Abstract
Abstract 1636
The erythrocyte redox environment may contribute to increased hemolysis and decreased nitric oxide (NO) bioavailability in pulmonary hypertension (PH) of sickle cell disease (SCD). Glutathione (GSH) is the principal thiol redox buffer in erythrocytes and its depletion has been linked to hemolysis. Glutamine plays an additional anti-oxidant role through preservation of the intracellular nicotinamide adenine dinucleotide (NAD) levels, required for reducing GSSG back to GSH. Altered GSH and glutamine metabolism will promote hemolysis and contribute to altered redox homeostasis. Glutamine depletion and low levels of the obligate NO substrate L-arginine are associated with pulmonary hypertension (PH) in SCD. Low arginine bioavailability is also associated with increased mortality risk. Pilot data of L-glutamine therapy has been associated with improved vasculopathy and increased NAD redox potential in SCD. Targeting these deficiencies in SCD has generated interest, however, little information on the pharmacokinetics (pK) is currently available.
We performed pK studies to determine the metabolic fate of glutamine supplementation on plasma and erythrocyte amino acids in patients with SCD. Patients fasting for > 8 hours received 10 grams of L-glutamine powder mixed with Gatorade. Blood was analyzed at baseline, 30, 60, 90, 2hr, 3hr, 4hr and 8hrs after ingesting study drug. A standardized diet was administered to all participants at 3 established time-points (after 2hr, 5hr and 7 hrs). A subset of patients also had pK studies performed without study drug to follow normal diurnal fluctuations in amino acids.
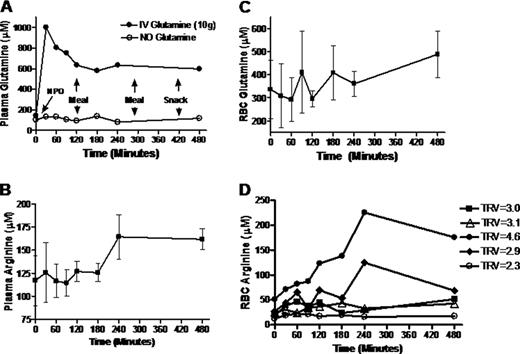
We report data on 5 patients with SCD, three of whom performed pK studies both with and without glutamine supplementation. Average age was 50.6 ±5.6 years, 60% were female, 40% SS, 60% SC, and 4 had PH (mean TRV=3.4±0.8m/s, range 3.0–4.6m/s) while one patient had a TRV=2.25 and no history of PH. Diurnal variations in many amino acids were observed. However plasma glutamine levels more than doubled after oral glutamine supplementation, compared to minimal fluctuations with diet. Plasma glutamine concentration peaked within 30 minutes of ingestion (p=0.01) before decreasing to a plateau by 2 hours that remained higher than baseline by 8 hours. Oral glutamine also increased plasma arginine concentration, which peaked by 4 hrs (p=0.03) and remained elevated through 8 hrs (FigB). Global arginine bioavailability (plasma arginine/(ornithine+ citrulline) also increased (0.67± 0.2 to 1.83±0.6, p=0.02). Erythrocyte glutamine levels began to increase by 8 hours, (Fig 1C), while erythrocyte arginine concentration peaked at 4 hours. Anecdotally, the greatest improvement in intracellular arginine bioavailability occurred in our patient with severe PH and a TRV=4.6 (Fig 1D). The erythrocyte glutamine/glutamate ratio peaked at 1–2 hrs, as did the erythrocyte glutathione concentration, although these trends did not reach statistical significance.
Oral glutamine supplementation (10 gm) acutely improves glutamine and arginine bioavailability in both plasma and erythrocytes. The clinical implications of these observations remain to be determined, however this represents a promising novel therapy for hemoglobinopathies. Phase II studies of glutamine supplementation targeting PH patients with SCD or thalassemia are ongoing at Children's Hospital & Research Center Oakland.
Off Label Use: L-glutamine - an amino acid that may improve arginine bioavailability. IND held by C. Morris.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal