Abstract
Bone components participate in the regulation of hematopoietic stem cells in the adult mammal. Vitamin D regulates bone mineralization and is associated with pleiotropic effects in many cell types, including putative roles in hematopoietic differentiation. We report that deletion of the vitamin D receptor (VDR) in hematopoietic cells did not result in cell autonomous perturbation of hematopoietic stem cell or progenitor function. However, deletion of VDR in the microenvironment resulted in a marked accumulation of hematopoietic stem cells in the spleen that could be reversed by calcium dietary supplementation. These data suggest that VDR participates in restricting splenic hematopoiesis through maintenance of bone calcium homeostasis and are consistent with the concept that calcium regulation through VDR is a central participant in localizing adult hematopoiesis preferentially to bone marrow.
Introduction
Hematopoietic stem cell (HSC) function is influenced in part by the cells, extracellular matrix, and minerals of bone.1-6 To further investigate the interplay of hematopoiesis and bone, we evaluated the impact of deleting the vitamin D receptor (VDR). Dihydroxyvitamin D is a well-known modulator of calcium homeostasis, bone integrity, and hematopoietic differentiation.7-9 Our studies indicate that the absence of the VDR has minimal effects on hematopoietic cell number and apparent differentiation but profound effects on primitive hematopoietic cell localization. The data suggest that localization is modulated by the effects of vitamin D on bone and calcium homeostasis.
Methods
VDR animals10 that were backcrossed 9 generations to C57Bl/6 were a kind gift of Marie Demay (Massachusetts General Hospital, Harvard Medical School, Boston, MA). Mice were maintained in a pathogen-free environment and fed irradiated chow: “normal diet” chow (supplied by the animal facility) contained 0% lactose, 1% calcium, and 2.4 IU vitamin D3 per gram chow, whereas “rescue diet” chow (Harlan Teklad) contained 20% lactose, 2.0% calcium, and 2.2 IU vitamin D per gram chow, as per previous publications.11,12 Detailed experimental protocols are described in the figure legends, and they are modified from those previously published.13-15 All animal procedures were approved by the Massachusetts General Hospital subcommittee on research animal care.
Results and discussion
To test whether VDR loss altered the location of primitive hematopoietic cells, we examined the spleens of VDR−/− mice fed a normal diet (“Methods”) for colony-forming unit cell (CFC) number. As shown in Figure 1A, VDR−/− mice have a significant increase in spleen CFCs. To test whether this phenotype extended to HSCs, we transplanted splenocytes in competition with bone marrow (BM) cells into irradiated recipients. As shown in Figure 1B, VDR−/− mice have a significant increase in long-term (16-week) total donor chimerism and in multilineage (B, T, and myeloid cell) engraftment. Because VDR−/− and VDR+/+ animals do not differ in total splenocyte number (Figure 1C), these data in combination with our transplantation data suggest that VDR−/− animals have increased HSC numbers in their spleens. However, this conclusion is not testable statistically because the transplantation experiments were performed using pooled donor cells, whereas the splenocyte counts were performed on individual mice. Together, our results indicate that VDR−/− have an increase in primitive splenic hematopoiesis.
VDR−/− mice have increased HSCs and HPCs in the spleen because of cell-extrinsic effects of VDR loss. (A) Increased HPCs in VDR−/− spleens. VDR−/− and VDR+/+ animals were fed a normal diet immediately after weaning. At 8 weeks of age, animals were killed, and the spleens were removed and crushed through a 40-μm filter. Total splenocyte mononuclear cell (MNC) numbers were obtained with a hemocytometer (trypan blue exclusion). To quantify HPC number, 1 to 2 × 105 splenocytes were plated in M3434 methylcellulose (StemCell Technologies) and scored 12 days later for total colony (defined by a cluster of ≥ 20 cells) number. Colony number was then corrected for total splenocyte number per animal. Bars represent averages; individual mice are represented by individual data points (n = 5). P value was calculated via an unpaired Student t test. (B) Increased HSC frequency in the spleens of VDR−/− animals. Animals were fed a normal diet immediately after weaning. After death at 8 weeks of age, 2 × 106 spleen MNCs from at least 3 pooled VDR+/+ or 3 pooled VDR−/− animals (WT and KO were both CD45.2+) were mixed with 2 × 105 BM MNCs from CD45.1+ (or CD45.1+/CD45.2+ double-positive) WT animals and transplanted into 8 to 10 lethally irradiated (9.5 Gy) CD45.1+ WT hosts. Sixteen weeks after transplantation, stable engraftment was measured by spleen donor contribution to the PB (obtained by tail vein nicking) in the total MNC fraction and in the B-cell (B220+), T-cell (CD4+ or CD8+), and myeloid (CD11b+ or Gr-1+) lineages. All antibodies were purchased from BioLegend, BD Biosciences PharMingen, and eBioscience. Antibody clones used are as follows: GK1.5 (anti-CD4), 53-6.7 (anti-CD8a), RA3-6B2 (anti-B220), RB6-865 (anti–Gr-1), and M1/70 (anti-CD11b). Open columns represent data from WT donors; and filled columns, data from KO donors. Error bars represent SE. P values were calculated using Student t tests. (C) No change in total splenocyte numbers. Animals were fed a normal diet immediately after weaning. After death at 8 weeks of age, spleens were removed and crushed through a 40-μm filter. Total splenocyte MNC numbers were obtained with a hemocytometer (trypan blue exclusion). Bars represent averages. Individual mice are represented by individual data points (n = 9). P value was calculated via an unpaired Student t test. (D) No change in splenic HPCs between VDR−/− and VDR+/+. VDR−/− and VDR+/+ animals were fed a rescue diet immediately after weaning. At 8 weeks of age, animals were killed and analyzed as per the protocol in panel A. Bars represent averages. Individual mice are represented by individual data points (n ≥ 4). P value was calculated via an unpaired Student t test. (E) Little change in HSC frequency after dietary correction. VDR−/− and VDR+/+ animals were fed a rescue diet immediately after weaning. At 8 weeks of age, animals were killed, and splenocytes were transplanted according to the protocol in panel B. Sixteen weeks after transplantation, stable engraftment was measured by spleen donor contribution to the PB (obtained by tail vein nicking) in the total MNC fraction and in the B-cell (B220+), T-cell (CD4+ or CD8+), and myeloid (CD11b+ or Gr-1+) lineages. All antibodies were purchased from BioLegend, BD Biosciences PharMingen, and eBioscience. Antibody clones used are as follows: GK1.5 (anti-CD4), 53-6.7 (anti-CD8a), RA3-6B2 (anti-B220), RB6-865 (anti–Gr-1), and M1/70 (anti-CD11b). Open columns represent data from WT donors; and filled columns, data from KO donors. Error bars represent SE. P values were calculated using Student t tests. (F) No change in total splenocyte numbers. VDR−/− and VDR+/+ animals were fed a rescue diet immediately after weaning. After death at 8 weeks of age, spleens were removed and crushed through a 40-μm filter. Total splenocyte MNC numbers were obtained with a hemocytometer (trypan blue exclusion). Bars represent averages. Individual mice are represented by individual data points (n ≥ 8). P value was calculated via an unpaired Student t test.
VDR−/− mice have increased HSCs and HPCs in the spleen because of cell-extrinsic effects of VDR loss. (A) Increased HPCs in VDR−/− spleens. VDR−/− and VDR+/+ animals were fed a normal diet immediately after weaning. At 8 weeks of age, animals were killed, and the spleens were removed and crushed through a 40-μm filter. Total splenocyte mononuclear cell (MNC) numbers were obtained with a hemocytometer (trypan blue exclusion). To quantify HPC number, 1 to 2 × 105 splenocytes were plated in M3434 methylcellulose (StemCell Technologies) and scored 12 days later for total colony (defined by a cluster of ≥ 20 cells) number. Colony number was then corrected for total splenocyte number per animal. Bars represent averages; individual mice are represented by individual data points (n = 5). P value was calculated via an unpaired Student t test. (B) Increased HSC frequency in the spleens of VDR−/− animals. Animals were fed a normal diet immediately after weaning. After death at 8 weeks of age, 2 × 106 spleen MNCs from at least 3 pooled VDR+/+ or 3 pooled VDR−/− animals (WT and KO were both CD45.2+) were mixed with 2 × 105 BM MNCs from CD45.1+ (or CD45.1+/CD45.2+ double-positive) WT animals and transplanted into 8 to 10 lethally irradiated (9.5 Gy) CD45.1+ WT hosts. Sixteen weeks after transplantation, stable engraftment was measured by spleen donor contribution to the PB (obtained by tail vein nicking) in the total MNC fraction and in the B-cell (B220+), T-cell (CD4+ or CD8+), and myeloid (CD11b+ or Gr-1+) lineages. All antibodies were purchased from BioLegend, BD Biosciences PharMingen, and eBioscience. Antibody clones used are as follows: GK1.5 (anti-CD4), 53-6.7 (anti-CD8a), RA3-6B2 (anti-B220), RB6-865 (anti–Gr-1), and M1/70 (anti-CD11b). Open columns represent data from WT donors; and filled columns, data from KO donors. Error bars represent SE. P values were calculated using Student t tests. (C) No change in total splenocyte numbers. Animals were fed a normal diet immediately after weaning. After death at 8 weeks of age, spleens were removed and crushed through a 40-μm filter. Total splenocyte MNC numbers were obtained with a hemocytometer (trypan blue exclusion). Bars represent averages. Individual mice are represented by individual data points (n = 9). P value was calculated via an unpaired Student t test. (D) No change in splenic HPCs between VDR−/− and VDR+/+. VDR−/− and VDR+/+ animals were fed a rescue diet immediately after weaning. At 8 weeks of age, animals were killed and analyzed as per the protocol in panel A. Bars represent averages. Individual mice are represented by individual data points (n ≥ 4). P value was calculated via an unpaired Student t test. (E) Little change in HSC frequency after dietary correction. VDR−/− and VDR+/+ animals were fed a rescue diet immediately after weaning. At 8 weeks of age, animals were killed, and splenocytes were transplanted according to the protocol in panel B. Sixteen weeks after transplantation, stable engraftment was measured by spleen donor contribution to the PB (obtained by tail vein nicking) in the total MNC fraction and in the B-cell (B220+), T-cell (CD4+ or CD8+), and myeloid (CD11b+ or Gr-1+) lineages. All antibodies were purchased from BioLegend, BD Biosciences PharMingen, and eBioscience. Antibody clones used are as follows: GK1.5 (anti-CD4), 53-6.7 (anti-CD8a), RA3-6B2 (anti-B220), RB6-865 (anti–Gr-1), and M1/70 (anti-CD11b). Open columns represent data from WT donors; and filled columns, data from KO donors. Error bars represent SE. P values were calculated using Student t tests. (F) No change in total splenocyte numbers. VDR−/− and VDR+/+ animals were fed a rescue diet immediately after weaning. After death at 8 weeks of age, spleens were removed and crushed through a 40-μm filter. Total splenocyte MNC numbers were obtained with a hemocytometer (trypan blue exclusion). Bars represent averages. Individual mice are represented by individual data points (n ≥ 8). P value was calculated via an unpaired Student t test.
To test whether VDR loss in the hematopoietic environment was the primary cause of the increased spleen residence of primitive hematopoietic cells, we repeated our analyses using mice that had been placed on the rescue diet (“Methods”) immediately after weaning, a protocol that corrects many of the nonhematopoietic abnormalities in the VDR−/− animals.11,12 As shown in Figure 1D-F, this protocol markedly mitigated or reversed the changes seen in the VDR−/− mice on a normal diet. Although this protocol did not completely reverse the change in the B-cell lineage after splenocyte transplantation, this may be the result of cell-intrinsic effects of VDR loss specifically on the B-cell lineage because the B-cell frequency in the spleens of untransplanted VDR−/− mice on a rescue diet was also significantly increased (data not shown). Hence, our results indicate that the increased spleen residence of primitive hematopoietic cells in VDR−/− animals was the result of loss of VDR−/− in the hematopoietic environment.
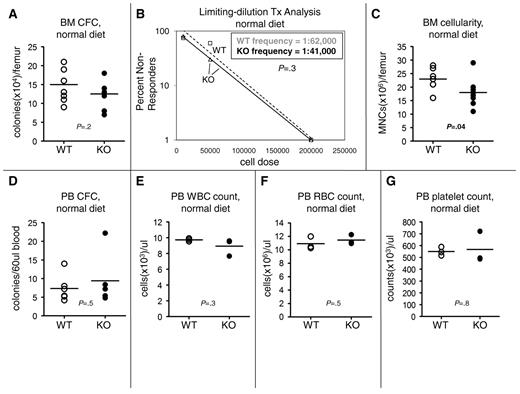
To test whether VDR loss also affected in the BM hematopoietic compartment, we quantified BM CFC numbers. As indicated in Figure 2A, we found no difference in absolute BM CFC numbers between wild-type (WT) and knockout (KO) animals fed a normal diet. Conversely, when we quantified the BM HSC frequency by limiting-dilution transplantation analysis in donor mice fed a normal diet, we found no difference between WT and KO animals (Figure 2B). We repeated our limiting-dilution transplantation analysis on another set of pooled mice fed a normal diet and again found no difference in BM HSC frequency between WT and KO animals (data not shown). In contrast, when we quantified BM cellularity, we found a significant decrease in VDR−/− mice (Figure 2C). When combined with the limiting-dilution transplantation analysis results, our data suggested that KO animals have an absolute decrease in HSCs in the BM. However, this conclusion is not testable statistically because the transplantation experiments were performed using pooled donor cells, whereas the femur counts were performed on individual mice. Finally, we found no difference in BM cellularity between WT and KO animals fed a rescue diet (data not shown), which indicated that the observed cellularity defect was the result of loss of VDR in the hematopoietic environment. Together, our BM analyses indicated that VDR loss has no effect on hematopoietic progenitor cells (HPCs) but may decrease BM HSC numbers, perhaps as a result of increased spleen HSC numbers.
VDR loss alters BM hematopoiesis but not PB hematopoiesis. (A) No change in BM-resident colony number. VDR−/− and VDR+/+ animals were fed a normal diet immediately after weaning. At 8 weeks of age, animals were killed, and the bones were removed and crushed with a mortar and pestle. Total femur MNC numbers were obtained with a hemocytometer (trypan blue exclusion). MNCs were plated in M3434 methylcellulose (Stem Cell Technologies) and scored 12 days later for total colony (defined by a cluster of ≥ 20 cells) number. Colony number was then corrected for total BM MNC number per femur. Bars represent averages. Individual mice are represented by individual data points (n = 8). P value was calculated via an unpaired Student t test. (B) No change in BM HSC frequency. VDR−/− and VDR+/+ animals were fed a normal diet immediately after weaning. At 8 weeks of age, animals were killed, and the bones removed and crushed with a mortar and pestle. BM MNCs from 3 pooled VDR+/+ animals or 3 pooled VDR−/− animals (WT and KO were both CD45.2+) were competed at various doses (2 × 106, 2 × 105, 5 × 104, and 1 × 104) with a constant (2 × 105) number of CD45.1+ WT BM MNCs and transplanted into lethally irradiated (9.5 Gy) hosts (8-10 recipients per cell dose). Sixteen weeks after transplantation, multilineage reconstitution (B220; Gr-1 or CD11b; CD4 or CD8). All antibodies were purchased from BioLegend, BD Biosciences PharMingen, and eBioscience. Antibody clones used are as follows: GK1.5 (anti-CD4), 53-6.7 (anti-CD8a), RA3-6B2 (anti-B220), RB6-865 (anti–Gr-1), and M1/70 (anti-CD11b) in the PB obtained by tail vein nicking by VDR+/+ and VDR−/− donor cells was assessed, and recipient mice, which possessed less than 1% VDR donor cells in any one of the 3 lineages, were scored as nonresponders. Each one of the dosage groups was evaluated for nonresponders. The frequency of multilineage reconstituting HSCs in the original donor marrow was calculated from these data using Poisson statistics in the L-Calc software package (StemCell Technologies). For ease of graphing on a logarithmic scale, groups in which zero nonresponders were present were arbitrarily assigned a “1%” value in panel B (□ represents WT data points; ▵, KO data points). Broken line represents WT trend line; and solid line, KO trend line. P value for the comparison of VDR−/− and VDR+/+ HSC frequency was also calculated in L-Calc using Poisson statistics. (C) Decreased BM cellularity in VDR−/− animals. VDR−/− and VDR+/+ animals were fed a normal diet immediately after weaning. At 8 weeks of age, animals were killed, and the bones removed and crushed with a mortar and pestle. Total femur MNC numbers were obtained with a hemocytometer (trypan blue exclusion). Bars represent averages. Individual mice are represented by individual data points (n = 8). P value was calculated via an unpaired Student t test. (D) No change in PB HPC number. VDR−/− and VDR+/+ animals were fed a normal diet immediately after weaning. At 8 weeks of age, PB was obtained by tail vein nicking. The red blood cells (RBCs) in a defined volume of blood were lysed in ammonium chloride lysis buffer, and the resultant PB MNCs were plated in M3434 methylcellulose (StemCell Technologies) and scored 12 days later for total colony (defined by a cluster of ≥ 20 cells) number. Bars represent averages. Individual mice are represented by individual data points (n = 6). P value was calculated via an unpaired Student t test. (E) No change in PB white blood cell (WBC) count. VDR−/− and VDR+/+ animals (n = 3) were fed a normal diet immediately after weaning. At 8 weeks of age, PB was obtained by tail vein nicking. Complete blood counts (CBCs) were obtained using a Hemavet 850 (Drew Scientific). (F) No change in PB RBC count. VDR−/− and VDR+/+ animals (n = 3) were fed a normal diet immediately after weaning. At 8 weeks of age, PB was obtained by tail vein nicking. CBCs were obtained using a Hemavet 850 (Drew Scientific). (G) No change in PB platelet count. VDR−/− and VDR+/+ animals (n = 3) were fed a normal diet immediately after weaning. At 8 weeks of age, PB was obtained by tail vein nicking. CBCs were obtained using a Hemavet 850 (Drew Scientific).
VDR loss alters BM hematopoiesis but not PB hematopoiesis. (A) No change in BM-resident colony number. VDR−/− and VDR+/+ animals were fed a normal diet immediately after weaning. At 8 weeks of age, animals were killed, and the bones were removed and crushed with a mortar and pestle. Total femur MNC numbers were obtained with a hemocytometer (trypan blue exclusion). MNCs were plated in M3434 methylcellulose (Stem Cell Technologies) and scored 12 days later for total colony (defined by a cluster of ≥ 20 cells) number. Colony number was then corrected for total BM MNC number per femur. Bars represent averages. Individual mice are represented by individual data points (n = 8). P value was calculated via an unpaired Student t test. (B) No change in BM HSC frequency. VDR−/− and VDR+/+ animals were fed a normal diet immediately after weaning. At 8 weeks of age, animals were killed, and the bones removed and crushed with a mortar and pestle. BM MNCs from 3 pooled VDR+/+ animals or 3 pooled VDR−/− animals (WT and KO were both CD45.2+) were competed at various doses (2 × 106, 2 × 105, 5 × 104, and 1 × 104) with a constant (2 × 105) number of CD45.1+ WT BM MNCs and transplanted into lethally irradiated (9.5 Gy) hosts (8-10 recipients per cell dose). Sixteen weeks after transplantation, multilineage reconstitution (B220; Gr-1 or CD11b; CD4 or CD8). All antibodies were purchased from BioLegend, BD Biosciences PharMingen, and eBioscience. Antibody clones used are as follows: GK1.5 (anti-CD4), 53-6.7 (anti-CD8a), RA3-6B2 (anti-B220), RB6-865 (anti–Gr-1), and M1/70 (anti-CD11b) in the PB obtained by tail vein nicking by VDR+/+ and VDR−/− donor cells was assessed, and recipient mice, which possessed less than 1% VDR donor cells in any one of the 3 lineages, were scored as nonresponders. Each one of the dosage groups was evaluated for nonresponders. The frequency of multilineage reconstituting HSCs in the original donor marrow was calculated from these data using Poisson statistics in the L-Calc software package (StemCell Technologies). For ease of graphing on a logarithmic scale, groups in which zero nonresponders were present were arbitrarily assigned a “1%” value in panel B (□ represents WT data points; ▵, KO data points). Broken line represents WT trend line; and solid line, KO trend line. P value for the comparison of VDR−/− and VDR+/+ HSC frequency was also calculated in L-Calc using Poisson statistics. (C) Decreased BM cellularity in VDR−/− animals. VDR−/− and VDR+/+ animals were fed a normal diet immediately after weaning. At 8 weeks of age, animals were killed, and the bones removed and crushed with a mortar and pestle. Total femur MNC numbers were obtained with a hemocytometer (trypan blue exclusion). Bars represent averages. Individual mice are represented by individual data points (n = 8). P value was calculated via an unpaired Student t test. (D) No change in PB HPC number. VDR−/− and VDR+/+ animals were fed a normal diet immediately after weaning. At 8 weeks of age, PB was obtained by tail vein nicking. The red blood cells (RBCs) in a defined volume of blood were lysed in ammonium chloride lysis buffer, and the resultant PB MNCs were plated in M3434 methylcellulose (StemCell Technologies) and scored 12 days later for total colony (defined by a cluster of ≥ 20 cells) number. Bars represent averages. Individual mice are represented by individual data points (n = 6). P value was calculated via an unpaired Student t test. (E) No change in PB white blood cell (WBC) count. VDR−/− and VDR+/+ animals (n = 3) were fed a normal diet immediately after weaning. At 8 weeks of age, PB was obtained by tail vein nicking. Complete blood counts (CBCs) were obtained using a Hemavet 850 (Drew Scientific). (F) No change in PB RBC count. VDR−/− and VDR+/+ animals (n = 3) were fed a normal diet immediately after weaning. At 8 weeks of age, PB was obtained by tail vein nicking. CBCs were obtained using a Hemavet 850 (Drew Scientific). (G) No change in PB platelet count. VDR−/− and VDR+/+ animals (n = 3) were fed a normal diet immediately after weaning. At 8 weeks of age, PB was obtained by tail vein nicking. CBCs were obtained using a Hemavet 850 (Drew Scientific).
To test whether VDR loss affected peripheral blood (PB) hematopoiesis, we quantified the HPC frequency in the PB and found no difference in CFC numbers between WT and KO (Figure 2D). We also measured hematopoietic maturation in the PB and found no change in white blood cell (Figure 2E), red blood cell (Figure 2F), or platelet (Figure 2G) frequency. In addition, no significant differences in neutrophil, monocyte, eosinophil, or basophil numbers, or in hemoglobin levels were observed (data not shown). Together, these data indicated that VDR loss has no effect on PB cell numbers.
The increased residence of primitive hematopoietic cells in the spleens of VDR−/− animals raises the question of the mechanism mediating this effect. Theoretically, an absolute increase in splenic HSCs and HPCs may be the result of increased egress of BM-resident cells to the spleen. However, such an egress would be expected to increase the level of such cells in the PB, and we did not observe increased HPCs in the PB (Figure 2D). Conversely, our results may be the result of increased spleen retention and decreased BM retention/lodgement6 of HSCs and HPCs that normally exit and re-enter the BM.16 This phenomenon would be expected to simultaneously decrease BM levels and increase splenic levels of primitive hematopoietic cells. Our data (Figures 1A-C, 2A-C) are consistent with such a model.
Our results also raise the question of the hematopoietic cell-extrinsic signals mediating our increased spleen residence phenomena. Although our data may immediately suggest a splenic source of the signal, prior studies have shown that VDR−/− mice have defects in multiple tissues. For example, VDR−/− animals have severe osteomalacia, and they fail to properly regulate serum calcium levels.10-12 Hence, the increased spleen residence of HSCs and HPCs may be emanating from an extrasplenic source.
Because extracellular calcium levels have been previously shown to regulate HSC behavior and have been speculated to specifically affect HSC lodgment in the BM,6 we suspect that loss of extracellular calcium regulation in the VDR−/− animals may result in loss of HSC and HPC retention in the BM and increased retention in the spleen. Conversely, during HSC ontogeny, calcium does not appear to be critical; however, calcium receptor function is essential to adult hematopoiesis.6 Hence, because the nonhematopoietic deficiencies in the VDR−/− animals appear after weaning10-12 and because we observed hematopoietic cell-extrinsic alterations in the VDR−/− animals in adult animals, our findings are consistent with an adult-specific role for VDR regulation of calcium in HSC physiology. Together, our results suggest that VDR loss in the hematopoietic environment results in increased splenic residence of HSCs and HPCs, possibly the result of dysregulation of extracellular calcium in the VDR−/− animals.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Brian Garrison, Stephanie Xie, Rod Martin, and the Massachusetts General Hospital and Harvard Stem Cell Institute flow cytometry core facilities for assistance with experiments, and Ying-Hua Wang and Dongsu Park for assistance with data retrieval and display.
D.T.S. was supported by the National Institutes of Health. N.T.J. was supported by the Massachusetts General Hospital.
National Institutes of Health
Authorship
Contribution: N.T.J. designed and performed experiments and wrote the paper; and D.T.S. designed experiments and wrote the paper.
Conflict-of-interest disclosure: D.T.S. is stockholder and consultant for Fate Therapeutics and consultant for Hospira, Genzyme and Bone Therapeutics. N.T.J. declares no competing financial interests.
Correspondence: David T. Scadden, Center for Regenerative Medicine, Massachusetts General Hospital, Harvard Stem Cell Institute, Rm 4265A, 185 Cambridge St, Boston, MA 02114; e-mail: scadden.david@mgh.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal