Abstract
Abstract 3412
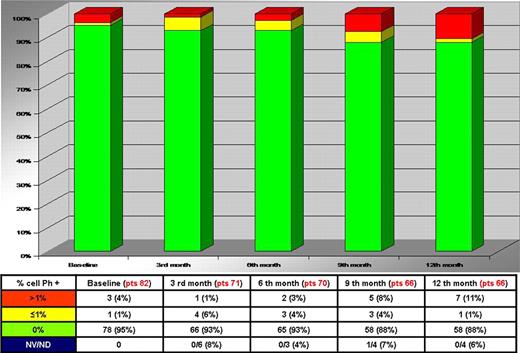
The phase II explorative study of intermittent Imatinib (IM) treatment (InterIM) in elderly patients with Ph + chronic myeloid Leukemia (CML) who achieved a stable complete cytogenetic response (CCgR) after at least 2-years standard IM therapy (any dose between 300 and 800 mg/day) was started in April 2008 and closed for the enrollment in August 2009, since more than 78 patients required by statistics were included into the study. The main objective of the study was to investigate if after 12 months (trial time) the CCgR achieved with standard (daily administration) IM therapy could be maintained with InterIM. For this purpose, the CgR status was assessed by Interphase Fluorescence In Situ Hybridization (I-FISH) on peripheral blood (≥ 200 cells counted) every 3 months. When I-FISH (% Ph + nuclei) increased more then 1%, chromosome banding analysis (CBA) on bone marrow was performed to confirm the loss of CCgR and to check for additional cytogenetic abnormalities (ACA). At the present time, out of the 95 patients who were enrolled, 82 patients were evaluable and out of them 77 (94%), 73 (89%), 71 (87%) and 70 (85%) completed 3, 6, 9 and 12 months of the treatment program, respectively. Therefore, the great majority of patients completed the study core and at the end of 2010 all the patients are expected to complete the trial time (12 mo). During the first 12 months of InterIM, 1% to 11% of the evaluable patients at 3, 6, 9 and 12 months showed an I-FISH >1% Ph+ nuclei (Figure 1).
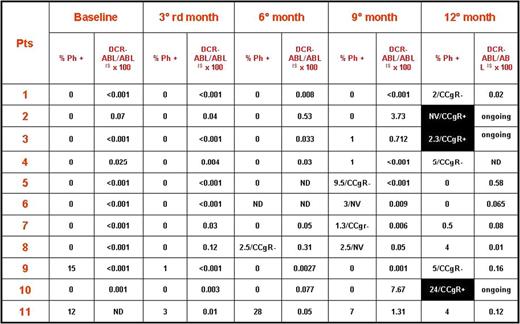
Totally, eleven (13%) out of 82 patients treated with InterIM showed an I-FISH >1% and they were checked by CBA on bone marrow (Figure 2). Out of them only 3 cases, that means 4% of the 82 evaluable patients, lost the CCgR and resumed standard IM therapy (daily administration), but none completed 3 months of therapy. All the patients lost the MMR and increased several folds the BCR-ABL transcript levels. Two pts had a low risk Sokal and one a high risk; age was 66, 69, 77 years; time from diagnosis was 29, 91 and 100 months; duration of IM therapy was 29, 83 and 84 months; the IM dose was 400mg in all cases.
Cytogenetic and molecular response in 11 cases who showed I-FISH >1% + nuclei and who were checked by CBA on bone marrow. Black boxes shows the 3 cases who lost the CCgR
Cytogenetic and molecular response in 11 cases who showed I-FISH >1% + nuclei and who were checked by CBA on bone marrow. Black boxes shows the 3 cases who lost the CCgR
As concern as molecular response, 99% of the patients had a major molecular response (MMR=<0.001-0.1 BCR-ABL/ABLISX 100) at the baseline. The proportion of the patients who maintained the MMR after 3, 6, 9 and 12 months of InterIM was 95%, 92%, 91%, 84%, respectively. Interestingly, we found a weak but significant correlation between the % of BCR-ABL + nuclei and the BCR-ABL transcript levels in the patients who completed the trial time (12 mo) (r=0.27; p=0.001).
In conclusion, the results of the InterIM study core (12 months), clearly show that Intermittent Imatinib (IM) treatment (InterIM) is sufficient to maintain the complete cytogenetic response (CCgR) previously achieved with standard IM therapy in elderly (≥ 65 years) Ph+ CML patients. The risk to loose the CCgR has been very low (4%), while the benefit either in terms of reduction of IM dose and of costs of therapy or in terms of compliance (data not shown) was very high.
This work was supported in part by CML-Leukemia Net and Progetto Regione Lombardia.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal