Abstract
Although individuals with homozygous sickle cell disease (HbSS) share the same genetic mutation, the severity and manifestations of this disease are extremely heterogeneous. We have previously shown that the microRNA expression in normal and HbSS erythrocytes exhibit dramatic differences. In this study, we identify a subset of HbSS patients with higher erythrocytic miR-144 expression and more severe anemia. HbSS erythrocytes are known to have reduced tolerance for oxidative stress, yet the basis for this phenotype remains unknown. This study reveals that miR-144 directly regulates nuclear factor-erythroid 2-related factor 2, a central regulator of cellular response to oxidative stress, and modulates the oxidative stress response in K562 cell line and primary erythroid progenitor cells. We further demonstrate that increased miR-144 is associated with reduced NRF2 levels in HbSS reticulocytes and with decreased glutathione regeneration and attenuated antioxidant capacity in HbSS erythrocytes, thereby providing a possible mechanism for the reduced oxidative stress tolerance and increased anemia severity seen in HbSS patients. Taken together, our findings suggest that erythroid microRNAs can serve as genetic modifiers of HbS-related anemia and can provide novel insights into the clinical heterogeneity and pathobiology of sickle cell disease.
Introduction
Sickle cell disease (SCD) is a hemolytic anemia resulting from a single amino acid substitution in the β-globin gene that renders erythrocytes susceptible to intracellular hemoglobin polymerization in the deoxygenated state. Individuals homozygous for the sickle mutation (HbSS) have the most severe form of SCD, with acute and chronic sequelae.1 Although many general principles of molecular genetics and cell biology have been established in SCD, its phenotypic heterogeneity and variable clinical severity has not been fully explained. Despite having the same HbS mutation, SCD patients display remarkably variable clinical courses in terms of incidence of painful events, severity of anemia, incidence of acute complications (such as stroke and acute chest syndrome), and frequency of end-organ damage (eg, heart disease, renal failure, leg ulcers, pulmonary hypertension).2-4
The erythrocyte is a chief oxidative sink and important mobile detoxifying system in the human body. The effective antioxidant capacity of the red blood cell allows it to serve as an antioxidant for itself as well as other cells and tissues. In doing so, it is susceptible to hemolysis due to various contributors of oxidative stress. One central regulator of antioxidant response is nuclear factor-erythroid 2-related factor 2 (NRF2), a basic leucine zipper transcription factor. Under oxidative stress, NRF2 binds to the antioxidant response element (ARE),5-8 found on the promoters of key genes involved in oxidative stress response. The binding of NRF2 to ARE is important for the coordinately inducible expression of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), GPX1, phase II detoxification enzymes such as NAD(P)H:quinone oxidoreductase (NQO1), and glutathione (GSH) synthesis and processing enzymes such as γ-glutamylcysteine synthetase and GSH reductase. NRF2-regulated expression of ARE-driven genes, especially those involved in GSH biosynthesis and recycling,9 has been shown to be critical for cell survival during oxidative stress in various in vivo and in vitro models.10-12
The importance of NRF2 in the cellular defense mechanism of the erythrocyte is illustrated in mice with targeted deletions of NRF2 or NRF2-dependent genes. NRF2-deficient mice develop hemolytic anemia, increased sensitivity to oxidative stress, decreased GSH, and decreased expression levels of NRF2-dependent genes.13 Targeted deletion of the peroxiredoxin PRDX1, a NRF2-dependent gene involved in preventing oxidative stress damage, also leads to severe hemolytic anemia characterized by an increase in erythrocyte reactive oxygen species (ROS), protein oxidation, hemoglobin instability, and decreased lifespan.14
The normally effective balance between oxidative stress and antioxidant capacity of the erythrocyte is significantly altered in SCD.15,16 Compared with normal erythrocytes, SCD erythrocytes have an increased level of oxidative stress and ROS.17 To prevent hemolysis under this environment, SCD erythrocytes require an even higher antioxidant capacity and enhanced repair mechanisms for oxidant damage. However, what is actually seen in SCD is a significantly lower antioxidative capacity in SCD erythrocytes, with reduced level of GSH18,19 and decreased SOD, CAT, and GPX1 activities.20,21 This reduced antioxidant capacity makes SCD erythrocytes especially susceptible to oxidative insult and hemolysis.16,18,22 The basis for such reduced capacity to defend against oxidative stress in SCD is currently unknown. Once the cells' GSH levels are insufficient, normally protected sulfhydryl groups can become exposed and oxidized, alterations in globin conformation can occur, heme can dissociate from globin, and oxidized membrane and cytoskeletal proteins can become sites for hemichrome binding,23 all of which can lead to hemolysis and are often seen in SCD erythrocytes.19,24,25
In this report, we have analyzed the erythrocyte microRNAs to identify a group of SCD patients with a more severe anemic phenotype. We have found that high erythrocytic miR-144 expression is associated with severe anemia in SCD and describe a potential mechanism supporting this association. Increased miR-144 expression leads to decreased expression of its target NRF2 and its downstream target genes important for oxidative stress tolerance, especially under high oxidative stress environments such as are seen in SCD. The dysregulated miR-144-NRF2 regulatory axis in HbSS cells may explain their compromised antioxidant capacity and susceptibility to oxidative stress, hemolysis, and severe anemia. In summary, our findings suggest that the global analysis of erythrocytic microRNA expression can provide novel insights into the clinical heterogeneity and pathobiology of SCD.
Methods
Analysis of erythrocytic microRNA expression by microarray
This study was conducted with the approval of the Duke University Institutional Review Board, and informed consent from each donor was obtained in accordance with the Declaration of Helsinki. Procedures for blood collection and RNA purification from mature erythrocytes and microarray processing were described.26
Cell culture, cell transfection, RNA, and protein extraction
K562 (ATCC) was maintained in RPMI1640 with 10% fetal calf serum (FCS), glutamine, and antibiotics. Transfection experiments with K562 cells were done using Amaxa nucleofection (Amaxa) or Lipofectamine LTX (Invitrogen) lipofection before RNA isolation using mirVana microRNA Extraction kit (Ambion). For protein analysis, cells were harvested 48 hours after transfection, washed with phosphate-buffered saline, pelleted by centrifugation, and used for indicated assays.
microRNA expression and target gene luciferase reporter constructs
The 3′ untranslated region (UTR) of the NRF2 was amplified using primers (forward: 5′-atttaggaggatttgacc-3′; reverse: 5′-tttttgccagagctaaacaattt-3′) and cloned into the XbaI site downstream of the firefly luciferase gene in the pGL3-control vector (Promega). The NRF2 3′UTR mutant reporters were constructed with QuikChange II Site-Directed Mutagenesis (Stratagene). Expression constructs encoding miR-144, miR-142-5p, and miR-320 were created by insertion into a cytomegalovirus-based pcDNA3 cloning vector (Invitrogen). The NRF2 cDNA clone plasmid was obtained from Origene and was used for the NRF2-without-3′UTR expression plasmid.
3′UTR and ARE-construct luciferase reporter assay
K562 cells were cotransfected with 0.5 μg indicated luciferase reporter, 20 ng Renilla luciferase construct, and 2 μg indicated microRNA expression constructs using Lipofectamine LTX (Invitrogen) in triplicate. After 24 hours, the transfected cells were lysed with lysis buffer (Promega), and the luciferase activity was measured using the Dual-Glo Luciferase assay (Promega) and a luminometer (Lumat; Berthold Technology).
Oxidative stress assay
The cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega). K562 cells were seeded at 2 × 104 cells/well and treated with H2O2 for 6 hours. Primary erythroid cells were stressed for 2 hours. Samples were treated and analyzed in quadruplicate. Results are expressed as a percentage mean of metabolically active cells with untreated control cells set at 100%.
Reverse transcribed–polymerase chain reaction quantification of gene expression
RNA was reverse-transcribed with SuperScript II (Invitrogen) and assayed with primers specific for SOD1, CAT, glutamate-cysteine ligase, catalytic subunit (GCLC), and glutamate-cysteine ligase modifier subunit (GCLM), with glyceraldehyde 3-phosphate dehydrogenase as an internal control.
Intracellular protein expression assay and ELISA
The cells were fixed in 0.025% (vol/vol) glutaraldehyde in phosphate-buffered saline, permeabilized with 0.01% saponin containing 1% (vol/vol) FCS, and incubated with rabbit anti–human SOD1 (Abcam), rabbit anti–human GCLC (Santa Cruz Biotechnology), or rabbit anti–human GCLM (Santa Cruz Biotechnology), and allophycocyanin-labeled goat anti–rabbit secondary antibody (Invitrogen). The enzyme-linked immunosorbent assay (ELISA) was done using the NRF2 TRANS-AM assay (Active-Motif) in triplicate.
Erythroid precursor isolation, differentiation, and analysis
Normal hemoglobin (HbAA) CD34+ cells (ALLCells) and HbSS CD34+ cells were purified from blood collected from apheresis with donor consent according to Duke Institutional Review Board protocol. Mononuclear cells were separated by Ficoll-Hypaque density gradient, and CD34+ cells were enriched by immunomagnetic selection (Miltenyi Biotec) and cultured in StemLine II (Sigma-Aldrich) medium with human serum albumin, insulin, and transferrin. For the first stage of erythroid differentiation (days 0-7), CD34+ cells were stimulated with 50 ng/mL stem cell factor (Invitrogen), 20 ng/mL interleukin-3 (Invitrogen), and 3 U/mL erythropoietin (EPO; Calbiochem). On day 7, the progenitor cells were further enriched for CD71+ using immunomagnetic selection (Miltenyi Biotec). During the second stage (days 8-12), interleukin-3 was removed, and the cells were maintained in both EPO and stem cell factor. In the final stage (days 12-14), the cells were cultured in the same media with the addition of EPO alone. May Grunwald Giemsa staining was used to assess cell morphology and differentiation status. On selected days, the cells were lysed for RNA and protein extraction. RNA expression in progenitor cells was measured relative to RNU6 expression control. Transfection of erythroid precursor cells was done by Amaxa nucleofection on day 8 of differentiation using the CD34 nucleofector kit.
Statistical analysis
Unless otherwise stated, data were analyzed using the unpaired Student t test. Data are expressed as mean plus or minus SEM. P values less than .05 were considered significant.
Results
The identification of SCD heterogeneity based on erythrocyte microRNA expression
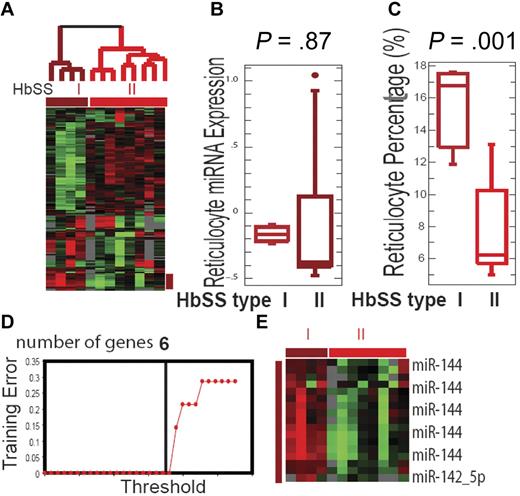
We have previously demonstrated the presence of microRNA in mature erythrocytes and their dysregulated expression in HbSS erythrocytes.26 Because global analysis of gene expression has uncovered novel subtypes of cancers,27,28 we reasoned that similar analysis of erythrocyte microRNA might also identify clinically relevant subtypes within erythrocyte disorders. We performed an unsupervised analysis of 12 HbSS individuals based on a hierarchical clustering of their microRNA expression. Two distinct groups of HbSS patients were readily identified based on the expression of the 141 microRNAs with greater than 1.6-fold variation in at least 2 samples (Figure 1A). Four HbSS samples consistently grouped into one branch (termed HbSS group I), while the remaining 8 samples grouped into a separate branch (termed HbSS group II). This grouping pattern was very robust and reproducible with several other filtering criteria.
Erythrocyte microRNA expression identifies SCD subtypes. (A) The separation of all HbSS patients into 2 groups (HbSS group I, brown, and HbSS group II, red) based on unsupervised analysis of the erythrocyte microRNA expression pattern. (B) The mean expression values of the 83 reticulocyte microRNAs were not significantly different between the 2 HbSS groups (P = .87). (C) The average reticulocyte percentages for group I were significantly higher than group II HbSS patients (P = .001). (D) HbSS subtype classification: training errors are shown as a function of the threshold parameter δ. The value δ = 4.34 is chosen and yields a subset of 6 selected genes and leads to zero error rate. (E) The heat map showing the expression values for the 6 top selected genes in the HbSS groups I and II.
Erythrocyte microRNA expression identifies SCD subtypes. (A) The separation of all HbSS patients into 2 groups (HbSS group I, brown, and HbSS group II, red) based on unsupervised analysis of the erythrocyte microRNA expression pattern. (B) The mean expression values of the 83 reticulocyte microRNAs were not significantly different between the 2 HbSS groups (P = .87). (C) The average reticulocyte percentages for group I were significantly higher than group II HbSS patients (P = .001). (D) HbSS subtype classification: training errors are shown as a function of the threshold parameter δ. The value δ = 4.34 is chosen and yields a subset of 6 selected genes and leads to zero error rate. (E) The heat map showing the expression values for the 6 top selected genes in the HbSS groups I and II.
To rule out reticulocyte contamination as a possible source of the differential microRNA expression seen between these 2 groups, we compared the mean expression of 83 previously defined reticulocyte-enriched microRNAs26 in each group (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and found no significant differences (Figure 1B). This result indicates that the expression differences between the 2 HbSS groups were not based on reticulocyte contamination.
To evaluate the clinical relevance of these HbSS subtypes, we compared the clinical profiles of the patients in these 2 groups. Compared with group II, group I patients had significantly lower hematocrit (Hct; 22% vs 28%, P = .04) and hemoglobin (Hb) level (7.77 vs 9.92, P = .04; supplemental Table 1). Group I patients also had a higher reticulocyte percentage (15.75% vs 7.74%, P = .001; Figure 1C) and absolute reticulocyte count (381 vs 230 × 109, P = .02). Taken together, these results indicated that group I patients exhibited a more severely anemic phenotype than group II patients, with lower Hb and Hct levels and a higher degree of reticulocytosis. On the other hand, these 2 groups did not vary significantly in terms of their gender, age, HbF level, or other clinical parameters (supplemental Table 1). High serum lactate dehydrogenase has been identified as a biomarker for a hemolytic subtype of SCD.29 To determine whether our HbSS groups, based purely on erythrocyte microRNA expression patterns, corresponded to this reported hemolytic subtype of HbSS, we compared serum lactate dehydrogenase and bilirubin but found no significant differences between the 2 groups.
MiR-144 is associated with the severe anemia phenotype in SCD
To identify the microRNA probes required to accurately separate these 2 HbSS groups based on erythrocyte microRNA expression, we used prediction analysis of microarray (PAM) to prioritize the differentially expressed microRNAs from array analysis.30 PAM is a class prediction tool based on the shrunken centroids of gene expression, which computes a standardized centroid for each class and selects the number of genes required to characterize each assigned class with a defined error rate. PAM analysis showed that only 6 probes were needed to achieve 100% accuracy of class prediction (Figure 1D). The top 5 probes selected by PAM were all miR-144 homologs from different species with identical sequences, while the sixth probe was miR-142-5p (Figure 1E). Neither of these microRNAs was reticulocyte-specific, further indicating that these 2 HbSS groups were not identified due to reticulocyte contamination. Both miR-144 and miR-142-5p were expressed at higher levels in all HbSS compared with normal erythrocytes (supplemental Figure 2A). During erythropoiesis, miR-144 is regulated by GATA-1 and transcribed as polycistronic pre-microRNA with miR-451.31 We found that while miR-451 was also expressed at a significantly higher level in the group I HbSS patients (P = .013), its ability to separate 2 HbSS groups was not as significant than that of miR-144 (supplemental Figure 2A). The higher miR-144 expression in HbSS compared with HbAA erythrocyes was further confirmed using real-time polymerase chain reaction (supplemental Figure 2B).
NRF2 is a direct target of miR-144
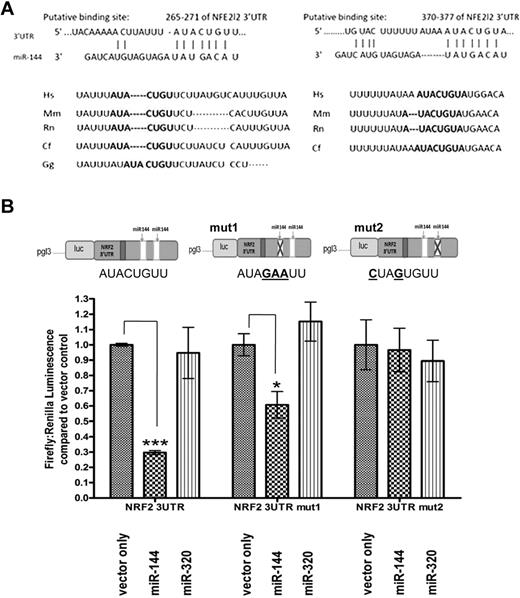
To understand the potential functional connection between miR-144 and anemia severity, we analyzed its predicted target genes using TargetScanS and MiRbase.32,33 Among the predicted targets of miR-144 (supplemental Table 2), we hypothesized that NRF2 could be a functionally relevant target for anemia severity due to its importance in mediating oxidative stress tolerance and to the presence of 2 evolutionarily conserved potential miR-144 target sites in the 3′UTR of NRF2 (positions 265-271 and 370-377; Figure 2A). To determine whether miR-144 directly regulates NRF2, we used a NRF2 3′UTR reporter construct consisting of firefly luciferase reporter followed by the full-length 3′UTR of NRF2 (Figure 2B). Expression constructs for miR-144, miR-320, or empty vector (supplemental Figure 3A) were cotransfected with the NRF2 3′UTR reporter into K562 cells to measure the effect of their respective overexpression on luciferase reporter activity. The expression constructs were made containing the genomic regions of human miR-144 and of miR-142-5p and tested to confirm the overexpression of these microRNAs after transient transfection into K562 cells (supplemental Figure 3B-D). The overexpression of the miR-144 expression construct led to miR-144 levels comparable with the observed difference in HbSS subtypes (supplemental Figures 2B,3B). We found that miR-144 overexpression was able to repress luciferase activity by 70.3% (± 1.37 SEM, P < .0001) relative to the empty vector control, while miR-320, which does not have a target site in the NRF2 3′UTR, did not have a significant effect on luciferase activity (Figure 2B). Thus, NRF2 is a direct regulatory target of miR-144 through the specific interaction between miR-144 and the NRF2 3′UTR. Similar results were also found using HEK293 cells, further confirming this direct miR-144-NRF2 interaction (supplemental Figure 4). To test which of the 2 predicted miR-144 target sites in the 3′UTR of NRF2 were relevant for miR-144–mediated repression, we mutated the 2 binding sites individually on the reporter construct. The mutation of the first target site (position 265-271) led to reduced repression by miR-144 (from 70.3% to 39.3%; ± 8.69 SEM, P = .022), whereas the mutation of the second binding site (position 370-377) completely abolished the repression by miR-144 (Figure 2B). MiR-320 overexpression did not have a significant effect on luciferase activity after mutation of either of these binding sites.
NRF2 is a direct regulatory target of miR-144. (A) Sequence alignment and evolutionary conservation between miR-144 and its 2 putative binding sites in the 3′UTR of NRF2 mRNA from several indicated species, with sequences recognized by miR-144 seed sequence shown in bold. (B) NRF2 3′UTR luciferase reporters (upper panel) were cotransfected with empty vector, miR-144, or miR-320 expression constructs (detailed in supplemental Figure 3). The overexpression of miR-144 significantly repressed the luciferase activity of a NRF2 3′UTR reporter construct. While mutation of the first miR-144 binding site (mut1) only modestly decreased the miR-144–mediated repression, mutation of the second site (mut 2) abolished this effect. MiR-320 does not contain a NRF2 3′UTR binding site and did not significantly alter the luciferase activity of any of the 3 reporters compared with empty vector control. *Significantly different by Student t test: *P ≤ .05, ***P ≤ .0001.
NRF2 is a direct regulatory target of miR-144. (A) Sequence alignment and evolutionary conservation between miR-144 and its 2 putative binding sites in the 3′UTR of NRF2 mRNA from several indicated species, with sequences recognized by miR-144 seed sequence shown in bold. (B) NRF2 3′UTR luciferase reporters (upper panel) were cotransfected with empty vector, miR-144, or miR-320 expression constructs (detailed in supplemental Figure 3). The overexpression of miR-144 significantly repressed the luciferase activity of a NRF2 3′UTR reporter construct. While mutation of the first miR-144 binding site (mut1) only modestly decreased the miR-144–mediated repression, mutation of the second site (mut 2) abolished this effect. MiR-320 does not contain a NRF2 3′UTR binding site and did not significantly alter the luciferase activity of any of the 3 reporters compared with empty vector control. *Significantly different by Student t test: *P ≤ .05, ***P ≤ .0001.
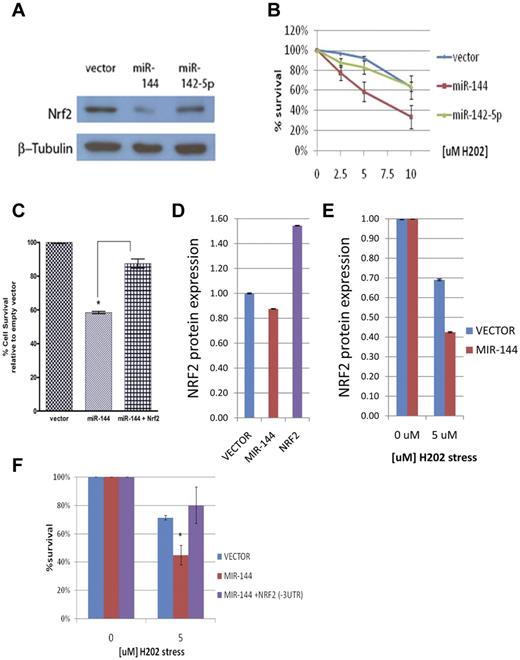
We then sought to test how these microRNAs affect endogenous NRF2 protein levels. Compared with empty vector control and miR-142-5p, miR-144 overexpression led to a detectable change in NRF2 protein level under baseline conditions (supplemental Figure 5A). However, under H2O2-induced oxidative stress, miR-144 led to a significant and reproducible decrease in NRF2 protein level (Figure 3A and supplemental Figure 5B). Taken together, these results indicate that NRF2 is a valid target gene of miR-144, especially under oxidative stress.
MiR-144 modulates oxidative stress tolerance
Given the importance of NRF2 in the transcriptional response to oxidative stress, we hypothesized that miR-144 could directly influence the cellular tolerance to oxidative stress through modulation of NRF2 expression. Although microRNAs have been shown to be modulators of cellular stress,34,35 this has not been reported in erythroid cells. To test the functional effect of miR-144 on oxidative stress tolerance, we transfected miR-144 into K562 cells and measured their survival under the oxidative stress of different hydrogen peroxide (H2O2) concentrations (0, 2.5, 5, and 10μM). We found that miR-144 overexpression leads to a 19.78%, 36.41%, and 47.19% (± 11.6 SEM, P = .0049) increase in sensitivity to 2.5, 5, and 10μM H2O2-induced oxidative stress, respectively (Figure 3B). In contrast, overexpression of miR-142-5p does not lead to significant differences in oxidative stress sensitivity. To test whether this effect is mediated through NRF2, we cotransfected a NRF2 cDNA expression construct with miR-144 and found that this significantly rescued miR-144–mediated sensitivity to 10μM H2O2 oxidative stress (P = .0037; Figure 3C). Furthermore, we used Amaxa nucleofection to transfect primary erythroid cells (supplemental Figure 6) and found that miR-144 overexpression also leads to a detectable reduction of NRF2 protein expression compared with vector control (Figure 3D). The effect of miR-144 on NRF2 protein was more pronounced under oxidative stress (Figure 3E), as seen in K562 cells. Importantly, miR-144 overexpression in primary erythroid cells also leads to significantly increased sensitivity to H2O2-induced oxidative stress (P = .012), and cotransfection of a NRF2 cDNA construct without its 3′UTR rescued this miR-144–mediated sensitivity (Figure 3F). These results indicate that miR-144–mediated NRF2 repression can play a direct and important role in the reduction of oxidative stress tolerance in erythroid cells.
MiR-144 modulates cellular response to oxidative stress. (A) Western blot analysis of NRF2 protein levels after the overexpression of miR-144 and miR-142-5p in K562 cells and H2O2-induced oxidative stress. (B) MiR-144 overexpression leads to significantly increased sensitivity to oxidative stress at indicated concentrations of H2O2 as measured by MTS assay. Each curve represents the indicated sample relative to its unstressed (baseline) control. (C) MiR-144–mediated sensitivity to H2O2 oxidative stress is partially rescued by NRF2 overexpression. (D) ELISA analysis of baseline NRF2 protein levels in primary erythroid cells after miR-144 overexpression compared with vector control. NRF2 overexpression is shown as a positive control. (E) ELISA analysis of NRF2 protein levels demonstrates decreased NRF2 levels after H2O2-induced oxidative stress in the miR-144 transfected primary erythroid cells compared with vector control. (F) MiR-144–mediated sensitivity to H2O2 oxidative stress is fully rescued by NRF2 overexpression without its 3′UTR. *Significantly different by Student t test: *P ≤ .05.
MiR-144 modulates cellular response to oxidative stress. (A) Western blot analysis of NRF2 protein levels after the overexpression of miR-144 and miR-142-5p in K562 cells and H2O2-induced oxidative stress. (B) MiR-144 overexpression leads to significantly increased sensitivity to oxidative stress at indicated concentrations of H2O2 as measured by MTS assay. Each curve represents the indicated sample relative to its unstressed (baseline) control. (C) MiR-144–mediated sensitivity to H2O2 oxidative stress is partially rescued by NRF2 overexpression. (D) ELISA analysis of baseline NRF2 protein levels in primary erythroid cells after miR-144 overexpression compared with vector control. NRF2 overexpression is shown as a positive control. (E) ELISA analysis of NRF2 protein levels demonstrates decreased NRF2 levels after H2O2-induced oxidative stress in the miR-144 transfected primary erythroid cells compared with vector control. (F) MiR-144–mediated sensitivity to H2O2 oxidative stress is fully rescued by NRF2 overexpression without its 3′UTR. *Significantly different by Student t test: *P ≤ .05.
Mir-144 reduces ARE-driven gene expression
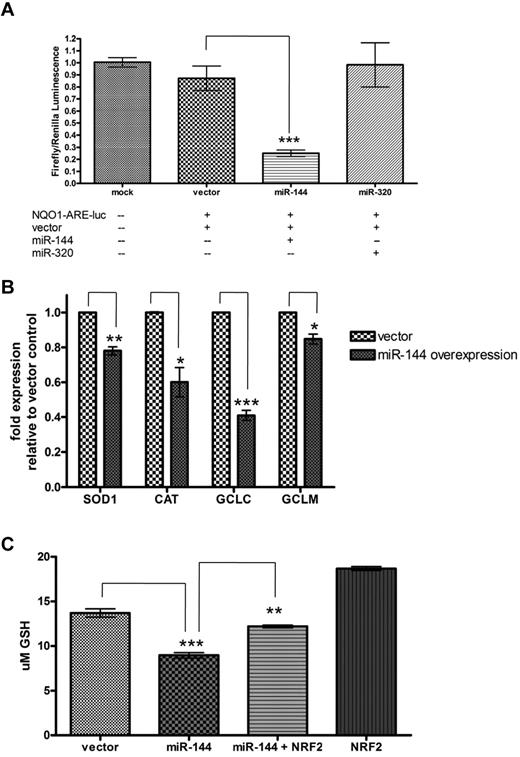
The transcriptional activity of NRF2 in oxidative stress states is mediated by its binding to the antioxidant response elements (AREs) located in the promoter regions of many genes encoding antioxidants and detoxification enzymes. This promoter-enhancer element regulates the basal transcription and induction of these genes through the binding of NRF2 in response to ROS and other forms of oxidative stress. This process is vital to the cellular stress response and survival of many cell types, including erythrocyte precursors. To determine whether miR-144 can have an inhibitory effect on the activation of genes that undergo ARE-mediated up-regulation, we used a luciferase reporter consisting of an ARE promoter element upstream of luciferase cDNA (NQO1-ARE-Luc; a kind gift from Jeffrey A. Johnson). This reporter construct was responsive to the level of NRF2 protein, because the reporter activity was shown to be increased by NRF2 overexpression and reduced with knockdown of endogenous NRF2 by si-NRF2 (supplemental Figure 7A). Next the NQO1-ARE-Luc reporter was cotransfected into K562 cells with empty vector, miR-144, or miR-320 expression constructs to measure their respective effects on ARE-driven gene expression. We found that miR-144 significantly inhibited activity of the ARE luciferase reporter by 62.1% (± 2.64 SEM, P < .0001) relative to the empty vector control and miR-320 did not significantly inhibit reporter activity (Figure 4A). Taken together, this suggests that miR-144 overexpression leads to the subsequent inhibition of ARE-driven gene expression, presumably by its inhibitory effect on NRF2 expression. Similar results were found in HEK293 cells (supplemental Figure 7B).
MiR-144 reduces ARE-driven gene expression and cellular GSH levels. (A) MiR-144, but not miR-320, repressed reporter activity of an antioxidant response element (ARE) luciferase construct in K562 cells. (B) MiR-144 overexpression decreased gene expression of indicated ARE-driven genes and (C) led to significant reduction in cellular GSH after H2O2-induced oxidative stress. This GSH reduction is partially rescued by exogenous NRF2 expression. *Significantly different by Student t test: *P ≤ .05, **P ≤ .001, ***P ≤ .0001.
MiR-144 reduces ARE-driven gene expression and cellular GSH levels. (A) MiR-144, but not miR-320, repressed reporter activity of an antioxidant response element (ARE) luciferase construct in K562 cells. (B) MiR-144 overexpression decreased gene expression of indicated ARE-driven genes and (C) led to significant reduction in cellular GSH after H2O2-induced oxidative stress. This GSH reduction is partially rescued by exogenous NRF2 expression. *Significantly different by Student t test: *P ≤ .05, **P ≤ .001, ***P ≤ .0001.
To further confirm the effect of miR-144 overexpression on the RNA expression of several endogenous ARE-driven genes under oxidative stress, we measured the gene expression of SOD1, CAT, and the catalytic (GCLC) and modulatory (GCLM) subunits of γ-glutamylcysteine synthetase in K562 cells at baseline (supplemental Figure 8A) and after treatment with H2O2-induced oxidative stress (Figure 4B). Compared with empty vector control, miR-144 overexpression led to a 22.0% decrease (± 2.3 SEM, P = .0007) in SOD1 expression, 39.9% decrease (± 8.4 SEM, P = .009) in CAT expression, 59.1% decrease (± 3.0 SEM, P = .0001) in GCLC expression, and 15.2% decrease (± 2.9 SEM, P = .0083) in GCLM expression under oxidative stress (Figure 4B).
MiR-144 decreases cellular GSH concentration under oxidative stress
NRF2-regulated GSH regeneration is critical for the generation and maintenance of cellular response to oxidative stress and for cell survival.9 Because mature erythrocytes lack regenerative capacity after oxidative damage, GSH synthesis and recycling is even more vital to the mature erythrocyte. The role of GSH is to maintain the redox state of protein sulfhydryl moieties and reduce hydrogen and lipid peroxides in erythrocytes before permanent oxidative damage occurs. Given the ability of miR-144 to reduce NRF2 protein and ARE-driven gene expression, we reasoned that miR-144 may also affect cellular GSH concentration. To test this possibility, we overexpressed empty vector, miR-144, miR-144 cotransfected with NRF2, and NRF2 alone (as a positive control) in K562 cells. Total reduced GSH was measured after 2 hours under basal conditions or under 10μM H2O2-induced oxidative stress. Under basal conditions, NRF2 overexpression led to an increase in GSH concentration ([GSH]) of 73.5% (supplemental Figure 8B), while miR-144 overexpression did not lead to significant changes in [GSH]. However, under oxidative stress, there was a significant decrease in [GSH] (34.6% ± 0.6%, P < .0001) in the cells with miR-144 overexpression compared with empty vector control (Figure 4C). This effect was partially rescued by the cotransfection of NRF2, which led to a 23.9% increase (± 3.67%, P = .0007) in [GSH]. Therefore, high miR-144 expression may compromise GSH recycling and restoration during oxidative stress.
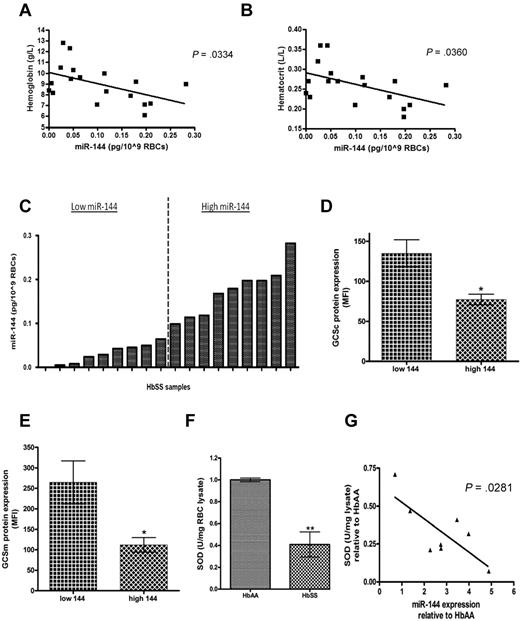
MiR-144 expression levels correlate with antioxidant protein expression in HbSS cells
Because miR-144 was found to be overexpressed in all SCD erythrocytes, particularly among the group with more severe anemia, and was also found to repress the key regulator of oxidative stress response, we sought to determine the relationship between miR-144 expression and antioxidant capacity in SCD erythrocytes using an independent set of 18 HbSS patient samples (supplemental Table 2). Among this cohort, high miR-144 expression was also correlated with the severity of anemia as indicated by lower hemoglobin and hematocrit levels (Figure 5A-B). We measured expression of several antioxidant proteins (GCLC, GCLM, and SOD1) in these SCD erythrocytes using intracellular staining followed by flow cytometric analysis to obtain quantitative single-cell measurements. When we separated the samples into 2 groups (high vs low miR-144) based on miR-144 expression (Figure 5C), we noted that the intracellular expression of GCLC and GCLM was 1.74-fold and 2.37-fold higher, respectively, in low miR-144′ group compared with the high miR-144′ group (Figure 5D-E and supplemental Figure 9).
MiR-144 expression levels correlate with antioxidant protein expression in HbSS mature erythrocytes. (A) Hemoglobin and (B) hematocrit levels correlated inversely with miR-144 expression in mature erythrocytes. (C) Eighteen HbSS samples were grouped into 2 groups based on miR-144 expression. (D) GCLC and (E) GCLM intracellular protein expression in mature erythrocytes were compared and shown as mean fluorescence intensity (MFI) for samples from each group (n = 5 in panel D, n = 4 in panel E). (F) HbSS erythrocytes (n = 8) expressed significantly decreased SOD activity compared with normal HbAA erythrocytes (n = 4). (G) MiR-144 expression in HbSS erythrocytes had a negative correlation with superoxide dismutase activity. Each HbSS sample miR-144 expression (fold relative to HbAA) is shown with corresponding SOD activity.
MiR-144 expression levels correlate with antioxidant protein expression in HbSS mature erythrocytes. (A) Hemoglobin and (B) hematocrit levels correlated inversely with miR-144 expression in mature erythrocytes. (C) Eighteen HbSS samples were grouped into 2 groups based on miR-144 expression. (D) GCLC and (E) GCLM intracellular protein expression in mature erythrocytes were compared and shown as mean fluorescence intensity (MFI) for samples from each group (n = 5 in panel D, n = 4 in panel E). (F) HbSS erythrocytes (n = 8) expressed significantly decreased SOD activity compared with normal HbAA erythrocytes (n = 4). (G) MiR-144 expression in HbSS erythrocytes had a negative correlation with superoxide dismutase activity. Each HbSS sample miR-144 expression (fold relative to HbAA) is shown with corresponding SOD activity.
Although SOD1 protein expression was higher in the low miR-144′ group, this difference did not reach statistical significance (supplemental Figure 10A). Because the SOD1 is the predominant SOD isoform present in mature erythrocytes, we used SOD enzymatic activity in erythrocytes as an alternative means of determining SOD1 levels in HbSS erythrocytes. We first compared SOD1 activity in a cohort of 4 HbAA and 8 HbSS samples and found significantly decreased SOD activity (−40.9%, P < .05) in HbSS samples (Figure 5F). As expected, mature HbSS erythrocytes also demonstrated significantly decreased GSH levels (−32.3%, P < .0001; supplemental Figure 10B), and higher (+83%, P < .0001) levels of ROS (supplemental Figure 10C) compared with HbAA erythrocytes, consistent with previous observations.16,18-20,36 Importantly, even among the HbSS erythrocyte samples, we found that miR-144 expression was significantly inversely correlated with SOD activity (Figure 5G). These results demonstrate that higher miR-144 in erythrocytes is associated with lower levels of NRF2-driven antioxidant proteins among HbSS patients, supporting the role of miR-144 in the modulation of oxidative stress tolerance in SCD.
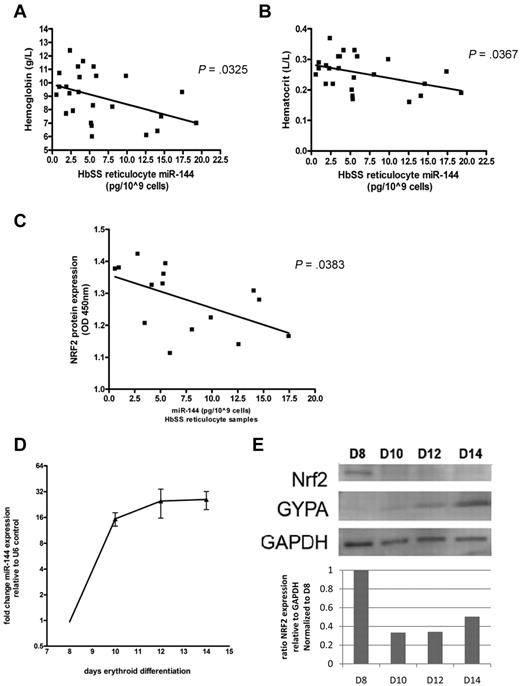
Inverse correlation of miR-144 and NRF2 protein level in HbSS reticulocytes
Because high miR-144 was correlated with poor antioxidant capacity in HbSS mature erythrocytes, we sought to determine the relationship between miR-144 expression and NRF2 protein levels in CD71+ HbSS reticulocytes, using an independent set of 25 HbSS patient samples (supplemental Table 3). Because reticulocytes have recently undergone the process of enucleation, there are still detectable levels of residual NRF2 protein. Among this cohort, high miR-144 expression in HbSS reticulocytes was also correlated with the severity of anemia as indicated by lower hemoglobin and hematocrit levels (Figure 6A-B). Analysis of the level of NRF2 proteins in a subset of this cohort (n = 15) using quantitative ELISA revealed a significant inverse correlation of NRF2 protein levels with miR-144 expression (P = .0383; Figure 6C) in reticulocytes, the precursor cells prior to terminally differentiated erythrocytes.
Correlation of miR-144 and NRF2 protein level in HbSS reticulocytes and during in vitro differentiation. (A) Hemoglobin and (B) hematocrit levels were inversely correlated with miR-144 expression in an independent cohort of reticulocyte samples from 25 HbSS patients. (C) NRF2 protein levels measured by NRF2 ELISA assay in HbSS reticulocytes (n = 15). (D) The induction of miR-144 expression during indicated days of erythroid maturation of HbSS CD34+ progenitors. (E) NRF2 protein levels (upper panel) with densitometric analysis (lower panel) during the corresponding days of erythroid maturation in HbSS progenitors were determined by Western blot and normalized to glyceraldehyde 3-phosphate dehydrogenase. Glycophorin A expression is shown as an additional control for erythroid maturation.
Correlation of miR-144 and NRF2 protein level in HbSS reticulocytes and during in vitro differentiation. (A) Hemoglobin and (B) hematocrit levels were inversely correlated with miR-144 expression in an independent cohort of reticulocyte samples from 25 HbSS patients. (C) NRF2 protein levels measured by NRF2 ELISA assay in HbSS reticulocytes (n = 15). (D) The induction of miR-144 expression during indicated days of erythroid maturation of HbSS CD34+ progenitors. (E) NRF2 protein levels (upper panel) with densitometric analysis (lower panel) during the corresponding days of erythroid maturation in HbSS progenitors were determined by Western blot and normalized to glyceraldehyde 3-phosphate dehydrogenase. Glycophorin A expression is shown as an additional control for erythroid maturation.
Inverse correlation of miR-144 and NRF2 protein level in HbSS erythroid progenitors
To determine the relationship between miR-144 and NRF2 during the erythroid maturation stages prior to the reticulocyte, we performed ex vivo differentiation studies with HbSS CD34+ cells (supplemental Figure 11A). In the purified erythroid precursor cells, we found significant up-regulation of miR-144 expression (Figure 6D) and a corresponding decrease in NRF2 protein level during erythroid maturation (Figure 6E). In addition, we also found that the degree of miR-144 induction was significantly higher in HbSS than HbAA cells during differentiation in identical culture conditions and cytokine milieu (supplemental Figure 11B), suggesting that high miR-144 levels could be a cell-autonomous com-ponent of HbSS erythrocytes. Importantly, NRF2 protein levels were lower in HbSS compared with HbAA erythroid cells at all 4 differentiation stages (supplemental Figure 11C).
Taken together, our study suggests a model in which erythroid precursor miR-144 expression modulates NRF2 expression, alters oxidative stress tolerance, and predetermines the reduced ability of mature red blood cells to tolerate oxidative stress present in SCD, thus contributing to the clinical severity and erythrocyte properties seen in HbSS patients (Figure 7).
Proposed model for biologic consequences of miR-144 overexpression in SCD. We propose that miR-144 is a genetic modifier of oxidative stress tolerance in HbSS erythrocytes and can contribute to the varying clinical severity of SCD.
Proposed model for biologic consequences of miR-144 overexpression in SCD. We propose that miR-144 is a genetic modifier of oxidative stress tolerance in HbSS erythrocytes and can contribute to the varying clinical severity of SCD.
Discussion
SCD is a monogenic disorder with remarkable phenotypic diversity. The microRNAs in erythrocytes may help the understanding of such diversity because they reflect the complex and dynamic nature of the production (erythropoiesis) and destruction (turnover kinetics) that determine erythrocyte phenotypes and clinical manifestations. Here, we have identified the association of high miR-144 expression with a severe anemia phenotype in SCD and further implicated NRF2 and oxidative stress tolerance as the potential mechanistic basis for this association. The high level of miR-144 expression in SCD erythroid progenitors may lead to low levels of NRF2 during erythropoiesis and subsequently to the increased susceptibility of mature HbSS erythrocytes to oxidative damage and hemolysis. This observation is consistent with the hemolytic anemia phenotype seen in mice with disrupted NRF2 loci13 and provides a plausible explanation for the known reduced oxidative stress tolerance in SCD erythrocytes.
In healthy adults, erythrocyte production in the bone marrow maintains the steady-state level of circulating erythrocytes. In SCD, it is important that this process remain effective to compensate for increased hemolysis (termed stress erythropoiesis), which occurs in to quickly counteract this loss. We wonder why stress erythropoiesis in SCD can better compensate for anemia in some patients than in others. There is evidence that this stress erythropoiesis response is distinct from normal erythropoiesis and involves different microenvironmental factors, progenitor cell characteristics, cytokine growth factors, and signaling pathways.37,38 Our analysis of the microRNA expression profile from mature erythrocytes may be illustrative of this difference in ability to compensate for anemia among SCD patients.
Recent studies have found that the miR-144/451 locus is a major downstream effector of GATA-1 in erythroid cells31 and is important in the erythroid differentiation, homeostasis, and fine-tuning of gene expression in erythroid cells in zebrafish.31,39 Recent reports have also shown its importance in mice,40 as disruption of the miR-144/451 locus leads to a mild anemia and is critical for homeostasis under stress conditions.41 The significantly higher miR-144 expression during the in vitro differentiation of HbSS erythroid progenitor cells also suggests that this may be a cell-autonomous phenomenon. In SCD, the average younger age of red cells may also play a role in the dysregulation of miR-144 expression. For example, the anemia and hemolysis in SCD can trigger both stress erythropoiesis42 and an erythropoietin response, which can lead to the erythropoietin-dependent GATA-1 gene expression program and increased induction of miR-14431 in sickle red cell progenitors. In addition, the posttranscriptional stability of individual microRNAs may also be regulated by nontemplated adenines43 or RNA-binding proteins.44 In addition, the steady state of miR-144 level is determined by the balance of novel transcription, processing, and decay. Although miR-144 is part of a polycistronic precursor with miR-451, we show that only miR-144 is associated with anemia severity in SCD. This difference sug-gests that differential processing may play a role. Given the processes of differential regulation are just beginning to be understood in other model organisms,45,46 such differential regulation presents an attractive model to dissect microRNA-specific processing and decay.
Here, we provide evidence of the importance of the dysregulated miR-144-NRF2 regulatory axis in the oxidative stress tolerance among SCD patients with a more severely anemic phenotype. Erythroid cells with high miR-144 expression exhibit lower NRF2 levels, decreased GSH, and greater susceptibility to oxidative damage and hemolysis. In SCD, these properties can make SCD erythrocytes unusually susceptible to the high level of oxidative stress typical in SCD and suggest the presence of a vicious cycle of anemia, stress erythropoiesis, and the generation of erythrocytes with reduced oxidative stress tolerance.
Because NRF2 is a transcription factor that functions in the nuclei, it is unlikely that NRF2 is functional in mature erythrocytes. However, NRF2 and oxidative stress tolerance may play an important role in the developmental stages of erythroid cells, their survival to maturity, and the quality of the mature erythrocyte. In addition, antioxidant capacity is a feature whose importance persists until maturity and in the mature cell is of vital importance to survival and resistance to hemolysis in the absence of the ability to regenerate machinery damaged by oxidative stress. Therefore, the high level of miR-144 expression, reduced levels of NRF2, and antioxidants in the mature erythrocyte may explain the higher susceptibility to hemolysis as at least a partial mechanism for the different anemia severity.
Our findings have several important clinical implications. First, these observations indicate that erythroid microRNAs may contribute to the complex regulatory network of disease modifiers in SCD pathobiology and can aid in the identification and dissection of heterogeneity. When combined with genetic polymorphisms associated with risk for certain SCD clinical manifestations such as stroke,47-49 microRNA expression profiles are likely to enhance our ability to further identify risk of specific complications and disease severity. Second, our data show that miR-144 expression in erythrocytes may serve as useful molecular markers to identify patients who are likely to benefit from antioxidant treatments. Third, in addition to SCD, other anemia disorders (eg, thalassemia and glucose-6-phosphate dehydrogenase deficiency) also manifest enhanced oxidative stress and impaired antioxidant status in their erythrocytes. It will be important to determine whether the dysregulation of miR-144 may also play a role in their reduced ability to deal with oxidative stress and susceptibility to hemolysis. Lastly, these results indicate the potential of using microRNA expression profiles as an indicator for the quality of mature erythrocytes, either in vivo during physiologic and pathologic adaptations or in vitro during the storage of blood products. The unknown functions and the regulation of expression of other erythroid-specific microRNAs could provide further clues to elucidate the remaining mysteries of SCD and other anemia disorders.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the members of the Chi laboratory for feedback, Mardee Delahunty, Darlene Oakley, and the Duke Apheresis Center for assistance with sample collection, and Dr Joanne Kurtzberg and Sophia Avrutsky for assistance and constructive feedback regarding the erythroid progenitor studies.
This research was funded by National Institutes of Health R21DK080994, R01HL079915, and Roche Foundation for Anemia Research (RoFAR). C.S. is supported by the Duke University Program in Genetics and Genomics and the Duke Medical Scientist Training Program.
National Institutes of Health
Authorship
Contribution: C.S. and J.T.C. conceived and designed the experiments; C.S., M.J.T., and J.T.C. performed the experiments and analyzed the data; and C.S., M.J.T., and J.T.C. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jen-Tsan Chi, 101 Science Dr, DUMC 3382, CIEMAS 2177A, Duke Medical Center, Durham, NC 27708; e-mail: chi00002@mc.duke.edu.