Abstract
Abstract 4296
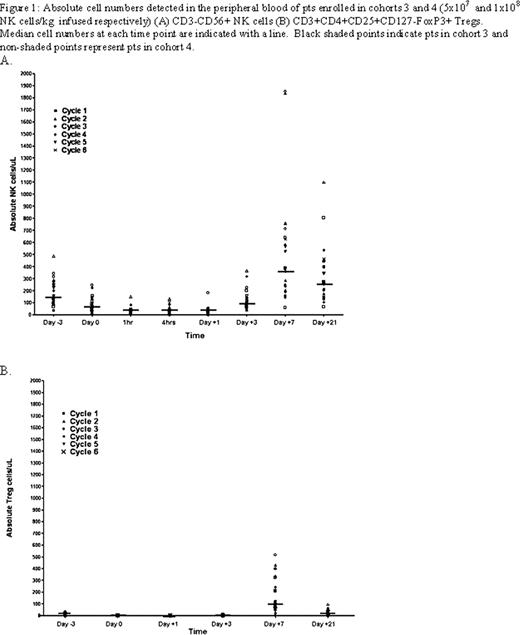
The proteosome inhibitor bortezomib (bort) augments NK cell TRAIL and perforin/granzyme-mediated caspase-8 activity in tumor cells. We have observed that bort sensitizes human tumors to autologous NK cell cytotoxicity in vitro and enhances the antitumor effects of adoptively transferred NK cells in vivo in tumor bearing mice (Lundqvist A et al, Blood 2009). Based on these data, we initiated a clinical trial to explore the safety and antitumor efficacy of infusing escalating doses of adoptively infused ex vivo expanded autologous NK cells following bort treatment in patients with advanced malignancies. Patients (pts) age 18–70 with hematological malignancies or metastatic tumors with progressive disease refractory to conventional therapy were eligible for study. Pts underwent a 15–20 liter apheresis to isolate NK cells that were enriched using immuno-magnetic beads to deplete CD3+ T-cells followed by CD56+ selection. Enriched NK cells (5-8 × 107cells) were expanded in vitro over 2 weeks using an irradiated clinical grade EBV-LCL feeder cell line. Three days prior to NK cell infusion, subjects received a single injection of pentostatin (4mg/m2) to deplete regulatory T-cells followed by a single injection of bort (1.3 mg/m2) 24 hours prior to the infusion of autologous ex vivo expanded NK cells given on day 0. To maintain NK cell viability and TRAIL surface expression, 2 million IU/m2 of IL-2 was given s.c. for 7 days (daily for cohorts 1–2 and q 12 hours for cohorts 3–4). Pts were restaged at 3 weeks and those with disease regression or stable disease were eligible to receive additional cycles. Cohorts of 3–6 pts were enrolled into escalating NK cell dose levels (5 × 106, 1× 107, 5×107, and 1×108 NK cells/kg). To date, 4 dose levels have been completed with a total of 43 adoptive NK cell infusions given to 14 pts. Following a 2 week culture, NK cells expanded a median 184 fold (range 58–683) with 42/43 cultures expanding successfully to achieve the target NK cell dose. NK cells harvested a median 14 days after expansion (range 14–16) contained a median 99.7% (range 96–100) CD3-/CD56+ NK cells, 0% CD3+ T-cells and had a median 87% (range 71–93) viability by 7AAD/Annexin V staining. The absolute lymphocyte count nadired on day +1 at a median 160 cells/ul (range 0–430) and peaked on day 7 at a median 2060 cells/ul (range760-6090). For patients in cohorts 3 and 4, circulating numbers of CD3-/CD56+ NK cells in the blood peaked at day 7 at a median of 382cells/uL (range 60–1851 cells/uL-Figure 1A). Despite an initial decline in circulating Treg (CD3+CD4+CD25+CD127-FoxP3+) numbers following pentostatin therapy, IL-2 administration in these pts was associated with an increase in Treg cells which peaked on day 7 at 112 cells/uL (range 0–700 cells/uL-Figure 1B). The most common adverse events related to treatment were attributable to IL-2 therapy and included grades I-II fever, renal insufficiency, edema and hypotension. Two pts had unexplained and transient grade I and II elevations in hepatic enzymes and one patient developed zoster. Two pts developed elevated free T4 levels and low TSH levels following NK cell therapy (one in dose level 2 and one in dose level 4) consistent with acute thyroiditis; both subsequently developed hypothyroidism with one developing myopathy requiring thyroid replacement therapy. Best clinical response to date includes 6 pts with progressive disease, 7 pts with stable disease (including two pts with metastatic tumors who had more than a 30% decline in serum tumor markers), and 1 minor response in a patient with metastatic kidney cancer. 9/14 (64%) pts received more than 1 NK cell infusion including 3/14 pts who received 3 cycles, 2/14 pts who received 4 cycles, 3/14 pts who received 5 cycles, and 1 patient who received 6 cycles of NK cells before going off study for progressive disease. The trial continues to accrue pts and is currently evaluating the effects of infusing increasing numbers of expanded NK cells given sequentially on days 0 and +5. Conclusions: With the exception of thyroiditis, infusions of up to 1 × 108 ex vivo expanded NK cells/kg have been well tolerated and have provided preliminary clinical evidence for mediating anti-tumor immunity in pts with cancer.
No relevant conflicts of interest to declare.
This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal