Abstract
Abstract 1028
L-Asparaginase (ASNase) is a key component of multi-agent chemotherapy regimens used in the treatment of pediatric Acute Lymphoblastic Leukemia (ALL). This bacterially-derived enzyme lowers blood asparagine levels by catalyzing the hydrolysis of L-asparagine, leading to specific killing of leukemic blasts. However, relapses and associated therapy resistance occur in about 20% of the patients. Although the molecular mechanisms that contribute to ASNase resistance are not well understood, increased expression of asparagine synthethase (ASNS), the enzyme responsible for asparagine synthesis, has been linked to ASNase resistance both in ALL cell lines and in primary leukemias. We recently reported that micro-deletions affecting the B-cell translocation gene 1 (BTG1) occur in about 10% of pediatric pre-B ALL cases. Here we show that BTG1 loss contributes to ASNase resistance by up-regulation of ASNS.
Using RNA interference, we show that loss of BTG1 expression promotes cell growth and renders pre-B ALL cells completely refractory to ASNase induced cell death. Resistance to ASNase in the BTG1 knockdown cells is accompanied by increased expression of ASNS, while knockdown of this metabolic enzyme is sufficient to reverse therapy resistance, indicating that upregulation of ASNS is required for the observed resistance phenotype. We further show that BTG1 associates with and regulates the activity of the transcription factor ATF4, a key regulator of metabolic stress responses and a central component in the regulatory network that controls ASNS expression.
Together, our experiments identify BTG1 as an important regulator of ASNS expression which acts by modulating ATF4 function. We expect that a detailed molecular understanding of how BTG1 loss contributes to ASNase resistance, will lead to the identification of pharmacological targets that can be used to improve treatment responses in therapy-resistant ALL.
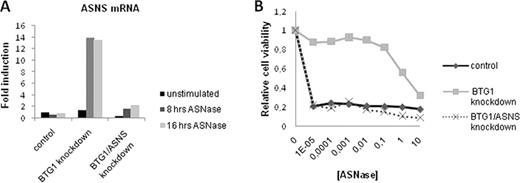
(A) BTG1 knockdown leads to induction of ASNS expression in response to ASNase treatment. Expression of ASNS is efficiently suppressed in the BTG1/ASNS double knockdown cells.
(B) Induction of ASNS expression in the BTG1 knockdown cells causes resistance to ASNase, which is completely reversed by silencing of ASNS mRNA expression.
(A) BTG1 knockdown leads to induction of ASNS expression in response to ASNase treatment. Expression of ASNS is efficiently suppressed in the BTG1/ASNS double knockdown cells.
(B) Induction of ASNS expression in the BTG1 knockdown cells causes resistance to ASNase, which is completely reversed by silencing of ASNS mRNA expression.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal