Abstract
Abstract 3610
Chronic Lymphocytic Leukemia (CLL) is incurable with conventional chemotherapy treatments. Nurturing of leukemic CLL B cells by marrow microenvironment appears to be one important method of chemoresistance (NEJM 2005).
Epigallocatechin 3 gallate (EGCG), the major catechin in green tea, induces caspase-dependent death CLL B-cells and down regulates anti-apoptotic proteins (Mcl-1; XIAP) critical for enhanced apoptosis resistance of CLL B-cells (Blood 2004). In Phase I and II clinical testing, we found EGCG induced a decline in absolute lymphocyte count and/or lymphadenopathy in the majority of Rai stage 0-II CLL patients (JCO 2009; ASCO 2010).
In these phase I/II studies, trough plasma EGCG levels as high as 8 mM were achieved. Since it does not cause myelosuppression, EGCG has the potential to be combined with other chemotherapeutic agents used for CLL. We previously reported that pharmacologically achievable plasma levels of EGCG have an additive or synergistic effect when combined with fludarabine (F), chlorambucil (C), and FC in the majority of patients (ASH 2009). In this study, we evaluated i) the effect of pharmacologically achievable doses of EGCG when combined with bendamustine (B) and ii) the ability of pharmacologically achievable doses of EGCG to overcome in vitro stromal rescue of CLL B-cells exposed to B or FC.
We first evaluated the effects of EGCG combined with B to establish if this partnership would be additive or synergistic. Primary CLL cells were treated with B (12.5-100 mM) with or without 4 uM EGCG. After 48 hours cells were stained with annexin/PI, and viability analyzed by flow cytometry. Data were analyzed using the CalcuSyn program (Chou & Talay method) to determine the combination index (indicating antagonistic, additive or synergistic effects). Next, we evaluated the ability of 4 mM EGCG to overcome stromal rescue of CLL cells treated with B or FC. To eliminate the cytotoxic effects of FC and B on stromal cells, we treated CLL cells with FC or B for 24 hours, washed with PBS, and then cultured these drug exposed cells directly on the HS-5 human stromal cell line with or without 4 mM EGCG for 48 hours (FC) or 24 hours (B). Cells were harvested and viability of CD19+ cells was assessed by annexin/PI staining. To specifically evaluate the ability of 4 mM EGCG to overcome stromal protection, co-culture samples in which no stromal protection was observed were excluded. Similar experiments were conducted using primary bone marrow mesenchymal stromal cells (MSC) from CLL patients (Leuk Res, 2007). In these experiments the fludarabine/chlorambucil combination was used as an approximation of fludarabine/cyclophosphamide since the active form of cyclophosphamide (4-hydroxycyclophosphamide) rapidly breaks down in aqueous buffer and is not readily available for in vitro use and.
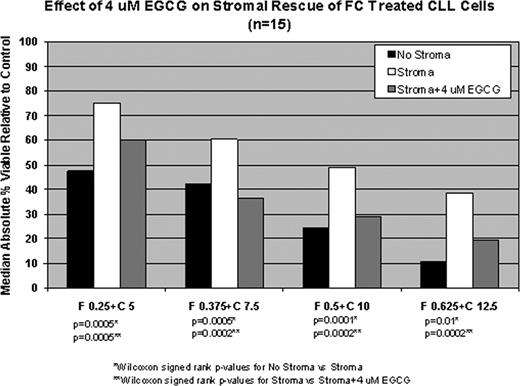
We previously observed additive or synergistic FC and EGCG interactions in a majority of patient samples (ASH 2009). 4 mM EGCG was less consistently beneficial when combined with increasing doses of B in the absence of stroma. Synergistic or mixed synergistic/additive effect was seen in 5 patients, mixed additive/antagonistic effects in 3, and antagonistic effects in 2. Given previously observed synergy with FC and additive/synergistic interactions with at least some B treated samples, we next explored the effects of EGCG on stromal cell rescue. Co-culture with HS-5 stromal cells significantly reduced both B and FC mediated cell death. However the addition of 4 μM EGCG significantly attenuated stromal cell rescue of CLL B-cells exposed to B and FC therapy (Fig. 1A and 1B respectively). Remarkably in the case of FC, 4 mM EGCG almost completely abrogated stromal rescue. Experiments using primary bone marrow MSC cultures from CLL patients yields similar results (n=3, data not shown).
Pharmacologically achievable doses of EGCG appear to reduce stromal rescue of CLL cells treated with B or combinations of purine nucleoside analog/alkylating agents. This occurs in the absence of detectable stromal cell kill by EGCG. These findings, coupled with the favorable toxicity profile and lack of myelosuppression in phase I/II trials of EGCG make it an attractive agent to test in combination with bendamustine or purine analogue/alkylating agent chemo-immunotherapy for patients with CLL.
Shanafelt:Polyphenon Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal