Abstract
Abstract 898
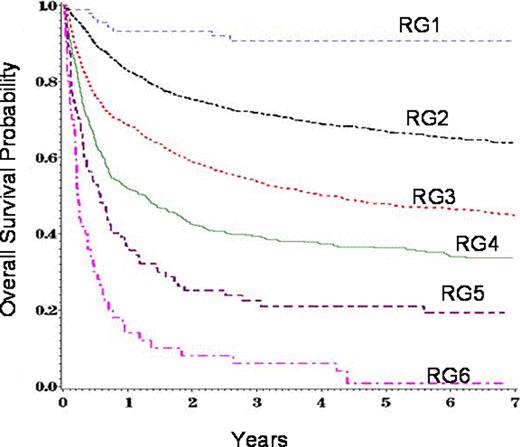
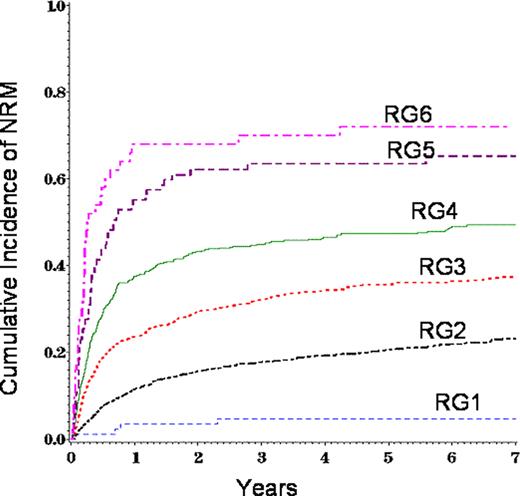
Chronic graft-versus-host disease (cGvHD) remains a major barrier to allogeneic (allo) hematopoietic cell transplant (HCT). Previous studies have identified several variables associated with high mortality in persons with cGvHD. The most consistent risk-factors for mortality are thrombocytopenia and the progressive type of cGvHD onset. We evaluated subject-, disease-, and transplant related variables at diagnosis of cGvHD to develop a risk score in 5343 patients with cGvHD. All patients received first allo-transplant for acute myeloid leukemia (AML), acute lymphoid leukemia (ALL), chronic myeloid leukemia (CML) and myelodysplastic syndrome (MDS) between 1995 and 2004 and reported to CIBMTR. 10 variables were significantly correlated with cGvHD in multivariate analysis of overall survival and non-relapse mortality (NRM) and were used to build the risk score (Table 1). Variable-specific risk scores (VSRC) were constructed for each factor based on the relative risk (RR) of overall or NRM associated with each factor category. Scores were summed for each subject to assign an overall risk score (ORS). Risk groups (RG) of 1–6 were assigned based on the overall risk score. RG1: ORS 0–2, RG2: ORS 3–6, RG3: ORS 7–8, RG4: ORS 9–10, RG5: ORS: 11, RG6: ORS ≥ 12. Survival and NRM were significantly different between RGs (Figure 1 and Figure 2). Although this cGvHD risk score requires independent validation, knowledge of the above HCT factors and cGvHD-specific factors at the time of initial diagnosis provides important prognostic information for clinical care and clinical trial planning. Future analysis will validate this risk score in a more recent allo-transplant cohort assembled after 2005.
| Variable . | Overall Survival . | NRM . | VSRC . | ||
|---|---|---|---|---|---|
| RR | P | RR | P | ||
| Recipient age (baseline: <10 years) | <0.0001 | <0.0001 | Age < 30: 0; 30–59: 1; > 60: 2 | ||
| 10–19 | 1.35 | 0.0026 | 1.41 | 0.008 | |
| 20–29 | 1.31 | 0.0057 | 1.46 | 0.003 | |
| 30–39 | 1.41 | 0.0003 | 1.66 | <0.0001 | |
| 40–49 | 1.53 | <0.0001 | 1.90 | <0.0001 | |
| 50–59 | 1.73 | <0.0001 | 2.17 | <0.0001 | |
| > 60 | 2.18 | <0.0001 | 2.75 | <0.0001 | |
| Prior Acute GVHD (baseline: no AGVHD) | <0.0001 | <0.0001 | No acute GVHD: 0; Prior acute GVHD: 1 | ||
| Grade I-II | 1.23 | 0.0003 | 1.31 | 0.0001 | |
| Grade III-IV | 1.55 | <0.0001 | 1.71 | <0.0001 | |
| Time from HCT to cGvHD (baseline: <5 mo) | > 5 mo: 0; < 5 mo: 1 | ||||
| > 5mo | 0.79 | <0.0001 | 0.84 | 0.0016 | |
| S. Bilirubin at cGvHD (baseline: < 1mg/dl) | <0.0001 | <0.0001 | Bilirubin ≤ 2: 0; Bilirubin > 2: 2 | ||
| 1–2 mg/dl | 1.11 | 0.0557 | 1.22 | 0.0035 | |
| > 2 mg/dl | 1.65 | <0.0001 | 1.88 | <0.0001 | |
| KPS (cGvHD onset) (baseline: < 80) | KPS ≥ 80: 0 KPS <80: 1 | ||||
| 80–100 | 0.57 | <0.0001 | 0.47 | <0.0001 | |
| Platelet count(cGvH onset) (baseline: < 100) | Platelets ≥ 100:0; Platelets < 100: 1 | ||||
| > 100 × 10^9/L | 0.64 | <0.0001 | 0.53 | <0.0001 | |
| HLA group (baseline: HLA-identical sib) | <0.0001 | <0.0001 | HLA –identical sib, well-matched or partially matched URD:0; Other related or mismatched URD: 1 | ||
| Other related | 1.38 | 0.0019 | 1.37 | 0.0137 | |
| Well matched URD | 1.03 | 0.6405 | 1.0 | 0.9863 | |
| Partially matched URD | 1.11 | 0.0931 | 1.17 | 0.0360 | |
| Mismatched URD | 1.37 | <0.0001 | 1.51 | <0.0001 | |
| Disease status at HCT (Baseline: Early) | <0.0001 | <0.0001 | Early: 0; Intermediate: 1; Advanced: 2 | ||
| Intermediate | 1.22 | 0.0001 | 0.99 | 0.0137 | |
| Advanced | 1.86 | <0.0001 | 1.83 | <0.0001 | |
| GvHD prophylaxis (Baseline: T-cell depletion) | <0.0001 | <0.0001 | CSA + methotrexate +/− other: 0; Other categories: 1 | ||
| CSA + methotrexate+/− other | 0.81 | <0.0001 | 0.77 | <0.0001 | |
| Tacrolimus + methotrexate +/− other | 1.12 | 0.1108 | 1.07 | 0.4099 | |
| Sex match (Baseline: male to male) | 1.94 | <0.0001 | <0.0001 | Male to male, male to female, female to female: 0; Femal to male: 2 | |
| Male to female | 0.91 | 0.1096 | 0.89 | 0.1087 | |
| Female to male | 1.94 | 0.0009 | 1.25 | 0.0006 | |
| Female to female | 1.10 | 0.1203 | 1.15 | 0.0428 | |
| Variable . | Overall Survival . | NRM . | VSRC . | ||
|---|---|---|---|---|---|
| RR | P | RR | P | ||
| Recipient age (baseline: <10 years) | <0.0001 | <0.0001 | Age < 30: 0; 30–59: 1; > 60: 2 | ||
| 10–19 | 1.35 | 0.0026 | 1.41 | 0.008 | |
| 20–29 | 1.31 | 0.0057 | 1.46 | 0.003 | |
| 30–39 | 1.41 | 0.0003 | 1.66 | <0.0001 | |
| 40–49 | 1.53 | <0.0001 | 1.90 | <0.0001 | |
| 50–59 | 1.73 | <0.0001 | 2.17 | <0.0001 | |
| > 60 | 2.18 | <0.0001 | 2.75 | <0.0001 | |
| Prior Acute GVHD (baseline: no AGVHD) | <0.0001 | <0.0001 | No acute GVHD: 0; Prior acute GVHD: 1 | ||
| Grade I-II | 1.23 | 0.0003 | 1.31 | 0.0001 | |
| Grade III-IV | 1.55 | <0.0001 | 1.71 | <0.0001 | |
| Time from HCT to cGvHD (baseline: <5 mo) | > 5 mo: 0; < 5 mo: 1 | ||||
| > 5mo | 0.79 | <0.0001 | 0.84 | 0.0016 | |
| S. Bilirubin at cGvHD (baseline: < 1mg/dl) | <0.0001 | <0.0001 | Bilirubin ≤ 2: 0; Bilirubin > 2: 2 | ||
| 1–2 mg/dl | 1.11 | 0.0557 | 1.22 | 0.0035 | |
| > 2 mg/dl | 1.65 | <0.0001 | 1.88 | <0.0001 | |
| KPS (cGvHD onset) (baseline: < 80) | KPS ≥ 80: 0 KPS <80: 1 | ||||
| 80–100 | 0.57 | <0.0001 | 0.47 | <0.0001 | |
| Platelet count(cGvH onset) (baseline: < 100) | Platelets ≥ 100:0; Platelets < 100: 1 | ||||
| > 100 × 10^9/L | 0.64 | <0.0001 | 0.53 | <0.0001 | |
| HLA group (baseline: HLA-identical sib) | <0.0001 | <0.0001 | HLA –identical sib, well-matched or partially matched URD:0; Other related or mismatched URD: 1 | ||
| Other related | 1.38 | 0.0019 | 1.37 | 0.0137 | |
| Well matched URD | 1.03 | 0.6405 | 1.0 | 0.9863 | |
| Partially matched URD | 1.11 | 0.0931 | 1.17 | 0.0360 | |
| Mismatched URD | 1.37 | <0.0001 | 1.51 | <0.0001 | |
| Disease status at HCT (Baseline: Early) | <0.0001 | <0.0001 | Early: 0; Intermediate: 1; Advanced: 2 | ||
| Intermediate | 1.22 | 0.0001 | 0.99 | 0.0137 | |
| Advanced | 1.86 | <0.0001 | 1.83 | <0.0001 | |
| GvHD prophylaxis (Baseline: T-cell depletion) | <0.0001 | <0.0001 | CSA + methotrexate +/− other: 0; Other categories: 1 | ||
| CSA + methotrexate+/− other | 0.81 | <0.0001 | 0.77 | <0.0001 | |
| Tacrolimus + methotrexate +/− other | 1.12 | 0.1108 | 1.07 | 0.4099 | |
| Sex match (Baseline: male to male) | 1.94 | <0.0001 | <0.0001 | Male to male, male to female, female to female: 0; Femal to male: 2 | |
| Male to female | 0.91 | 0.1096 | 0.89 | 0.1087 | |
| Female to male | 1.94 | 0.0009 | 1.25 | 0.0006 | |
| Female to female | 1.10 | 0.1203 | 1.15 | 0.0428 | |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal