Abstract
Abstract 2512
Eltrombopag, an oral, nonpeptide thrombopoietin receptor agonist, increased platelet counts, reduced bleeding, and reduced the need for concomitant and rescue ITP medications compared with placebo in the 6-month, phase 3, double-blind, RAISE study (Cheng, 2008). Eltrombopag data specifically in splenectomized patients (pts) are not widely published. We compared splenectomized versus nonsplenectomized pt data from the RAISE study, which is of particular interest because relapsed splenectomized pts have limited treatment options.
In RAISE, 197 adults with previously treated chronic ITP and platelet counts <30,000/μL were randomized 2:1 to local standard of care plus eltrombopag or placebo. Randomization was stratified by splenectomy status, baseline use of concomitant ITP medication, and platelet count ≤15,000/μL. Eltrombopag was initiated at 50 mg/day with dose modifications based on platelet counts. After 6 weeks, baseline concomitant ITP treatments could be tapered or interrupted if clinically indicated.
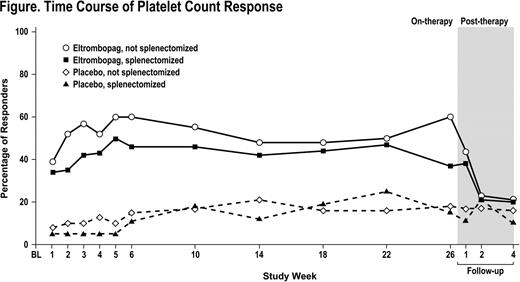
At study entry, 37% (50/135) of eltrombopag pts and 34% (21/62) of placebo pts had been splenectomized. No differences were seen at baseline between subgroups with respect to age, weight, number of prior ITP medications, concomitant ITP medications, or median platelet counts. Median daily dose of eltrombopag was 64 mg and 53 mg for splenectomized and nonsplenectomized pts. Treatment with eltrombopag resulted in an increased percentage of responders (≥50,000/μL and ≤400,000/μL) across all on-therapy nominal visit assessments in each eltrombopag subgroup compared with placebo (Figure). There was no evidence of a difference between splenectomized and nonsplenectomized pts in the odds of responding to eltrombopag vs placebo over the 6-month treatment period (P value for interaction = 0.562; Odds Ratio [OR] (99% confidence interval [CI]) = 6.02 (1.31, 27.57), P=0.002; and OR (99% CI) = 9.41 (3.58, 24.72), P<0.001, respectively). The median maximum duration of continuous response was greater in splenectomized (34 days) and nonsplenectomized (64 days) eltrombopag pts compared to placebo (0 days for each subgroup). No significant difference was seen between splenectomized and nonsplenectomized pts on eltrombopag relative to placebo with respect to: 1) achieving a response for ≥75% of on-treatment assessments (P value for interaction = 0.704); 2) requiring rescue treatments (P value for interaction = 0.988); and 3) reducing concomitant ITP medications (P value for interaction = 0.113). Among nonsplenectomized pts, eltrombopag treatment significantly reduced the use of rescue treatments (P=0.013) and concomitant ITP medications (P=0.006) compared with placebo. Similar trends among splenectomized pts were observed albeit not reaching statistical significance. Eltrombopag treatment resulted in fewer bleeding events (WHO grades 1–4) at all on-therapy assessments in both splenectomized and nonsplenectomized pts compared with placebo. In both splenectomized (P=0.041) and nonsplenectomized (P=0.020) pts, fewer eltrombopag pts experienced clinically significant (WHO grades 2–4) bleeding (38% 19/50 and 29% 25/85, respectively) compared with placebo (70% 14/20 and 45% 18/40, respectively).
Time Course of Platelet Count Response
In both the eltrombopag and placebo groups, more splenectomized pts (placebo: 100%, 20/20 and eltrombopag: 98%, 49/50) experienced adverse events (AEs) than nonsplenectomized pts (placebo: 88%, 36/41 and eltrombopag: 81%, 69/85). The AE profile was similar between splenectomized and nonsplenectomized eltrombopag pts with the most common AEs being headache, diarrhea, nausea, fatigue, and nasopharyngitis. AEs were primarily grade 1–2 in severity.
Eltrombopag increased platelet counts and reduced bleeding symptoms vs placebo regardless of splenectomy status, although median dose was slightly higher in splenectomized pts. A similar effect of eltrombopag vs placebo was seen on duration of response and reduction of concomitant and rescue treatments in splenectomized and nonsplenectomized pts. Although AEs were more common in splenectomized pts for eltrombopag and placebo groups, eltrombopag was generally well tolerated. These results suggest eltrombopag is safe and efficacious regardless of splenectomy status, potentially filling an important unmet need for pts with limited treatment options.
Olney: GlaxoSmithKline: Consultancy. Fogarty: GlaxoSmithKline: Honoraria, Research Funding. Tarantino: GlaxoSmithKline, Novo Nordisk, Talecris, Baxter Cangene: Honoraria, Research Funding, Speakers Bureau. Mayer: GlaxoSmithKline: Employment, Equity Ownership. Vasey: GlaxoSmithKline: Employment. Brainsky: GlaxoSmithKline: Employment.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal