Abstract
Abstract 3375
Telomere are effective sensors of cell integrity and their accelerated shortening is a marker of genetic and/or proliferative stress in several tissues including the hematopoietic compartment. Severe telomere attrition has been indeed observed in aplastic anemia and post-transplant setting. Little is currently known on the genetic integrity of Ph-negative hematopoietic cells (HCs) repopulating the bone marrow (BM) after successful chronic myeloid leukemia (CML) treatment. We thus decided to verify whether severe telomere shortening might occur in this setting and to assess whether its presence might correlate to genetic and functional impairment of Ph-negative hematopoiesis.
We investigated 81 CML patients with persistent (≥12 months) complete cytogenetic remission (CCyR). Median age was 62 years (23-88), M/F ratio was 1.5. Median time from diagnosis and CCyR were 4 years (1-18) and 3 years (1-12) respectively. 15 patients had acquired cytogenetic abnormalities (CA) (del7: 4 patients, +8: 5 patients, del5q: 2 patients, del or +Y: 2 patients, other CA: 2 patients). Telomere length (TL) analysis was performed by Southern Blotting on polymorphonucleates (PMN) and on monocyte-depleted PBMC (MD-PBMC) to monitor both the myeloid and lymphoid compartments. As control group we analyzed 76 age-matched healthy donors. Prospective follow-up monitoring of TL was performed on 56 CML patients with a median time of 22 months from the first determination (range 12–20).
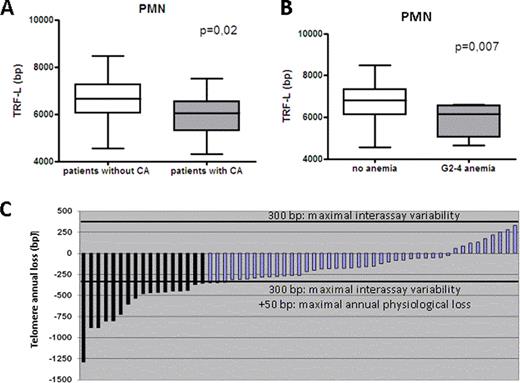
PMN (but not MD-PBMC) from CML patients showed a major erosion of their telomeric DNA (median loss 1294 bp p<0.001). Correlations were sought by using a multivariate general linear model on the whole population (CML patients and controls) and then exclusively on the CML population. In the whole population a previous history of CML was a predictor of TL attrition together with age (both p <0.001). In the CML-only population we found no association between TL and sex, Sokal score, or treatment schedule. Most notably we found a correlation between TL attrition and presence of acquired CA (p=0.02, figure 1A), particularly in case of del7 and +8. Somehow more surprisingly we found an increased TL shortening among patients lacking complete molecular remission (CMolR) (p=0.001). The physiological correlation between age and TL persisted also among CML patients (p=0.003). Moreover we found an association between the presence of short telomeres and G≥2 hematological toxicity of any kind (p=0,005), anemia (p=0,007) and a trend with the presence of neutropenia (p=0,080). The association persisted also when G1-4 toxicities were considered (hematological toxicity of any kind p=0.030, anemia p=0.010). We than made a prospective assessment of the telomere dynamics over time performing a second TL determination after at least 12 months on 56 patients. The overall population showed further significant ongoing telomere shortening that was superior to the expected yearly loss for healthy subjects (median annual telomeric loss of 261 bp). We then performed a patient by patient analysis of TL dynamics over time. None of the patients had evidence of telomere recovery. Moreover even considering the maximal recorded interassay variability of 300 bp and the maximal physiological annual telomeric loss (50 bp), a non-physiological telomeric loss was observed in 17 patients (30% of CML population, median loss of 534 bp, range 1290-357 bp, figure 1C).
i) Ph-negative HCs display severe telomeric loss, compared to healthy controls; ii) telomere erosion is more pronounced in patients with CA and without CMolR; iii) a strong association between shorter telomeres and hematological toxicity (particularly anemia) was observed; iv) telomere loss is persistent over time in the whole population. Moreover one third of them has a clear evidence of ongoing telomere erosion during the remission phase. Our results indicate that Ph-negative hematopoiesis emerging after successful CML treatment suffers from severe and ongoing telomeric stress whose biological and clinical consequences need to be carefully investigated.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal