Abstract
Abstract 221
A functional variation in the NKG2D gene regulates NKG2D receptor expression and is associated with better transplant outcomes after fully-HLA-matched unrelated bone marrow transplantation
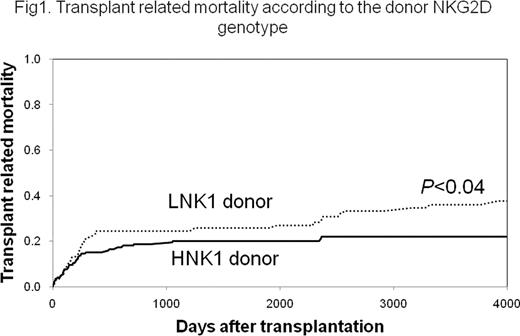
NKG2D, an activating receptor expressed by NK cells and some T cells, plays important roles in both immunity to microbial infections and cancer immune surveillance. A single nucleotide polymorphism (SNP rs1049174) in the 3′-untranslated region (3′UTR) of the NKG2D gene determines two genotypes, HNK1 genotype with high NK cytotoxicity, and the LNK1 genotype with low NK cytotoxicity. Individuals with the HNK1 genotype have reduced risk of cancer (Cancer Res 2006; 66(1):63-70). In the present study, we analyzed the impact of SNP rs1049174 on transplant outcomes after HLA-matched unrelated bone marrow transplantation. The NKG2D genotypes were retrospectively analyzed in a cohort of 360 pairs of patients and their unrelated donors transplanted through the Japan Marrow Donor Program. The donor HNK1 genotype was associated with lower transplant related mortality (TRM) in patients with standard risk (hazard ratio [HR], 0.58; 95% confidence interval [CI], 0.35–0.97; P=0.04) (Fig.1). The donor HNK1 genotype was also associated with a trend toward better OS (HR, 0.69; 95% CI, 0.46–1.04; P=0.08). The host NKG2D genotypes did not significantly influence the transplant outcomes. NK cells from HNK1 donors expressed higher levels of NKG2D on the cell surface, and also expressed higher levels of NKG2D mRNA. Luciferase reporter gene constructs transfected into NK cells showed that the LNK1 construct more effectively attenuated gene expression compared with the HNK1 construct (60% vs. 35%). Bioinformatics tools predicted an interaction between the rs1049174 SNP motif with the micro-RNA-1245 (miR1245). Next, in an mRNA/miRNA binding assay, the LNK allele showed higher affinity to the miR1245 than the HNK1 allele. Moreover transfection of NK cells possessing the LNK1 allele with a miR1245 mimic resulted in 25 fold decrease in mRNA NKG2D level versus only 2 fold decrease in mRNA NKG2D expression in HNK1 allele derived NK cells, thus suggesting that the 3′UTR SNP may regulate the expression of NKG2D through its binding to the putative repressor miR1245. These findings provide evidence that the NKG2D 3′UTR polymorphism is functional, and that the NK cells from donors with the HNK1 genotype exert higher cytotoxicity through their increased expression of NKG2D, thereby resulting in an improved outcome for bone marrow transplantation.
Disclosures:
No relevant conflicts of interest to declare.

This icon denotes an abstract that is clinically relevant.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2010 by The American Society of Hematology
2010

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal