyond 3 to 4 fields-of-view, equivalent to approximately 400-500 μm. The mean speed of propagation of the activation wave front was 15 ± 2 μm/s. Translated to an in vivo setting this rate of propagation would cross a 50 μm diameter arteriole from one side to the other in 5.2 seconds. Laser-stimulation of endothelial cells in vitro results in both rapid activation of the target cell and neighboring cells. These data confirm that the Ca2+ flux observed in vivo after laser-induced endothelial cell injury is a consequence of the injury.
Laser-activated endothelial cells can induce thrombin generation
To determine whether laser-induced activation of endothelial cells alters the coagulant potential of the cells we activated cultured endothelial cells in the presence of human plasma. Plasma was anticoagulated with sodium citrate, and subjected to centrifugation twice to remove platelets. The supernatant plasma was subjected to ultracentrifugation to remove any subcellular elements. Corn trypsin inhibitor was added at a final concentration of 0.1 mg/mL to inhibit factor XIIa-mediated initiation of blood coagulation, and specific antibodies or control antibodies were added to the plasma. No fibrin clot formed in this plasma for more than 45 minutes after recalcification. Plasma was overlayed upon washed, confluent, Fluo-4–loaded HUVECs growing on gelatin-coated photo-etched coverslips and calcium was added. The cells were subjected to laser injury or sham. Cells were fixed in situ 15 minutes after addition of calcium and the presence of fibrin detected by immunofluorescence using an Alexa 647–labeled fibrin specific antibody. Background signal was calculated using a similarly labeled isotype matched control antibody (Figure 6D). With unstimulated cells no fibrin was generated (Figure 6A). In contrast, laser stimulation of cells in the presence of plasma led to rapid formation of fibrin strands visible over the stimulated cells and neighboring cells (Figure 6B). Fibrin formation was blocked when an inhibitory monoclonal anti–tissue factor antibody, cH36, was included in the plasma but not when an isotype matched control antibody was included (Figure 6C and E, respectively).
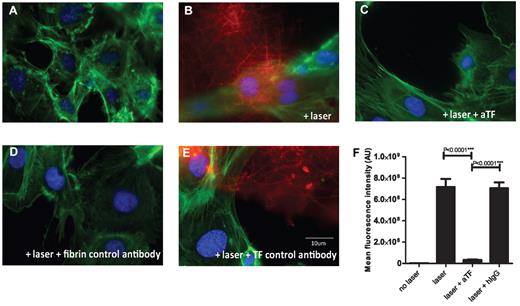
Laser activated endothelial cells can induce thrombin generation. HUVECs plated on photo-etched coverslips were loaded with Fluo-4 AM and incubated with plasma in presence of calcium and corn trypsin inhibitor for 15 minutes. Cells were either stimulated with laser or left unstimulated in the presence or absence of various antibodies. After incubation with plasma, cells were fixed and immunostained for fibrin (red), phalloidin (green) and DAPI (blue). (A) Representative images of cells not stimulated by laser and incubated with recalcified plasma show minimal fibrin specific Alexa 647 signal (red). (B) Detection of fibrin-specific signal (red) after laser induced injury of a single cell in a field in presence of recalcified plasma. (C) Representative image showing lack of fibrin formation after laser injury when the cells were incubated with recalcified plasma containing 100 μg/mL function blocking tissue factor monoclonal antibody cH36. (D) No signal was detected when laser stimulated cells were immunostained with an isotype matched control IgG instead of fibrin antibody in the presence of plasma. (E) Fibrin meshwork was detected on cells activated with laser and incubated with recalcified plasma in the presence of an isotype matched control human IgG instead of the monoclonal antibody cH36. (F) Mean integrated fluorescence signal intensity of fibrin (n = 29). Data are from 2 independently performed experiments. The mean ± SEM is plotted for each group. A background mask was created for all images from panel D. Mean of the maximum signal intensities from 29 images in panel D was used as a constant background number to create a threshold segment mask for all other conditions and integrated fluorescence intensity was calculated. The means of the integrated fluorescence intensity show a significant decrease in fibrin generation when laser stimulated endothelial cells are incubated with recalcified plasma in presence of function blocking tissue factor antibody.
Laser activated endothelial cells can induce thrombin generation. HUVECs plated on photo-etched coverslips were loaded with Fluo-4 AM and incubated with plasma in presence of calcium and corn trypsin inhibitor for 15 minutes. Cells were either stimulated with laser or left unstimulated in the presence or absence of various antibodies. After incubation with plasma, cells were fixed and immunostained for fibrin (red), phalloidin (green) and DAPI (blue). (A) Representative images of cells not stimulated by laser and incubated with recalcified plasma show minimal fibrin specific Alexa 647 signal (red). (B) Detection of fibrin-specific signal (red) after laser induced injury of a single cell in a field in presence of recalcified plasma. (C) Representative image showing lack of fibrin formation after laser injury when the cells were incubated with recalcified plasma containing 100 μg/mL function blocking tissue factor monoclonal antibody cH36. (D) No signal was detected when laser stimulated cells were immunostained with an isotype matched control IgG instead of fibrin antibody in the presence of plasma. (E) Fibrin meshwork was detected on cells activated with laser and incubated with recalcified plasma in the presence of an isotype matched control human IgG instead of the monoclonal antibody cH36. (F) Mean integrated fluorescence signal intensity of fibrin (n = 29). Data are from 2 independently performed experiments. The mean ± SEM is plotted for each group. A background mask was created for all images from panel D. Mean of the maximum signal intensities from 29 images in panel D was used as a constant background number to create a threshold segment mask for all other conditions and integrated fluorescence intensity was calculated. The means of the integrated fluorescence intensity show a significant decrease in fibrin generation when laser stimulated endothelial cells are incubated with recalcified plasma in presence of function blocking tissue factor antibody.
Discussion
Intravital models are an essential tool in the process of investigating hemostasis and thrombosis. They provide an in vivo system of verifying in vitro data and a more holistic means of investigation of a process that has many interdependent components. Most intravital thrombosis models lead to disruption or denudation of the endothelium, and studies in these models have concentrated on responses to the subendothelial matrix.16 The subendothelial matrix undoubtedly plays a critical role in hemostasis. However, we were interested in exploring cases where the endothelium may be fully intact but diseased or activated in some other way. In this study, we have extended our ability to image platelet activation, fibrin generation and thrombus formation to include monitoring of activation of the endothelium in vivo.
Activation of the endothelium and its subsequent interactions with platelets may be important in cases of venous thrombosis, as well as in failure of arterially transplanted vein grafts, stents or artificial valves. We thus employed a laser-induced injury model of thrombosis to examine thrombus formation in the presence of intact but activated endothelium.16 We have previously demonstrated in our model that collagen-exposure and GPVI do not play a role in platelet aggregation and thrombus development.7 Therefore, the initiating events of thrombus formation in this model appear to be dominated by thrombin generation.
In this study we have investigated the possible role of endothelial cell activation in thrombus formation and have demonstrated rapid activation of the vascular endothelium prior to thrombus formation in vivo in response to laser injury. Both Ca2+ mobilization and granule secretion leading to LAMP-1 antigen surface expression were detected after laser injury but before platelet accumulation in our intravital model. The activation propagated locally but was limited to the region immediately adjacent to the injury site. These findings were supported by the results of laser-induced endothelial cell activation in vitro that led to rapid calcium mobilization in the injured cell and surrounding cells.
Sammak and colleagues have previously shown that mechanical disruption of a cultured endothelial monolayer leads to Ca2+ mobilization within seconds of injury and subsequent propagation of activation to surrounding cells.17 As in our studies, these authors showed that cell–cell contact was not required for propagation of the Ca2+ flux to neighboring cells. Rather these authors observed that addition of culture medium from injured cells could elevate Ca2+ levels in unwounded reporter cultures, suggesting a soluble mediator secreted from activated endothelial cells. Dispersion of a soluble mediator from the injured cell would be consistent with our in vitro results that show a decrease in level of calcium mobilization in cells circumferentially from the point of the injured cell in a nonflow system. If a similar mechanism occurs in vivo, then blood flow would be expected to rapidly disperse the signaling molecule consistent with our observation that endothelial cell activation is limited to a small region of the injured vessel around the injury site.
Our results suggest that the endothelium plays a role in thrombus initiation or the early stages of thrombus formation. We have previously demonstrated that in our laser-induced thrombosis model resting platelets are recruited to the site of vessel injury where they become activated.18 In the current study we show that both Ca2+ mobilization and LAMP-1 expression precede platelet accumulation at the site of injury in the cremaster arteriolar endothelium. While we observe both calcium elevation and LAMP-1 expression on both sides of the vessel around the injury site, thrombus formation only occurs on the side of the injury. One explanation for focal thrombus growth at the injury site may be the magnitude of the activation of the cells. In our analysis of calcium mobilization along the vessel wall the endothelium shows greater activation closer to the site of injury indicating that there may be a threshold level of activation needed to initiate platelet adhesion.
A number of mechanisms are known to support recruitment of resting platelets to the endothelial cell surface. For example apoptotic endothelial cells that may be present under some inflammatory or prothrombotic conditions have been shown to be proadhesive for resting platelets.19 However, induction of apoptosis is a slow process not consistent with the kinetics of the events observed after laser vessel wall injury. Endothelial cell P-selectin has been implicated in recruitment of platelets to endothelium stimulated with inflammatory mediators such as tumor necrosis factor-α20 and in response to ischemia-reperfusion injury21 but platelets accumulate after laser induced injury in P-selectin−/− mice making it unlikely that this protein is playing an important role in recruiting platelets to the activated endothelium in this model.11 Like P-selectin, von Willebrand factor is stored in the Weibel-Palade bodies of endothelial cells and is released when these cells are activated. Although von Willebrand factor is known to play a role in platelet adhesion to the endothelium it is not required for recruitment of platelets after laser injury.18 Thus, the mechanism of recruitment of resting platelets to laser-activated endothelium remains to be elucidated.
LAMP-1, a lysosomal membrane protein, is not present on the cell surface under basal conditions; secretion is required to translocate the protein to the plasma membrane. Therefore we suggest that activation and subsequent secretion of endothelial cell granule contents upon laser injury leads to the release of mediators and surface presentation of proteins that locally transform the endothelium into a pro-thrombotic surface. One such mediator could be the thiol isomerase protein disulfide isomerase, which has recently been shown to play a critical role in thrombus formation and fibrin generation in vivo.22 Protein disulfide isomerase is present in endothelial cell granules and is secreted upon laser-induced vessel wall injury in our mouse model of thrombosis.23
The activated platelet surface is generally considered the primary site for assembly of the tenase and prothrombinase complexes required for thrombin production. Thus, it was perplexing to find that fibrin generation was normal in Par4−/− mice—lacking the platelet thrombin receptor—in our laser-induced thrombosis model where only a minimal platelet aggregate of unactivated platelets forms at the site of injury.9 Our in vitro results indicate that laser-induced activation of endothelial cells can convert these cells from a quiescent noncoagulant state to an activated procoagulant state that supports thrombin generation indicated by fibrin deposition. The components of this thrombin generation system include washed cultured HUVECs and platelet depleted plasma that has been treated with corn trypsin inhibitor to inhibit the intrinsic pathway to thrombin generation. Alternative sources of membranes from plasma were removed by ultracentrifugation. In the absence of another source of membrane, our results demonstrate that activated endothelial cells support the formation of the tenase and prothrombinase complexes. Endothelial cells activated with thrombin, phorbol 12-myristate 13-acetate, or tumor necrosis factor-α have been shown to support assembly of these complexes.24,25
The fibrin deposition on HUVECs was demonstrated to be tissue factor dependent by use of an inhibitory anti–tissue factor antibody. The absence of clot formation after recalcification of the citrated ultracentrifuged plasma used in these experiments indicates that any tissue factor activity retained in the plasma is below the level required to form fibrin. Thus, our in vitro results may also implicate activated endothelial cells as a source of the tissue factor required to form the initiating tissue factor-factor VIIa complex necessary for thrombin generation. In vivo thrombin formed from the enzymatic reactions supported by the activated endothelial cell membrane may initiate activation of the initial platelets recruited to the activated endothelial surface. Taken together these results suggest an important role for the activated endothelium in thrombus formation through provision of active tissue factor and a membrane surface for tenase and prothrombinase formation under conditions where extravascular cells and vascular matrix components are not exposed.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Glenn Merrill-Skoloff for expert technical assistance.
This work was supported by grants from the National Institutes of Health to B.F. and B.C.F. R.J. is a recipient of a fellowship from the American Heart Association and V.M.C. is a recipient of a grant from the National Health and Medical Research Council of Australia.
National Institutes of Health
Authorship
Contribution: B.T.A., R.J, and V.M.C. designed and performed the experiments, analyzed the results, and wrote the manuscript; P.N. performed the experiments and analyzed the results; and B.F. and B.C.F. designed the experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Barbara C. Furie, Beth Israel Deaconess Medical Center, 330 Brookline Ave, E/CLS 905, Boston, MA 02215; e-mail: bfurie1@bidmc.harvard.edu.