Abstract
Abstract 1379
Although chemoimmunotherapy (CIT) has substantially improved response rates, treatment free survival, and overall survival in patients with chronic lymphocytic leukemia (CLL), only 40–50% of patients achieve a complete remission and the majority have residual disease when evaluated using sensitive assays for minimal residual disease (MRD). While this observation created interest in consolidation therapy, previous alemtuzumab based consolidation trials have demonstrated excess morbidity/mortality. We conducted a lenalidomide based consolidation trial for patients with previously untreated CLL who received 6 cycles of induction with pentostatin, cyclophosphamide and rituximab (PCR).
Eligible patients were previously untreated and had CLL in need of treatment according to the NCI-WG criteria (Blood 111:5446). Treatment schema consisted of 6 cycles of pentostatin (2 mg/m2), cyclophosphamide (600 mg/m2) and rituximab (375 mg/m2) given every 21 days (Blood 109:405). All patients completing 6 cycles of PCR, underwent complete restaging and evaluation for MRD using sensitive flow cytometry (Leukemia 21:956). Following restaging and adequate recovery of blood counts, patients received 6 mos of lenalidomide consolidation by continuous daily administration. The starting lenalidomide dose was 5 mg/day with escalation to 10 mg/day after the first cycle as tolerated. Patients again underwent complete restaging and MRD evaluation after 6 mos of lenalidomide. At the time of this restaging, MRD negative patients entered observation. Those with residual disease continued on lenalidomide and underwent repeat MRD assessment every 3 mos with cessation of lenalidomide when an MRD negative state achieved.
45 patients were enrolled at Mayo Clinic between 3/2008 and 11/2009. 44 patients were eligible for treatment: 71% male, median age 65 (range: 44–78); intermediate Rai risk 61%; high Rai risk 39%. On prognostic testing 32% were CD38+, 52% Zap-70 +, 52% IGHV unmutated, and 16% had high risk FISH (del 17p13; del 11q22).
38 of 44 eligible patients (86%) completed 6 cycles of PCR induction. Adverse events deemed at least possibly related to PCR induction included 19 (43%) patients with grade 3+ hematologic toxicity and 9 (20%) with grade 3+ non-hematologic toxicity. The Overall response rate to induction was 98% with 15 CR/CRi, 3 CCR, 9 nPR, and 16 PR. Four patients with CR were also MRD negative at the completion of induction.
Of 38 patients who completed induction, 34 initiated lenalidomide consolidation. Thus, by protocol intent to treat analysis, 34/44 (77%) patients starting CIT with PCR were able to receive consolidation therapy. The remaining patients were unable to initiate consolidation due to: failure to complete induction (6 patients), disease progression (1), adverse event (1) and patient refusal (2). Among the 34 patients who initiated consolidation, the median number of cycles of lenalidomide received was 5.5 (range: 1–19). Adverse events deemed at least possibly related to lenalidomide consolidation included 22 (65%) patients with grade 3+ hematologic toxicity and 4 (12%) with grade 3+ non-hematologic toxicity. Among the 34 patients who received at least 1 cycle of lenalidomide, 4 patients have improved the quality of their response to date with 11 patients still on lenalidomide at the time of this report.
After median follow-up of 16 mos, 42/44 patients are alive and median duration of response has not been reached. To date 3/44 (7%) of patients have progressed to require additional treatment.
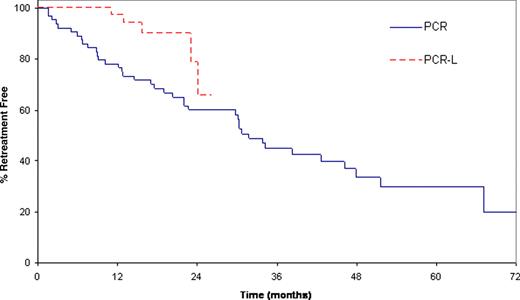
Finally, since eligibility was nearly identical to our historic trial of PCR without lenalidomide consolidation, we compared the 44 patients in the present trial to the 64 patients previously treated on our PCR trial (Blood 109:405). Demographic and prognostic characteristics of the patients in the two studies were similar. With the caveat it represents comparison across phase II trials, the proportion of patients free of retreatment at 12 mos was 97% (95%CI: 91–100) for PCR-L vs. 78% (95%CI: 68–89) for PCR (Figure 1).
We report here the first results of lenalidomide based consolidation for CLL patients receiving first-line CIT induction. Although lenalidomide consolidation appears to improve the quality of response and prolong time to retreatment, longer follow-up is necessary to determine the clinical benefit of this strategy.
Shanafelt:Celgene: Research Funding; Hospira: Research Funding; Genentech: Research Funding. Off Label Use: Lenalidomide. Kay:Celgene: Research Funding; Hospira: Research Funding; Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal