Abstract
Erdheim–Chester disease (ECD) pathophysiology remains largely unknown. Its treatment is not codified and usually disappointing. Interferon (IFN)-α therapy lacks efficacy for some life-threatening manifestations and has a poor tolerance profile. Because interleukin (IL)-1Ra synthesis is naturally induced after stimulation by IFN-α, we hypothesized that recombinant IL-1Ra (anakinra) might have some efficacy in ECD. We treated 2 patients who had poor tolerance or contraindication to IFN-α with anakinra as a rescue therapy and measured their serum C-reactive protein, IL-1β, IL-6, and monocytic membranous IL-1α (mIL-1α) levels before, under, and after therapy. Another untreated ECD patient and 5 healthy subjects were enrolled as controls. After treatment, fever and bone pains rapidly disappeared in both patients, as well as eyelid involvement in one patient. In addition, retroperitoneal fibrosis completely or partially regressed, and C-reactive protein, IL-6, and mIL-1α levels decreased to within the normal and control range. Beside injection-site reactions, no adverse event was reported. Therefore, our results support a central role of the IL-1 network, which seemed to be overstimulated in ECD. Its specific blockade using anakinra thereby opens new pathophysiology and therapeutic perspectives in ECD.
Introduction
Erdheim–Chester disease (ECD) is a rare non–Langerhans cell histiocytosis (approximately 350 described cases worldwide) characterized by tissue infiltration with lipoid granulomas composed of foamy CD68-positive but CD1a-negative macrophages, in contrast to Langerhans cells. These macrophages are also usually negative for the S-100 protein.1,2 Its classification as a tumoral or inflammatory disease remains controversial, and its etiology and pathophysiologic mechanisms are poorly understood, even though several studies have demonstrated a role of proinflammatory cytokines such as interleukin (IL)-1 and IL-6.3,4
Clinical features range from indolent and isolated bone involvement to more systemic and potentially life-threatening forms. Patients more typically present with symmetrical sclerosis of the diaphysometaphyseal regions of the long bones, but the involvement of other bones and pseudo-infarction or lytic osseous lesions have also been described. Extraskeletal manifestations include pituitary gland and retroperitoneal or periureteral infiltrations, potentially leading to diabetes insipidus or hydronephrosis, respectively. Constitutional symptoms, like hectic fever, are frequent. The prognosis of ECD is poor; 59% of patients die after a mean follow-up of 32 months.2
Several therapeutic approaches have been tested, including corticosteroids, bisphosphonates, and cytotoxic drugs, which are sometimes followed by autologous hematopoietic stem-cell transplantation, but only as case reports or in small series. Results are variable, often with incomplete and/or transient responses and frequent toxicity.3,5,6 More recently, interferon-α (IFN-α) therapy has been suggested as a valuable first-line treatment with variable efficacy and a limited tolerance profile. Moreover, cardiovascular and central nervous system involvements do not seem to be responsive.7-10
The IL-1 receptor antagonist (IL-1Ra) is the natural negative regulator of IL-1, acting via competitive binding to its membranous receptor with resulting anti-inflammatory effects. Subcutaneous administration of IFN-α or IFN-β increases both molecular transcription and the secretion of IL-1Ra,11-15 suggesting that IFN-α therapy might indeed act, at least in part, through modulation of the IL-1 pathway. Therapy with the recombinant form of IL-1Ra (anakinra) has been reported to be effective and safe in several auto-inflammatory diseases, such as cold auto-inflammatory periodic fevers and familial Mediterranean fever, in which IL-1 has a pivotal role.16-20
We successfully treated 2 ECD patients with anakinra because of a contraindication, an incomplete response, or a poor tolerance to IFN-α therapy. We measured some pro-inflammatory cytokines levels before, during, and after anakinra therapy, as well as in another ECD patient who did not receive anakinra and 5 healthy controls to further support our hypothesis regarding the pivotal role, and effective and save therapeutic target of IL-1 in ECD.
Methods
Patients and controls
Two ECD patients with intractable disease and intolerance or contraindication to IFN-α were treated with anakinra as a salvage treatment. ECD diagnosis relied on compatible clinical and radiologic presentations and was histologically proven. Patient 1 had a 15 year history of psychosis, including chronic depression with 6 suicide attempts and dissociation, and had received antipsychotic and antidepressant drugs. IFN-α therapy was thereby contraindicated and she failed to respond permanently to cladribine. Patient 2 had an incomplete response to IFN-α therapy with retroperitoneal involvement and renovascular hypertension; he suffered an IFN-α–related flu-like syndrome and depression, leading to withdrawal. Both patients as well as their families refused combined cytotoxic regimens. Before starting anakinra, patients received information on its potential benefit and toxicity, and they signed consent forms. A third ECD patient was taken as a control. He had stabilized disease with limited bilateral tibial involvement and received no specific treatment. Table 1 summarizes the main disease characteristics and treatments of these 3 patients.
Characteristics of the 3 ECD patients.
| . | Patient 1 (Female, 46 y old, 65 kg) . | Patient 2 (Male, 55 y old, 94 kg) . | Patient 3 (Male, 41 y old, 65 kg) . |
|---|---|---|---|
| Associated disease(s) | Psychosis and severe depression | Obstructive sleep apnea syndrome | Any disease |
| ECD duration | 7 y before starting anakinra | 9 y before starting anakinra | 5 y |
| Clinical and biological manifestations at diagnosis | Recurrent hectic fever, asthenia, leg and lumbar pains, eyelids xanthelasma, thickening, and permanently increased CRP (80-375 mg/L; n < 5 mg/L) | Episodic fevers, asthenia, leg and lumbar pains, and renovascular hypertension | Recurrent and transient diaphyso-metaphyseal pains in the legs |
| Permanently increased CRP (20-40 mg/L), increased serum creatinine level (156μM) | Episodic slight increases of CRP | ||
| Radiological findings at diagnosis | X-ray: tibial inferior metaphyseal pseudo-infarcts | Urography, ultrasonography, computed tomography scan, renal MRI: retroperitoneal fibroinflammatory mass with right ureteral stenosis and hydronephrosis | X-ray: tibial superior metaphyseal condensation and diffuse cortical thickening |
| Computed tomography scan: retroperitoneal and periureteral fibrosis with bilateral hydronephrosis and mesenteric lymph nodes | Arteriography: periarterial lesion with stenosis of the right renal artery | Scintigraphy: tibial increased uptake | |
| Scintigraphy: widespread areas of increased uptake (skull, legs, pelvis bone; liver, lacrimal glands, eyelids, and retroperitoneal space) | Scintigraphy: increased radionuclide uptake of tibias and distal portion of femurs and retroperitoneal spaces | Computed tomography scan: normal | |
| Cerebral MRI: normal | Aorta angio-MRI: abdominal coated aorta | ||
| Positive biopsy | Retroperitoneal tissue and lymph node | Periureteral infiltrate | Tibial metaphysis |
| Treatments and outcomes | Corticosteroids and zoledronic acid: not effective | Corticosteroids: not effective | No specific treatment (stable and limited disease) but episodic prescription of indomethacin |
| Bilateral ureteral stenting | Bilateral ureteral stenting and bilateral renal artery angioplasty, but consequent non-functional right kidney | ||
| Cladribine (2 monthly courses): regression of eyelid involvement and disappearance of mesenteric lymph nodes, regression of skeleton scintigraphic uptake, but persistent retroperitoneal and periureteral fibrosis with bilateral hydronephrosis; severe thrombocytopenia | IFN-α (for 5 y): good response on periureteral infiltration, allowing removal of ureteral stents after 3 mo, but persistent flu-like signs, asthenia; mild mood disturbances | ||
| Interval between last treatment and initiation of anakinra (ECD status) | 13 mo after stopping cladribine (constitutional symptoms, eyelids thickening, persistent retroperitoneal and periureteral fibrosis with bilateral hydronephrosis and aggravation of legs, and pelvis bone involvement on scintigraphy) | 2 y after stopping IFN-α (reappearance of all manifestations, requiring left ureteral stenting) | No treatment with Anakinra |
| . | Patient 1 (Female, 46 y old, 65 kg) . | Patient 2 (Male, 55 y old, 94 kg) . | Patient 3 (Male, 41 y old, 65 kg) . |
|---|---|---|---|
| Associated disease(s) | Psychosis and severe depression | Obstructive sleep apnea syndrome | Any disease |
| ECD duration | 7 y before starting anakinra | 9 y before starting anakinra | 5 y |
| Clinical and biological manifestations at diagnosis | Recurrent hectic fever, asthenia, leg and lumbar pains, eyelids xanthelasma, thickening, and permanently increased CRP (80-375 mg/L; n < 5 mg/L) | Episodic fevers, asthenia, leg and lumbar pains, and renovascular hypertension | Recurrent and transient diaphyso-metaphyseal pains in the legs |
| Permanently increased CRP (20-40 mg/L), increased serum creatinine level (156μM) | Episodic slight increases of CRP | ||
| Radiological findings at diagnosis | X-ray: tibial inferior metaphyseal pseudo-infarcts | Urography, ultrasonography, computed tomography scan, renal MRI: retroperitoneal fibroinflammatory mass with right ureteral stenosis and hydronephrosis | X-ray: tibial superior metaphyseal condensation and diffuse cortical thickening |
| Computed tomography scan: retroperitoneal and periureteral fibrosis with bilateral hydronephrosis and mesenteric lymph nodes | Arteriography: periarterial lesion with stenosis of the right renal artery | Scintigraphy: tibial increased uptake | |
| Scintigraphy: widespread areas of increased uptake (skull, legs, pelvis bone; liver, lacrimal glands, eyelids, and retroperitoneal space) | Scintigraphy: increased radionuclide uptake of tibias and distal portion of femurs and retroperitoneal spaces | Computed tomography scan: normal | |
| Cerebral MRI: normal | Aorta angio-MRI: abdominal coated aorta | ||
| Positive biopsy | Retroperitoneal tissue and lymph node | Periureteral infiltrate | Tibial metaphysis |
| Treatments and outcomes | Corticosteroids and zoledronic acid: not effective | Corticosteroids: not effective | No specific treatment (stable and limited disease) but episodic prescription of indomethacin |
| Bilateral ureteral stenting | Bilateral ureteral stenting and bilateral renal artery angioplasty, but consequent non-functional right kidney | ||
| Cladribine (2 monthly courses): regression of eyelid involvement and disappearance of mesenteric lymph nodes, regression of skeleton scintigraphic uptake, but persistent retroperitoneal and periureteral fibrosis with bilateral hydronephrosis; severe thrombocytopenia | IFN-α (for 5 y): good response on periureteral infiltration, allowing removal of ureteral stents after 3 mo, but persistent flu-like signs, asthenia; mild mood disturbances | ||
| Interval between last treatment and initiation of anakinra (ECD status) | 13 mo after stopping cladribine (constitutional symptoms, eyelids thickening, persistent retroperitoneal and periureteral fibrosis with bilateral hydronephrosis and aggravation of legs, and pelvis bone involvement on scintigraphy) | 2 y after stopping IFN-α (reappearance of all manifestations, requiring left ureteral stenting) | No treatment with Anakinra |
MRI indicates magnetic resonance imaging.
Five healthy adults (blood donors) were taken as controls for peripheral blood mononuclear cell (PBMC) isolation and flow cytometric analyses.
Approval for this study was obtained from the Comité de Protection des Personnes-Ile de France II institutional review board. Informed consent was provided according to the Declaration of Helsinki.
Treatment
Anakinra (Kineret, Amgen Europe BV) was administered to the 2 initial ECD patients through daily subcutaneous injections of 100 mg. The dosage was 1.5 mg/kg for patient 1 and 1.06 mg/kg for patient 2. Treatment was to be stopped in case of poor tolerance or a serious adverse event or at the patient's request and would eventually be stopped after a sustained, complete response was achieved.
Serum cytokines and C-reactive protein measurements
Serum levels of IL-1β, IL-2, IL-6, IL-10, tumor necrosis factor (TNF)-α, and IFN-γ were assessed for all 3 ECD patients by using enzyme-linked immunosorbent assay (ELISA) tests (BioSource Europe). Measurements were made on frozen serum samples obtained before and/or after anakinra treatment from the 2 ECD anakinra recipients and on fresh serum samples from controls and the untreated ECD patient. C-reactive protein (CRP; normal < 5 mg/L) levels were measured at serial intervals.
PBMC isolation and flow cytometry
PBMCs were isolated by Ficoll–Paque from whole blood samples taken before anakinra treatment after 11 months of ongoing treatment (patient 2) or 3 months after having ceased treatment (patient 1, who had previously received anakinra for 25 months). PBMCs were also isolated from one blood sample of untreated patient 3 and the 5 non-ECD controls. PBMCs were frozen at −80°C in dimethyl sulfoxide (DMSO) and then stored until staining. For staining, PBMCs were thawed for 8 hours in RPMI medium supplemented with 10% fetal bovine serum. Staining was performed on 1.5 × 105 cells using fluorescein isothiocyanate (FITC)-coupled anti-CD14 (clone M5E2, Becton Dickinson), allophycocyanin (APC)-coupled anti-CD4 (clone RPA-T4, Becton Dickinson), phycoerythrin (PE)-coupled anti–IL-1α (clone 364-3B3-14, Biolegend), or an appropriate isotype PE control (Beckman Coulter). After cells were washed, 5 × 104 events were analyzed by flow cytometry (FACSCanto, Becton Dickinson). Data were analyzed using FlowJo software Version 9.0.2 (TreeStar) and GraphPad Prism software Version 5.01 (GraphPad Software). Statistical comparisons between the characteristics of healthy donors and patients were based on unpaired t tests. All reported P values are 2-tailed with confidence intervals (CIs) of 95%.
Results
Patient outcomes
Patients 1 and 2 achieved complete and good clinical responses, respectively. Constitutional symptoms totally resolved, leading to a withdrawal or decrease of pain control drugs and a rapid decrease of leg and lumbar pains as soon as the third and fifth days after anakinra therapy in patients 1 and 2, respectively. Episodic fever disappeared in both patients, with patient 1 becoming afebrile within the first 24 hours after anakinra onset. Both patients reported no more asthenia. Patient 1 also experienced a complete resolution of eyelid involvement. Furthermore, the latter while recovering her original performance status also improved both psychiatric and social behaviors.
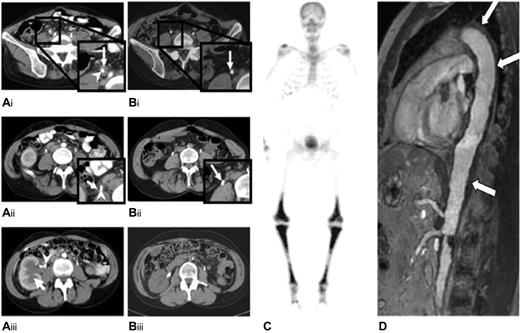
Abdominal computed tomography scans of patient 1 showed a regression of both bilateral fibrotic and stenosing periureteral infiltration and hydronephrosis 3 months after starting anakinra and then a complete disappearance of the infiltrates at 6 months of therapy (Figure 1A-B). Skeletal 99technetium scintigraphy showed a complete disappearance of the previously observed sites of increased uptakes, which had slightly persisted after cladribine treatment, but X-ray lesions on the leg bones remained unaltered. Kidney function returned to normal, and patient 1 stopped anakinra at month 11 on her own initiative because she felt improvement. She subsequently suffered a fever after 10 days off treatment; anakinra restart was allowed, and the patient again had a rapid and complete disappearance of fever. She remained well, and ureteral stents were withdrawn at month 18. She eventually decided on her own to stop the treatment at month 25. At month 32, she started to suffer recurrent mild and intermittent leg pains that were well controlled with paracetamol but had no fever spikes. Treatment with anakinra was reinstituted at month 36 with the same response.
Radiologic findings in patients 1 and 2. Abdominal computed axial tomography scan of patient 1, before (A) and after (B) anakinra. Before starting anakinra, a soft tissue mass was observed surrounding the right ureter (white arrow; A1, A2). Image A3 shows the dilatation of the right renal cavities (white arrow), which required the insertion of a JJ-ureteric catheter (white arrowhead). After 6 months of anakinra treatment, the retroperitoneal fibrosis surrounding the right JJ-catheter had disappeared (B1, B2; white arrow), and the kidney pyelo-ureteral cavities had returned to their normal sizes. (C) A 99m technetium-Methyl diphosphonate bone scan of patient 2 shows symmetrically increased uptake in the diaphyses and metaphyses of the femurs and tibias. (D) Magnetic resonance angiography of the aorta of patient 2 shows periaortic fibrosis (white arrow) with a coated aorta appearance.
Radiologic findings in patients 1 and 2. Abdominal computed axial tomography scan of patient 1, before (A) and after (B) anakinra. Before starting anakinra, a soft tissue mass was observed surrounding the right ureter (white arrow; A1, A2). Image A3 shows the dilatation of the right renal cavities (white arrow), which required the insertion of a JJ-ureteric catheter (white arrowhead). After 6 months of anakinra treatment, the retroperitoneal fibrosis surrounding the right JJ-catheter had disappeared (B1, B2; white arrow), and the kidney pyelo-ureteral cavities had returned to their normal sizes. (C) A 99m technetium-Methyl diphosphonate bone scan of patient 2 shows symmetrically increased uptake in the diaphyses and metaphyses of the femurs and tibias. (D) Magnetic resonance angiography of the aorta of patient 2 shows periaortic fibrosis (white arrow) with a coated aorta appearance.
Patient 2 had a similarly good response with urologic involvement, leading to the withdrawal of the left ureteral stent. Ureteropyelography performed 10 months after anakinra onset found that the stenosis had totally subsided, but that some abnormalities persisted, including slight residual segmental ureteral hypotonia and dilatation. Conversely, no radiologic improvement was noticed on skeletal scintigraphy, and the periaortic infiltrate shown in Figure 1C-D, remained unchanged at months 8 and 11, respectively. On this basis, treatment with anakinra was continued, with 26 months of follow-up from its onset.
CRP measurements
Both treated ECD patients exhibited a dramatic decrease and normalization of previously permanently increased CRP levels as soon as the 5th day of anakinra therapy. Thereafter, CRP values remained within the normal range for the entire follow-up period while under treatment. When patient 1 stopped treatment at month 11, her CRP level slightly increased to 10 and 18 mg/L at days 9 and 15 after ending therapy, respectively. A new and rapid normalization of the CRP level was seen 3 days after restarting treatment. After the second treatment cessation at month 25 the CRP level slightly increased again, ranging between 7 and 16 mg/L for the following 9 months off therapy; stable normalization was observed after restarting treatment.
For patient 3, who received no specific therapy, the CRP levels fluctuated between normal values and 6 to 10 mg/L during his entire 35 months of follow-up.
Tolerance of anakinra
Patient 1 reported intermittent diffuse pruritus without cutaneous lesions or rash. Patient 2 had some cutaneous redness and tenderness at the injection sites. Clinical and usual biological monitoring (including blood cell counts, electrolytes, and liver parameters) revealed no adverse effects from the treatment.
Cytokine measurements
IL-2, IL-10, and IFNγ levels were within the normal ranges at all times in all 3 ECD patients. For the inactive-untreated patient (patient 3) and before treatment for patients 1 and patient 2, 2 levels of IL-1β performed at 4- to 8-week intervals were within the normal range. One additional dosage for patients 1 (during a fever spike) and 3 (under a nonclinically active manifestation of the disease) were found to increase 1.36-fold (20.4 pg/mL) and 1.5-fold (22.5 pg/mL) compared with the normal value, respectively. TNF-α and IL-6 levels were increased in all 3 ECD patients: from 1.1 to 2.90 times (22-58 UI/mL) the normal value for TNF-α, with the highest value observed for patient 2; and from 1.4 to 28.3 times (12-243 pg/mL) the normal value for IL-6, with the highest increase observed for patient 1.
Under treatment with anakinra, cytokines levels were performed only for patient 1 (Table 2). Only IL-6 levels appeared to parallel the CRP decrease in patient 1. Indeed, CRP and IL-6 levels were highest before treatment with anakinra and then decreased at the same time to normal values during treatment. TNFα values were increased during both before and ongoing anakinra treatment periods, and reached the normal value only at the last measurement under treatment. IL-1β levels ranged between normal and high values for both before and during treatment periods.
TNF-α, IL-1β, IL-6, and CRP levels for patient 1.
| . | Before treatment (1) . | Before treatment (2) . | D1 anakinra . | M3 anakinra . | M5 anakinra . | M6 anakinra . |
|---|---|---|---|---|---|---|
| TNF-α (n < 20 UI/mL) | 26.4 | 57.2 | 33 | 48 | 40 | 7.4 |
| IL-1β (n < 15 pg/mL) | 3.6 | 2.2 | 20.4 | 19.5 | 12 | 45.6 |
| IL-6 (n < 8.6 pg/mL) | 243 | 38.7 | 101 | 2.6 | 0.9 | 0 |
| CRP (N < 5 mg/L) | 275 | 232 | 256 | 3 | 4 | 5 |
| . | Before treatment (1) . | Before treatment (2) . | D1 anakinra . | M3 anakinra . | M5 anakinra . | M6 anakinra . |
|---|---|---|---|---|---|---|
| TNF-α (n < 20 UI/mL) | 26.4 | 57.2 | 33 | 48 | 40 | 7.4 |
| IL-1β (n < 15 pg/mL) | 3.6 | 2.2 | 20.4 | 19.5 | 12 | 45.6 |
| IL-6 (n < 8.6 pg/mL) | 243 | 38.7 | 101 | 2.6 | 0.9 | 0 |
| CRP (N < 5 mg/L) | 275 | 232 | 256 | 3 | 4 | 5 |
D1: day 1 (just before anakinra injection); M3: month 3; N: normal values; Numbers written in bold-faced type correspond to levels outside normal values
IL-1α expression on peripheral monocytes
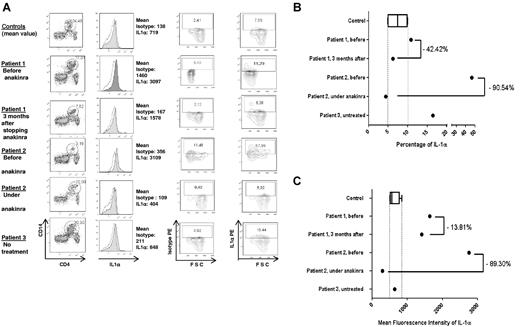
Active and untreated ECD patients had a high expression of IL-1α on monocytes cell surface (Figure 2A-C).
IL-1α expression in PBMCs in 3 ECD patients and 5 healthy controls (CT). (A) Results of flow cytometric analysis for 1 healthy control and the 3 ECD patients. For patient 1, analysis was performed before and 3 months after the end of anakinra treatment, and for patient 2, analysis was conducted before and after 11 months of ongoing treatment. Patient 3 had no treatment. The first column shows the percentage of CD14+CD4dim PBMCs. The second column represents membranous IL-1α expression (colored histogram) compared with the control isotype, with the mean fluorescence intensity (MFI) of each antibody. Column 4 shows the percentage of IL-1α–positive monocytes, and column 3 shows that of the control isotype in the same cells. (B-C) Respectively, the percentage of IL-1α–positive monocytes and the MFI of IL-1α on monocytes for the 5 healthy controls compared with that of the 2 ECD patients before and after treatment with anakinra (patients 1 and 2) and the untreated patient 3. The final levels on (B) and (C) were calculated by subtracting the measured levels of isotype from those of IL-1α on (A); second column for the MFI; third and fourth columns for the percentage.
IL-1α expression in PBMCs in 3 ECD patients and 5 healthy controls (CT). (A) Results of flow cytometric analysis for 1 healthy control and the 3 ECD patients. For patient 1, analysis was performed before and 3 months after the end of anakinra treatment, and for patient 2, analysis was conducted before and after 11 months of ongoing treatment. Patient 3 had no treatment. The first column shows the percentage of CD14+CD4dim PBMCs. The second column represents membranous IL-1α expression (colored histogram) compared with the control isotype, with the mean fluorescence intensity (MFI) of each antibody. Column 4 shows the percentage of IL-1α–positive monocytes, and column 3 shows that of the control isotype in the same cells. (B-C) Respectively, the percentage of IL-1α–positive monocytes and the MFI of IL-1α on monocytes for the 5 healthy controls compared with that of the 2 ECD patients before and after treatment with anakinra (patients 1 and 2) and the untreated patient 3. The final levels on (B) and (C) were calculated by subtracting the measured levels of isotype from those of IL-1α on (A); second column for the MFI; third and fourth columns for the percentage.
Before anakinra, the mean fluorescence intensity (MFI) of IL-1α expression on monocytes was increased in patients 1 and 2 (1637 and 2753, respectively) compared with the healthy controls (mean 613.3 ± 76.86) and untreated inactive-ECD patient 3 (637; Figure 2C).
After treatment, the MFI decreased for both patients 1 and 2 (1411 and 295, respectively) even though the reduction was less pronounced for patient 1. Of note for patient 1, the analysis was performed 3 months after a 25-month period of treatment. At this time the patient had a slight disease flare with an increase of CRP level to 15 mg/L. In contrast, patient 2 was under anakinra ongoing therapy at the time of IL-1α analysis and had a normal CRP level. On Figure 2B, like for MFI measurements, the percentages of IL-1α–positive monocytes decreased for both treated patients 1 and 2 (from 10.87% to 6.26% and from 46.51% to 4.4%, respectively) after treatment with anakinra to the levels of those of healthy controls (7.45% ± 1.38%). It is interesting to note that, unlike the MFI, the percentage of IL-1α–positive monocytes of the untreated inactive-ECD patient 3 (16.42%) was higher than the values of those of healthy controls.
Discussion
Whereas the pathophysiologic mechanisms involved in ECD are largely unknown, our results support the use of anakinra to treat ECD patients and, therefore, our hypothesis on the pivotal role of the IL-1 pathway in ECD. Our 2 ECD patients treated with anakinra indeed achieved both clinically and biologically complete or good responses, notably for constitutional symptoms and fever, as soon as 24 hours to a few days after the first injection. A response was also observed in retroperitoneal fibrosis; although this response was not complete for one of the patients, it allowed for the removal of ureteral stents in both patients.
Although the use of IFN-α therapy in ECD was based on its multifunctional, redundant and pleiotropic activities for regulating the immune system and responses,7,8 several authors have shown, in other contexts, that IFN-α therapy can indeed up-regulate and increase the synthesis of IL-1Ra.11-15,21 Wan et al demonstrated that both IFN-α and -β led to enhanced transcription and synthesis of IL-1Ra through the initiation of signal transducers and transcription activators (STAT)-6.22 In addition, Kovalovsky et al23 showed that IFN-α was able to reverse the inhibitory effects of corticosteroids on IL-1Ra expression and secretion and that the effects of IFN-α were blocked by pretreatment of monocytes with anti–IL-1β blocking antibodies. In an ECD patient, Myra et al3 used quantitative reverse transcription polymerase chain reaction analyses to find higher PBMC expression of IL-1β, IL-1α, IL-2, and IL-8 compared with healthy controls. Our results similarly suggest the overexpression of monocytic mIL-1α in active ECD patients, which decreased after treatment with anakinra.
Thus, justification is emerging for treatments targeting monocytes and their pro-inflammatory cytokines, especially IL-1, in ECD, Langerhans cell histiocytosis, and related disorders. IL-1 family cytokines mainly include IL-1α and IL-1β, which are essentially produced by monocytes and macrophages, and their natural competitive antagonist receptor IL-1Ra.24,25 IL-1α and IL-1β both play a leading role in the inflammatory process as the most pyrogenic fever-inducing cytokines besides their downstream IL-6 cytokine,26 leading to the increase of CRP level. IL-1α is expressed at the membrane surface after cell stimulation and acts through autocrine and juxtacrine (ie, intercellular contacts) actions.17 In contrast, IL-1β is a circulating protein with paracrine actions, for which activation is controlled by the so-called “inflammasome complex.” Anakinra was shown to be effective in several autoinflammatory systemic diseases, including cryopyrin-associated periodic syndrome (CAPS),17-20,27 in which the “IL-1β inflammasome” function is abnormally increased because of a NLRP3 receptor gene mutation within monocytes, leading to a greater amount of circulating IL-1β.16,17,28-30 Although anakinra was effective, we did not observe any significant increase of IL-1β levels in our patients. Hence, the efficacy of anakinra could also be related to the blockade of juxtacrine interactions between monocytic cells leading to down-regulation of membranous IL-1α overexpression. Likewise under treatment, the number of monocytes expressing IL-1α and the level of this expression decreased for both patient 1 and patient 2. However, these effects were less pronounced for patient 1, probably because the levels of these parameters before anakinra therapy were lower. These differences could be related to previous treatments between both patients. Patient 1 had been treated 13 months before with cladribine, which may have induced long term reduction of monocytes number and activation, whereas patient 2 did not receive cytotoxic drugs.
Other mechanisms might be implicated in ECD, such as a decreased production or impaired function of IL-1Ra.31-33 2 research groups recently described an auto-inflammatory disease related to an IL-1Ra gene mutation named Deficiency of the Interleukin-1 Receptor Antagonist because of the deficit of IL-1Ra.34,35 This last phenomenon indeed shares peculiar fibrosclerosing and lytic bone lesions and exhibits similar outcomes under therapeutics (ie, no significant improvement with corticosteroids but a dramatic response with anakinra).2,7,36
The response to anakinra on ECD might be variable according to the organ(s) involved, as already reported with IFN-α, which appears to be slowly effective on bone lesions and diabetes insipidus and almost ineffective on cardiac, aortic, central nervous system,8,10 pulmonary, and sometimes mesenteric involvement.9 However, because ECD is a rare disease, the largest assessment of IFN-α is a retrospective study on only 8 patients.8 Skeletal involvement resolved completely but slowly on scintigraphy in the present patient 1 and incompletely in patient 2, whose periaortic infiltrate was not altered on serial angio-MRI.
Doses of anakinra for our ECD patients were chosen to correspond to the standard dose used for RA patients (ie, 100 mg daily). Higher doses may yield a better response, as reported with doses of 1.5 to 2 mg/kg per day for RA and 1 to 10 mg/kg per day for pediatric Deficiency of the Interleukin-1 Receptor Antagonist or CAPS patients without harm.37-39 Patient 1 had the best response in retroperitoneal fibrosis and received a higher dose for her body weight than did patient 2. The optimal duration of the treatment is unknown. Patients may need a prolonged treatment for several months or years when effective. No adverse event was observed except the usual reactions at the site of injection. Anakinra exhibits a good tolerability and safety profile, as assessed by large previous studies in rheumatoid arthritis patients40,41 and in our 2 patients. Because of its specific targeting properties, anakinra should become an interesting alternative to IFN-α therapy, which is often hampered by dose-limiting side effects.42,43 In addition IFN-α therapy might be less efficient in patients receiving immunosuppressants or corticosteroids that are usually ineffective for treating ECD and that impaired the endogenous production of IL-1Ra.
In conclusion, our results suggest that anakinra represents a potentially safe and effective treatment of ECD. Moreover, our results shed new light on the pathophysiology and therapeutic strategy of ECD by demonstrating the central role of IL-1 network, which appeared overstimulated in ECD like in autoinflammatory processes. However, a larger multicenter prospective study is warranted to confirm our findings and to assess precisely the role and the best use of anakinra or other IL-1 blockers in ECD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.A. proposed the study; A.A. and B.B. were responsible for study initiation and coordination; A.A., C.P., N.M.S., F.G.S., S.L.T., H.B., F.L., R.S., M.d.M., L.G., and B.B. performed study design and data collection; S.G.-L., A.R., S.S., N.P., S.C., and O.H. performed laboratory and radiologic data collection and analyses; and A.A., S.G.-L., and C.P. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Achille Aouba, Department of Adult Hematology, Hôpital Necker Enfants-Malades, Université de Paris Descartes, AP-HP 149 rue de Sèvres, 75015 Paris, France; e-mail: achille.aouba@orange.fr or achille.aouba@nck.aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal