Abstract
Abstract 1230
Chronic myeloid leukemia (CML) is characterized by the presence of the BCR-ABL fusion gene. Different types of BCR-ABL transcripts can be found, due to different genomic breakpoints and alternative splicing. The most frequent transcripts are the e13a2 (b2a2) and the e14a2 (b3a2), that codify for a p210 protein. Occasionally, both transcripts may be present. In the imatinib (IM) era, few data about the prognostic significance of the transcript type are available, particularly in the setting of early chronic phase (ECP): one study suggested that patients with the b2a2 transcript may be more sensitive to IM (de Lemos et al. Genet Mol Res 2005), while two larger studies suggested that patients with b3a2 transcript may have better responses to IM (Vega-Ruiz et al. ASH 2007; Lucas et al. Haematologica 2009). No systematic evaluations in the context of large prospective clinical trials have been performed. AIM: To investigate the influence of the type of BCR-ABL fusion transcript on the responses and the outcome of CML patients treated with IM in ECP.
We performed an analysis of 3 concurrent clinical trials of the GIMEMA CML Working Party (Clin Trials Gov. NCT00514488, NCT00510926 and the observational trial CML/023). Response monitoring was based on conventional cytogenetic examination (bone marrow) and quantitative molecular (Q-PCR) evaluation (peripheral blood). Definitions: Complete Cytogenetic Response (CCgR): 0% Ph+; Major Molecular Response (MMR): BCR-ABL/ABL ratio <0.1% (International Scale); failures: according to the revised European LeukemiaNet criteria (Baccarani et al. J Clin Oncol 2009).
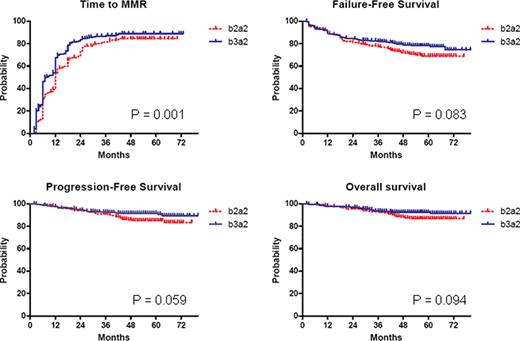
559 consecutive CML patients in early CP have been enrolled from January, 2004 to January, 2007. Patients expressing rare transcript types (e1a2 and e19a2) and patients with the presence of both b2a2 and b3a2 transcripts were excluded: 493 out of 559 patients were evaluable, 203 (41%) with a b2a2 transcript and 290 with a b3a2 transcript (59%). The 2 groups were comparable (no significant difference in sex, age, Sokal and Hasford scores, clonal chromosomal abnormalities in Ph+ cells before IM and imatinib dose). The median observation time is currently 60 (extremes 2–80) months. In patients with b2a2 and b3a2 transcript, the observed 12-months CCgR rates were 77% and 80%, respectively; the cumulative incidence of CCgR was 89% and 88%, respectively (no significant differences). The time to MMR was significantly shorter for patients with b3a2 transcript (fig.1), but the cumulative incidence of MMR was not significantly different (81% and 86% in patients with b2a2 and b3a2 transcript, respectively). The probabilities of Failure-Free Survival, Progression-Free Survival and Overall Survival were 69% and 75%, 83% and 89%, 87% and 92% in patients with b2a2 and b3a2 transcript, respectively (fig. 1); no difference was statistically significant.
In our experience, based on 493 early CP CML patients treated frontline with IM, the type of BCR-ABL fusion transcript had no relevant prognostic impact and no outcome differences have been observed so far.
Work supported by European LeukemiaNet, COFIN, University of Bologna and BolognAIL.
Time to MMR, Failure-Free Survival, Progression-Free Survival and Overall Survival.
Time to MMR, Failure-Free Survival, Progression-Free Survival and Overall Survival.
Castagnetti:Novartis: Honoraria; Bristol Myers Squibb: Honoraria. Gugliotta:Novartis: Honoraria. Martinelli:Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Pfizer: Consultancy. Saglio:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Baccarani:Novartis: Consultancy, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy, Research Funding; Wyeth: Consultancy, Research Funding. Rosti:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Honoraria, Speakers Bureau; Roche: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal