Abstract
Hematopoietic development during embryogenesis involves the interaction of extrinsic signaling pathways coupled to an intrinsic cell fate that is regulated by cell-specific transcription factors. Retinoic acid (RA) has been linked to stem cell self-renewal in adults and also participates in yolk sac blood island formation. Here, we demonstrate that RA decreases gata1 expression and blocks primitive hematopoiesis in zebrafish (Danio rerio) embryos, while increasing expression of the vascular marker, fli1. Treatment with an inhibitor of RA biosynthesis or a retinoic acid receptor antagonist increases gata1+ erythroid progenitors in the posterior mesoderm of wild-type embryos and anemic cdx4−/− mutants, indicating a link between the cdx-hox signaling pathway and RA. Overexpression of scl, a DNA binding protein necessary for hematopoietic development, rescues the block of hematopoiesis induced by RA. We show that these effects of RA and RA pathway inhibitors are conserved during primitive hematopoiesis in murine yolk sac explant cultures and embryonic stem cell assays. Taken together, these data indicate that RA inhibits the commitment of mesodermal cells to hematopoietic fates, functioning downstream of cdx4 and upstream of scl. Our studies establish a new connection between RA and scl during development that may participate in stem cell self-renewal and hematopoietic differentiation.
Introduction
Retinoic acid (RA), a derivative of vitamin A, is a potent regulator of patterning during development and tissue homeostasis such that excess or deficiency of vitamin A results in severe congenital malformations.1,2 Endogenous RA levels are modulated by the balance of biosynthesis by retinaldehyde dehydrogenases and catabolism by the cytochrome P450 enzyme cyp26. In the zebrafish (Danio rerio), retinaldehyde dehydrogenase 2 (raldh2) is principally responsible for biosynthesis, whereas cyp26a1 is the main RA catabolic enzyme.3,4 Retinoids bind to retinoic acid receptors (RARs) and retinoid X receptors, which dimerize to bind to RA responsive elements and activate gene transcription. Perturbing the delicate balance of RA signaling during development has profound effects on many tissues, including brain, heart, eye, limbs, and pancreas (reviewed by Duester1 ), making retinoids highly teratogenic compounds.
Although substantial data exist to support a role for RA to regulate hematopoiesis in adult animals, examining the effects of RA on embryonic hematopoiesis has been relatively unexplored. Murine mutants of many of the genes affecting RA signaling are lethal at embryonic or neonatal stages of development.5 Recently published data suggest that RA is not required for murine primitive hematopoiesis, because Raldh2 knockout mice that do not synthesize RA have normal-appearing hemoglobinized erythroid precursors in the yolk sac.6 In contrast, analysis of vitamin A–deficient quail embryos, which lack RA stores in the yolk, showed apoptosis of primitive erythroid cells in blood islands, indicating involvement of retinoids in yolk sac hematopoiesis.7 Reconciling these apparently contradictory results is difficult but may relate to the link between vascular and hematopoietic development.8-11 Vitamin A–deficient quail embryos display abnormal vascular development,7 whereas Raldh2 knockout mice have impaired formation of yolk sac hemogenic endothelium and decreased hematopoietic progenitors in the absence of RA.6 In addition, Raldh2 deletion is lethal by embryonic day 10.5 in the mouse because of a complex cardiac looping defect,12 limiting the evaluation of hematopoiesis in these mutants. Despite these 2 animal models, the role of RA in hematopoietic development remains unclear.
To further clarify the role of RA in hematopoietic development, we used the zebrafish, a versatile and powerful model for examining vertebrate development and hematopoiesis.13,14 Zebrafish embryos are externally fertilized, facilitating access to early stages of development and allowing investigation of the effects of RA on primitive hematopoiesis. As in all vertebrates, hematopoiesis arises in at least 2 apparently independent waves in the zebrafish.15 The first of these, termed primitive hematopoiesis, generates predominantly red blood cells and some myelomonocytic cells. Initiation of primitive blood cell production in the zebrafish embryo is characterized by the expression of scl and gata1 in the posterior lateral plate mesoderm starting in early somitogenesis, around 11 hours post fertilization (hpf). Gata1-expressing cells are visible as stripes in the posterior mesoderm and gradually merge toward the midline to form the intermediate cell mass around 22 hpf, an intraembryonic hematopoietic organ with similar function to the extraembryonic mammalian yolk sac. The second wave, termed definitive hematopoiesis, initiates around 36 hpf with the expression of runx1 and other markers of hematopoietic stem cells in the adjacent aorta-gonad-mesonephros. Definitive hematopoietic stem cells eventually populate the zebrafish kidney marrow and produce all the different blood cell lineages for the lifetime of the animal.
Given the potent effects of RA on many tissues in the developing embryo, we asked whether RA had any specific effects on the earliest stages of blood development, using an anemic blood mutant lacking expression of cdx4, the caudal-related homeobox gene.16 The cdx4 mutant embryos have altered anterior-posterior patterning and aberrant hox gene expression, as well as a severe defect in hematopoiesis. Overexpression of cdx4 in zebrafish embryos leads to greater hematopoietic potential, as measured by increased and ectopic expression of scl and gata1. Consistent with these findings in zebrafish, overexpression of cdx4 in murine embryonic stem cells (ESCs) leads to increased formation of multipotent hematopoietic progenitors (granulocyte, erythroid, megakaryocyte, and macrophage colony-forming units), supporting the importance of cdx4 in mammalian hematopoietic development.16 Because cdx4 mutants are known to have an abnormal expression pattern of raldh2 in the posterior mesoderm,4,17 we hypothesized that the cdx4 and RA signaling pathways might intersect to regulate primitive blood development.
We show here that primitive erythropoiesis is inhibited by RA, as measured by decreased expression of gata1. In contrast, evaluating posterior vascular and myeloid development by expression of fli1 and pu.1, respectively, shows these markers are not decreased. To assess whether the loss of gata1 in RA-treated embryos was a nonspecific result of abnormal patterning of the posterior mesoderm or a direct effect on blood development, we overexpressed scl or hoxa9a mRNA followed by exposure to exogenous RA. Overexpression of scl partially corrected the inhibition of early erythropoiesis in embryos treated with RA, whereas overexpression of hoxa9a had no effect on gata1 expression. Because scl expression is restricted to hematopoietic and vascular cells, this rescue result confirms that RA regulates primitive hematopoiesis in a specific manner. We also report that gata1+ erythroid progenitors are increased in wild-type and cdx4−/− embryos after treatment with RA inhibitors. This result is evident when embryos are exposed during gastrulation, but not when they are exposed during somitogenesis. We show that the effects of RA and RA pathway inhibitors are conserved in mammalian primitive hematopoiesis by examining murine yolk sac explant cultures and ESC assays. Taken together, our data indicate that RA maintains a critical balance during early mesodermal patterning that affects primitive erythropoiesis, functioning downstream of cdx4 and upstream of scl.
Methods
Zebrafish care and mutant lines
Wild-type AB strain zebrafish, and kugelig (kgg) heterozygotes carrying the cdx4 mutant allele were bred and maintained with the use of standard zebrafish husbandry.18,19 Developmental staging was done by embryo morphology.20 All zebrafish experiments and procedures were performed as approved by the Children's Hospital Boston Institutional Animal Care and Use Committee.
Chemicals
All-trans RA was purchased from Sigma-Aldrich, and a 10mM stock solution was prepared in 100% dimethyl sulfoxide (DMSO) or 100% ethanol and stored at −20°C. Appropriate vehicle controls were used for experiments with the use of the different preparations. 4-Diethylamino benzaldehyde (DEAB; Sigma-Aldrich) is a competitive inhibitor of aldehyde dehydrogenases, including raldh2, the rate-limiting enzyme in RA biosynthesis.21,22 A stock solution of DEAB was prepared in 100% DMSO at 1M concentration and stored at −20°C. AGN193109 is a pan-RAR antagonist23 and was kindly provided by Ron Evans (Salk Institute).
Genotyping embryos
Genomic DNA was isolated from fin clips or whole embryos as previously described,24 except embryos were first equilibrated in phosphate-buffered saline with 0.1% Tween and embryo lysis buffer, and proteinase K (50 μg/mL) was used in a 3-hour digestion. Kgg heterozygous fish and homozygous mutant embryos were genotyped as previously described.16
Whole-mount in situ hybridization
Whole-mount in situ hybridizations were performed as previously described25 with the use of antisense riboprobes labeled with digoxigenin and detected with antidigoxigenin antibody conjugated to alkaline phosphatase. In each case BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt/4-nitroblue tetrazolium chloride) was used as substrate to produce the purple colorimetric reaction. Photomicrographs were taken using a Zeiss Discovery V8 stereomicroscope with 63- to 80-fold magnification, an AxioCam HRc camera and AxioVision software (Carl Zeiss MicroImaging Inc). Digital images were further processed for brightness and contrast with Adobe Photoshop 7.0 software (Adobe Systems).
Microinjection of zebrafish embryos
RNA was generated from linearized pCS2 plasmid containing the zebrafish hoxa9a or scl gene with the use of the mMessage mMachine kit (Ambion). Embryos were injected into the yolk at the one-cell stage with 1 nL of RNA at a concentration of 6 to 10 ng/μL or 200 ng/μL for hoxa9a and scl, respectively.
Murine yolk sac explant cultures
Murine embryonic yolk sac cultures were performed as previously described.26 Timed pregnant ICR mice (Taconic Farms) were killed by CO2 inhalation at day 7.5 of gestation, and late streak to neural plate stage embryos were dissected into yolk sac and embryo proper fractions as previously described.27,28 Individual yolk sacs were cultured in 100 μL of Iscove modified Dulbecco medium supplemented with 15% serum replacement (Invitrogen), 5% plasma-derived serum (Antech), 2mM glutamine (Invitrogen), 0.1mM monothioglycerol, 2 U/mL erythropoietin, and 100 ng/mL stem cell factor in a 96-well plate for 24 to 72 hours in 5% CO2, room air, 37°C. Various concentrations of RA, DEAB, or vehicle (DMSO) alone were added to similarly staged individual explants at the start of the culture. At the end of the culture period, each yolk sac explant was trypsinized into single cells, and total cell number and cell viability were quantified. Eighty percent of the explanted cells were plated in methylcellulose supplemented with 10% plasma-derived serum, 5% protein-free hybridoma media (Gibco Invitrogen), 2mM glutamine, 2 U/mL erythropoietin, 100 ng/mL stem cell factor, 10 ng/mL interleukin-3 (IL-3), 10 ng/mL IL-6, and 5 ng/mL granulocyte-macrophage colony-stimulating factor and were cultured in 5% CO2, room air, 37°C. Primitive and definitive erythroid colonies were quantified at 5 and 7 days of culture, respectively, as previously described.26 The total cell number and number of erythroid colonies in RA-, DEAB- or AGN193109-treated explants were normalized to vehicle-treated control explants cultured in parallel.
ESC cultures
ESCs were maintained and differentiated in vitro according to published protocols.29,30 Embryoid bodies (EBs) were harvested at different time points and dissociated by incubation in EB dissociation buffer (1 mg/mL collagenase IV, 2 mg/mL hyaluronidase, and 80 U/mL DNase I) for 30 minutes at 37°C. Single-cell suspension was obtained by passing the mixture through an 18-guage needle several times. EB cells (5 × 104) were plated into 1.5 mL of M3434 Methyl cult (StemCell Technologies), and the number of hematopoietic colonies was counted days 4 to 10 after plating according to published protocols.31
Results
RA inhibits primitive erythropoiesis in zebrafish embryos but not vasculogenesis
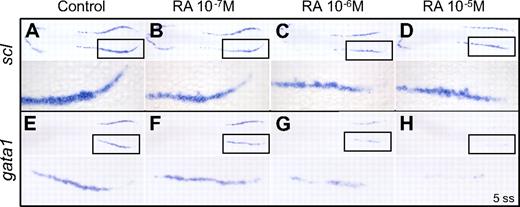
To test the role of RA signaling on primitive hematopoiesis, zebrafish embryos were incubated in various concentrations of RA from late gastrulation (90% epiboly stage, ∼ 9 hpf) until the 5 somite stage (ss; ∼ 11.7 hpf). Embryos were then assayed by whole-mount in situ hybridization for expression patterns of the erythroid specific transcription factor gata1 and the stem cell leukemia gene scl, a transcription factor required for hematopoiesis (Figure 1). A dose-dependent effect of RA on gata1 expression was observed, with RA at a concentration of 10−7M having little effect, whereas 10−5M almost completely ablated gata1 transcripts (Figure 1). In contrast, expression of scl was only mildly decreased by RA exposure. Many scl-expressing cells were still present after RA treatment, although the structure of the hematopoietic stripes was different, with slightly decreased length, thickness, and curvature. Because scl is expressed by both vascular and hematopoietic precursors, whereas gata1 is expressed by erythroid precursors only,10,32 this result suggests RA treatment negatively affects primitive erythroid development but not commitment to the vascular fate.
RA inhibits primitive erythropoiesis in WT zebrafish embryos. Dorsal view of flat-mounted 5-ss wild-type AB embryos. Head is oriented to the left, posterior embryo to the right. Higher power view in the bottom panels is indicated by the boxed region in the top panels. Representative embryos are shown from a single experiment. Similar results were obtained from 2 other replicate experiments. (A-D) Whole-mount in situ hybridization for scl expression in embryos treated with increasing concentrations of RA from 90% epiboly until 5 ss. (E-H) Whole-mount in situ hybridization for gata1 expression in embryos treated with increasing concentrations of RA from 90% epiboly until 5 ss. All embryos are fixed at 5 ss.
RA inhibits primitive erythropoiesis in WT zebrafish embryos. Dorsal view of flat-mounted 5-ss wild-type AB embryos. Head is oriented to the left, posterior embryo to the right. Higher power view in the bottom panels is indicated by the boxed region in the top panels. Representative embryos are shown from a single experiment. Similar results were obtained from 2 other replicate experiments. (A-D) Whole-mount in situ hybridization for scl expression in embryos treated with increasing concentrations of RA from 90% epiboly until 5 ss. (E-H) Whole-mount in situ hybridization for gata1 expression in embryos treated with increasing concentrations of RA from 90% epiboly until 5 ss. All embryos are fixed at 5 ss.
Ectopic RA is known to disturb tissue patterning along the anterior-posterior (A-P) axis during embryonic development, raising the possibility that the observed hematopoietic defect in the presence of RA may be secondary to a global loss of posterior tissue fates. To explore this, we analyzed markers of additional posterior cell types, including fli1, which marks angioblasts, pu.1, which is coexpressed with gata1 during early erythromyeloid development, and spadetail (spt) and hoxa9a, which are expressed in the posterior paraxial mesoderm and tailbud. Examination of fli1+ endothelial precursors in RA-treated embryos showed increased fli1+ cells in the posterior mesoderm, with absent fli1 in the head (Figure 2A). Similar results were observed with flk1 staining (data not shown). Given that RA had little effect on the early expression pattern of scl (Figure 1), it appears that RA is able to increase angioblast numbers after 5 ss. Posterior expression of the myeloid marker pu.1 was relatively unchanged in the RA-treated embryos compared with wild type, whereas the expression of pu.1 in the anterior of the embryo is ablated in the RA-treated embryos. Expression of spt and hoxa9a are only slightly decreased in RA-treated embryos (Figure 2A). In contrast, hoxb4 expression is markedly increased in the head of embryos treated with RA. Overall, these markers are variably altered by exogenous RA treatment, providing further support for the notion that generalized suppression of the posterior mesoderm by RA does not explain the decreased gata1 expression. Instead the increased expression of fli1 in the posterior of the embryo suggests a possible fate switch of potential gata1+ cells to a vascular fate after treatment with RA. Expression of fli1 or flk1 was also increased in RA-treated embryos at 24 hpf (Figure 2B; data not shown), further supporting the idea that RA skews the differentiation of bipotential hemangioblasts in the posterior mesoderm toward the vascular lineage.
RA has varying effects on other genes expressed in the posterior mesoderm. (A). Flat-mounted 12-ss embryos are shown after whole-mount in situ hybridization with the probes indicated. Treatment with 10−6M RA was applied to wild-type AB embryos from 90% epiboly until 12 ss. Representative embryos are shown from a single experiment. Replicate experiments showed comparable results in at least 2 other experiments with each probe. All the embryos from a given treatment group had similar expression patterns. (B). Whole-mounted embryos at 24 hpf. Treatment with 10−6M RA was applied from 60% epiboly until 10 ss, then washed out. Embryos were then fixed at 24 hpf before whole-mount in situ hybridization with fli1 riboprobe. Representative embryos from 1 of 3 experiments are shown.
RA has varying effects on other genes expressed in the posterior mesoderm. (A). Flat-mounted 12-ss embryos are shown after whole-mount in situ hybridization with the probes indicated. Treatment with 10−6M RA was applied to wild-type AB embryos from 90% epiboly until 12 ss. Representative embryos are shown from a single experiment. Replicate experiments showed comparable results in at least 2 other experiments with each probe. All the embryos from a given treatment group had similar expression patterns. (B). Whole-mounted embryos at 24 hpf. Treatment with 10−6M RA was applied from 60% epiboly until 10 ss, then washed out. Embryos were then fixed at 24 hpf before whole-mount in situ hybridization with fli1 riboprobe. Representative embryos from 1 of 3 experiments are shown.
Overexpression of scl, but not hoxa9a, increases gata1 expression in zebrafish embryos treated with RA
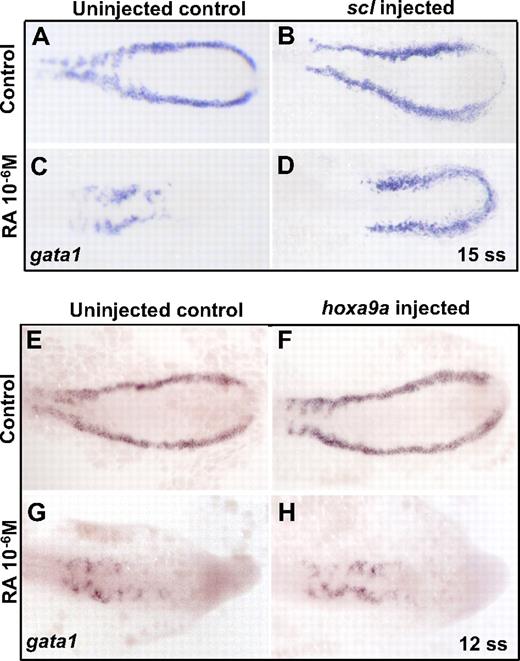
To further characterize the role of RA signaling in the emergence of hematopoietic cells during development and to examine whether this role was specific to hematopoietic cells, we treated embryos with RA and overexpressed the scl gene, known to have blood-inducing activities when ectopically expressed in the embryo. Wild-type embryos were injected with scl mRNA, then treated with RA (10−6M) from early gastrulation (60% epiboly) until 10 ss. As expected, embryos treated with RA alone have decreased gata1 expression in the posterior mesoderm (Figure 3C). However, overexpression of scl in the RA-treated embryos leads to increased gata1+ cells in the posterior mesoderm (64%; n = 16 of25 embryos; Figure 3D). The structure of the gata1+ stripes in the rescued embryos is abnormal, reflecting the aberrant posterior patterning in these embryos that is not rescued by scl overexpression. These data indicate that scl functions downstream of RA in primitive blood development and underscore that the inhibition of blood development by RA is specific to hematopoietic cells, not merely because of a global effect of RA on posterior patterning.
Overexpression of scl, but not hoxa9a, increases gata1 in RA-treated embryos. Representative embryos are shown for each experiment. Similar results were observed in at least 2 other replicate experiments. (A-D). Single-cell embryos were injected with scl mRNA, then incubated in 10−6M RA from early gastrulation (60% epiboly) until mid somitogenesis (15 ss). Expression of gata1 was measured by whole-mount in situ hybridization after embryos were fixed at 15 ss. (E-H) Single-cell embryos were injected with hoxa9a mRNA and then incubated in 10−6M RA from early gastrulation (50% epiboly) until mid somitogenesis (12 ss). Expression of gata1 was measured by whole-mount in situ hybridization after embryos were fixed at 12 ss. Similar experiments incubating injected embryos in 10−6M RA from late gastrulation (90% epiboly) until mid somitogenesis (12-15 ss) showed comparable results.
Overexpression of scl, but not hoxa9a, increases gata1 in RA-treated embryos. Representative embryos are shown for each experiment. Similar results were observed in at least 2 other replicate experiments. (A-D). Single-cell embryos were injected with scl mRNA, then incubated in 10−6M RA from early gastrulation (60% epiboly) until mid somitogenesis (15 ss). Expression of gata1 was measured by whole-mount in situ hybridization after embryos were fixed at 15 ss. (E-H) Single-cell embryos were injected with hoxa9a mRNA and then incubated in 10−6M RA from early gastrulation (50% epiboly) until mid somitogenesis (12 ss). Expression of gata1 was measured by whole-mount in situ hybridization after embryos were fixed at 12 ss. Similar experiments incubating injected embryos in 10−6M RA from late gastrulation (90% epiboly) until mid somitogenesis (12-15 ss) showed comparable results.
In contrast to the experiments with scl, microinjection of hoxa9a mRNA did not increase gata1 expression in RA-treated embryos (Figure 3H). Hoxa9a is a homeobox gene transcription factor known to facilitate leukemic transformation and plays a role in primitive blood cell development as regulated by cdx4.16,33 Wild-type embryos were injected with hoxa9a mRNA and treated with RA from early gastrulation (50% epiboly) or late gastrulation (90% epiboly) until 10 ss. Again, embryos treated with RA alone had very little expression of gata1 in the posterior mesoderm (Figure 3G), and the RA-treated embryos injected with hoxa9a mRNA had a similar low level of gata1 expression (Figure 3H) regardless of the timing of RA exposure. Hoxa9a injection also did not significantly increase the expression of gata1 in the untreated embryos. This result indicates that the effects of hoxa9a on hematopoiesis are not sufficient to overcome the inhibition of hematopoiesis by RA.
Gata1 expression is increased in cdx4 mutants and wild-type embryos when the RA pathway is blocked
The fact that RA inhibits gata1 expression in zebrafish embryos led us to hypothesize that blocking RA signaling might increase gata1, an effect that would be more evident in an anemic mutant embryo. The cdx4 mutants, known as kgg, are very anemic, displaying scant expression of gata1, as well as abnormal A-P patterning, and disrupted hox gene expression.16 As additional support for our hypothesis, loss of cdx4 has been linked to increased expression of raldh2 in the posterior mesoderm of zebrafish, which would presumably increase endogenous RA and, therefore, could explain the anemia in cdx4 mutants.4,17
DEAB, a competitive inhibitor of aldehyde dehydrogenases, inhibits raldh2 activity by blocking the active site of the enzyme and is commonly used as an inhibitor of the RA pathway.22,34 Inhibition of RA signaling by DEAB was confirmed in zebrafish embryos by microarray analysis comparing gene expression patterns after 10−6M RA or 10−5M DEAB (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), showing RA-responsive genes had significantly altered expression after RA and DEAB.
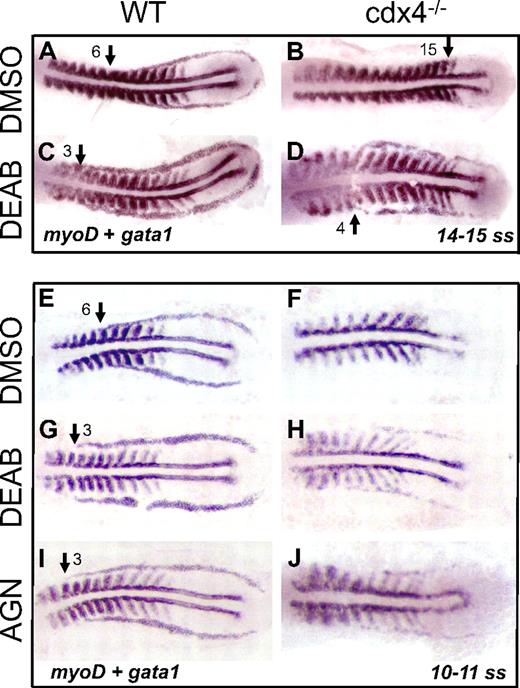
Wild-type and cdx4 mutant embryos were incubated in DEAB at 60% epiboly then fixed between 10 and 15 ss and examined by whole-mount in situ hybridization simultaneously for expression of gata1 and myoD (Figure 4). The cdx4 mutant embryos maintain relatively normal somitogenesis, as evidenced by almost normal myoD expression, but they have scant expression of gata1, which is pushed posteriorly compared with wild-type embryos, with the anterior margin of the gata1+ stripes starting around somite 15 (Figure 4B). By comparison, gata1 expression is markedly increased in cdx4 mutant embryos when RA biosynthesis is blocked by DEAB, and the anterior limit of the gata1+ stripes forms at the level of somite 4 (Figure 4D). Wild-type embryos also have increased expression of gata1 after exposure to DEAB, because the stripes in the posterior mesoderm are thicker and extend anteriorly, starting around somite 3 (Figure 4C), compared with somite 6 in the wild-type control embryos (Figure 4A).
Increased gata1 expression in wild-type and cdx4−/− embryos by blocking RA with the use of DEAB or AGN193109. Each panel shows representative embryos from one experiment. Comparable results were obtained in at least 3 replicate experiments. (A-D) Whole-mount in situ hybridization for gata1 and myoD expression in flat-mounted embryos 14 to 15 ss treated with DMSO control or DEAB (10−5M) to block RA biosynthesis. Embryos were treated with DEAB from 60% epiboly until 14 to 15 ss. (E-J) Whole-mount in situ hybridization for gata1 and myoD expression in flat-mounted embryos at 10 to 11 ss treated with DMSO control, DEAB (10−5M), or AGN193109 (2 × 10−5M), a pan-RAR antagonist. Embryos were treated from 60% epiboly until 10 ss, then fixed.
Increased gata1 expression in wild-type and cdx4−/− embryos by blocking RA with the use of DEAB or AGN193109. Each panel shows representative embryos from one experiment. Comparable results were obtained in at least 3 replicate experiments. (A-D) Whole-mount in situ hybridization for gata1 and myoD expression in flat-mounted embryos 14 to 15 ss treated with DMSO control or DEAB (10−5M) to block RA biosynthesis. Embryos were treated with DEAB from 60% epiboly until 14 to 15 ss. (E-J) Whole-mount in situ hybridization for gata1 and myoD expression in flat-mounted embryos at 10 to 11 ss treated with DMSO control, DEAB (10−5M), or AGN193109 (2 × 10−5M), a pan-RAR antagonist. Embryos were treated from 60% epiboly until 10 ss, then fixed.
Next, a pan-RAR inhibitor, AGN193109, was evaluated for rescue of gata1 expression in cdx4 mutant embryos. Similar to DEAB-treated cdx4−/− embryos tested in parallel (Figure 4H), when RA signaling was blocked at the receptor level by AGN193109, gata1 expression in the cdx4−/− embryos was increased (Figure 4J). The DMSO-treated cdx4−/− embryos had no gata1 expression (Figure 4F). The structure of the gata1+ stripes is similar for both DEAB and AGN193109, such that the stripes extend anteriorly and are almost parallel to each other, without the characteristic merging of the anterior segment toward midline as seen in wild-type embryos. These results indicate that blocking RA signaling in cdx4 mutants partially rescues primitive hematopoiesis, consistent with RA acting downstream of cdx4.
Exposure to DEAB during gastrulation is necessary to allow rescue of gata1 expression in cdx4 mutants
The timing and dose of retinoid exposure is known to be critical for the development of many tissues in mammalian systems.2,35 To examine whether the timing of DEAB exposure also affected the rescue of gata1 expression observed in the cdx4 mutants, embryos were exposed to DEAB during various timed intervals after the onset of gastrulation until mid somitogenesis. All embryos were fixed at 10 ss and then examined for gata1 expression by whole-mount in situ hybridization. DEAB treatment from the beginning of gastrulation (5.5-6 hpf, 50%-60% epiboly, shield stage) until mid somitogenesis (11-14 hpf, 5-10 ss) rescued 91% to 100% of the cdx4 mutant embryos (Table 1; Figure 5). Further experiments examined rescue of blood formation in cdx4 mutant embryos after DEAB treatment for shorter time windows within that developmental period. Mutant embryos treated from 5 ss to 10 ss, after somitogenesis was well under way, were not rescued and were indistinguishable from untreated controls. Embryos treated just after completing gastrulation, from 1 ss to 5 ss (10.3-11.7 hpf), had only scant rescue noted in 8% of the mutant embryos (Table 1; Figure 5). In contrast, RA inhibition from 90% epiboly until 1 ss (9-10.3 hpf) resulted in mildly increased (1+) gata1 expression for 86% of cdx4 mutant embryos. Treatment with DEAB throughout gastrulation yielded an increased level of rescue for almost all the mutant embryos. When treated from 50% epiboly until 1 ss (5.5-10.3 hpf), 96% of the mutant embryos had blood rescued. From these data we conclude that RA inhibition with DEAB at the end of gastrulation (around 90% epiboly; approximately 9 hpf) is the most critical time window to stimulate erythroid expansion in cdx4 mutant embryos, and the degree of gata1 expression increases if embryos are incubated throughout gastrulation. This conclusion agrees with our finding that exogenous RA inhibits erythropoiesis when added to embryos at 90% epiboly (Figure 1).
DEAB rescue of cdx4−/− embryos requires exposure during gastrulation
| Stage exposed . | Time exposed, hpf . | Mutant embryos with increased gata1/total mutants . | Mutant embryos with increased gata1, % . | Degree of rescue . |
|---|---|---|---|---|
| None | N/A | 0/23 | 0 | None |
| 50%-90* | 5.5-9 | 12/19 | 63 | 2+ |
| 50%-1 ss | 5.5-10.3 | 23/24 | 96 | 2+ |
| 90%-1 ss* | 9-10.3 | 18/21 | 86 | 1+ |
| 1 ss-5 ss* | 10.3-11.7 | 2/25 | 8 | Scant |
| 50%-5 ss | 5.5-11.7 | 21/23 | 91 | 3+ |
| 60%-10 ss* | 6-14 | 8/8 | 100 | 3+ |
| 90%-5 ss | 9-11.7 | 9/9 | 100 | 2+ |
| 90%-10 ss | 9-14 | 10/10 | 100 | 1+ |
| 5 ss-10 ss* | 11.7-14 | 0/6 | 0 | None |
| Stage exposed . | Time exposed, hpf . | Mutant embryos with increased gata1/total mutants . | Mutant embryos with increased gata1, % . | Degree of rescue . |
|---|---|---|---|---|
| None | N/A | 0/23 | 0 | None |
| 50%-90* | 5.5-9 | 12/19 | 63 | 2+ |
| 50%-1 ss | 5.5-10.3 | 23/24 | 96 | 2+ |
| 90%-1 ss* | 9-10.3 | 18/21 | 86 | 1+ |
| 1 ss-5 ss* | 10.3-11.7 | 2/25 | 8 | Scant |
| 50%-5 ss | 5.5-11.7 | 21/23 | 91 | 3+ |
| 60%-10 ss* | 6-14 | 8/8 | 100 | 3+ |
| 90%-5 ss | 9-11.7 | 9/9 | 100 | 2+ |
| 90%-10 ss | 9-14 | 10/10 | 100 | 1+ |
| 5 ss-10 ss* | 11.7-14 | 0/6 | 0 | None |
Embryos from incrosses of cdx4+/− heterozygotes were treated with DMSO or DEAB at various stages of early development. (5.5 hpf = 50% epiboly, 6 hpf = 60% epiboly, 9 hpf = 90% epiboly, 10.3 hpf = 1 ss, 11.7 hpf = 5 ss, and 14 hpf = 15 ss) The total number of mutant embryos in 2- to 3-treated groups were pooled for each incubation period. Embryos were incubated in DEAB (2 × 10−5M) and compared with control embryos incubated in DMSO to evaluate the degree of gata1 rescue. Each data point represented pooling of several (2-4) experiments. The degree of rescue was scored based on the number of gata1+ cells present in comparison to control embryos for that experiment. A full rescue was scored as 3+ and reflected a maximal increase of gata1+ cells as depicted in Figure 4D. When only a few more cells were present in comparison to the vehicle-treated control embryos, these embryos were scored as 1+ rescue. The level of rescue was deemed intermediate or 2+ if the number of gata1+ cells was less than maximal, or if there were mixed levels of rescue among the total number of mutant embryos scored.
N/A indicates not applicable.
Treatment groups depicted in Figure 5.
Percentage of cdx4−/− embryos rescued after treatment with DEAB at different stages of development. Embryos from incrosses of cdx4+/− heterozygotes were treated with DMSO or DEAB (2 × 10−5M) at various stages of development. The height of each bar indicates the percentage of total cdx4−/− embryos with increased gata1 expression after DEAB treatment compared with untreated embryos. The total number of mutant embryos for each incubation period was compiled from 2 to 3 separate experiments. The raw data are presented in Table 1.
Percentage of cdx4−/− embryos rescued after treatment with DEAB at different stages of development. Embryos from incrosses of cdx4+/− heterozygotes were treated with DMSO or DEAB (2 × 10−5M) at various stages of development. The height of each bar indicates the percentage of total cdx4−/− embryos with increased gata1 expression after DEAB treatment compared with untreated embryos. The total number of mutant embryos for each incubation period was compiled from 2 to 3 separate experiments. The raw data are presented in Table 1.
RA signaling regulates the number of primitive erythroid progenitors in murine yolk sac explants and ESC cultures
To determine whether RA signals have similar effects on mammalian primitive hematopoiesis, we incubated ex vivo cultures of murine yolk sac explants with RA, DEAB, or AGN193109. Embryonic day 7.5 murine yolk sacs were harvested and dissected, then pooled and cultured in RA, DEAB, or AGN193109 for 48 hours. This timing is analogous to the treatment of zebrafish embryos from gastrulation through early somitogenesis. The cultured cells were then plated for 5-day and 7-day colony assays to assess primitive erythroid colony-forming cells (EryP-CFCs) and definitive erythroid burst-forming units (BFU-Es), respectively. Treatment with RA significantly inhibited both types of erythroid progenitors in the yolk sac explants (Figure 6A). This was not because of generalized cytotoxicity because RA treatment did not affect the total number of cells. In contrast, inhibiting RA with either DEAB or AGN193109 increased the number of EryP-CFCs and BFU-Es by approximately 2-fold compared with treatment with vehicle alone, although this trend did not achieve statistical significance due to high variability inherent to this type of assay.
Primitive erythroid progenitors in murine yolk sac cultures and murine ESC cultures are inhibited after treatment with RA and are increased by DEAB. All statistical analyses used 1-way Student t tests to calculate P values comparing treated groups with the normalized control; *P < .01, **P < .001. (A) Pooled, stage-matched yolk sac explants were cultured in vehicle control, 10−5M AGN193109, 10−5M DEAB, or 10−6M RA, and plated in methylcellulose for colony-forming assays. The data are depicted as mean fold change in comparison to the vehicle control cultures ± SEM. After DEAB treatment, P = .1 for Ery-CFC and P = .08 for BFU-E colonies. For AGN193109 treatment, P = .06 for EryP-CFC and P = .08 for BFU-E colonies. There was no statistical difference in the total number of cells for each treatment. (B) EBs from ESC cultures were incubated in the presence of various concentrations of RA, 10−4M DEAB, or DMSO control, then plated for colony-forming assays as described in “Methods.” (C) 10−6M RA, DEAB, or DMSO was added to EB shaking cultures at various time points between days 2 and 6 of ESC differentiation and washed out after 24 hours of incubation. After the sixth day of ESC differentiation, cells were counted and plated for colony assays.
Primitive erythroid progenitors in murine yolk sac cultures and murine ESC cultures are inhibited after treatment with RA and are increased by DEAB. All statistical analyses used 1-way Student t tests to calculate P values comparing treated groups with the normalized control; *P < .01, **P < .001. (A) Pooled, stage-matched yolk sac explants were cultured in vehicle control, 10−5M AGN193109, 10−5M DEAB, or 10−6M RA, and plated in methylcellulose for colony-forming assays. The data are depicted as mean fold change in comparison to the vehicle control cultures ± SEM. After DEAB treatment, P = .1 for Ery-CFC and P = .08 for BFU-E colonies. For AGN193109 treatment, P = .06 for EryP-CFC and P = .08 for BFU-E colonies. There was no statistical difference in the total number of cells for each treatment. (B) EBs from ESC cultures were incubated in the presence of various concentrations of RA, 10−4M DEAB, or DMSO control, then plated for colony-forming assays as described in “Methods.” (C) 10−6M RA, DEAB, or DMSO was added to EB shaking cultures at various time points between days 2 and 6 of ESC differentiation and washed out after 24 hours of incubation. After the sixth day of ESC differentiation, cells were counted and plated for colony assays.
Murine ESCs cultured as EBs were also incubated with RA and DEAB to further examine their effects on mammalian primitive hematopoiesis. ESCs were differentiated in hanging-drop cultures for 2 days, then EBs were collected and incubated with DEAB or RA for 24 hours, followed by a washout and additional days in fresh culture media. On day 6 of EB formation, dissociated single cells were counted and plated in methylcellulose for colony assays. As shown in Figure 6B, low concentrations of RA inhibited the formation of hematopoietic progenitors. Even at 0.001μM (10−9M) RA, the colony counts are significantly lower than the control cultures. As found in the primary culture of yolk sac cells, DEAB inhibition of RA biosynthesis results in expansion of primitive erythroid progenitor numbers approximately 10-fold over control cultures. Definitive erythroid, granulocyte-macrophage and multipotent (granulocyte, erythroid, megakaryocyte, and macrophage) colonies were not significantly different from control cultures.
Because the timing of retinoid blockade in developing zebrafish embryos affects the level of increased blood development, we asked whether the timing of exposure during the EB culture resulted in differences in hematopoietic progenitor formation. We found the most potent effect occurred when DEAB was added to the shaking cultures in the first day after EB formation, that is, day 2 to 3 of ESC differentiation (Figure 6C). In this case, primitive erythroid progenitors were expanded approximately 7-fold (P = .006). In contrast, when DEAB was added at later time points, there was no difference in colony counts compared with control cultures. Our results with EB cultures showed that hematopoietic progenitor formation was blocked by RA, whereas primitive erythroid progenitor formation was specifically enhanced by DEAB when EBs were exposed at the earliest stages of hematopoietic specification. These results are consistent with the effects of RA and DEAB observed in vivo in zebrafish embryos and establish that RA has an inhibitory effect on primitive hematopoiesis in the mammalian system.
Discussion
In this study we show that exogenous treatment of embryos with RA inhibits erythropoiesis. This effect is not caused by a general suppression of all posterior mesoderm fates, because other cell types, including vascular, myeloid, and paraxial mesoderm, are formed in RA-treated embryos. The initial expression of raldh2 in the anterior paraxial mesoderm, adjacent to mesoderm that does not form erythroid cells, suggests a model in which localized diffusion of RA acts to inhibit hematopoiesis during the early stages of somitogenesis and thereby set the anterior limit of the blood stripes. In support of this hypothesis, blocking endogenous RA synthesis with DEAB leads to an anterior shift in the gata1+ stripes.
We cannot exclude the possibility that RA acts directly on the gata1 promoter to inhibit gata1 expression. The chicken gata1 gene is negatively regulated by binding of a gata1 hormone responselike element by a heterodimer of the thyroid hormone receptor α and the chicken ovalbumin upstream promoter transcription factor.36 It is conceivable that RA could function similarly, although an RA responsive element has not been reported in the gata1 promoter. However, because the effect of DEAB is most prominent when applied during gastrulation, before the onset of gata1 expression, it seems unlikely that direct inhibition of gata1 expression by ligand-bound RARs could be the only mechanism at work. In addition, for most RA-responsive genes, ligand binding to the RAR results in transcriptional activation, whereas transcriptional repression occurs in the absence of RA (reviewed in Rochette-Egly and Germain37 ).
Our experiments show that the effect of RA on gata1+ cells can be specifically rescued by exogenous expression of scl, a transcription factor that regulates the development of all hematopoietic lineages. Overexpression of scl has also been shown to rescue hematopoietic development from ESCs that lack expression of Oct-4.38 In that case, SCL failed to rescue hematopoiesis when Oct-4 was silenced at day 0 of differentiation. However, if Oct-4 was silenced at 48 hours, after mesodermal specification but before commitment to the hematopoietic lineage, SCL overexpression rescued hematopoietic development. In our studies, RA inhibits blood development, but not general mesodermal specification. We speculate that when exogenous RA inhibits hematopoiesis, scl may bypass this inhibition by acting on competent hematopoietic precursors poised to initiate the hematopoietic program. scl has also been shown to mediate mesodermal commitment to hematopoietic cell fates, by recruiting presumptive progenitors of other mesodermal lineages (eg, cardiac or paraxial mesoderm) to commit to hematopoietic cell fates.39 Therefore scl may act to recruit other mesodermal precursors in RA-treated embryos to drive them toward a hematopoietic cell fate. Our data indicate that fli1+ cells appear to be increased after RA treatment. Under the influence of forced overexpression of scl, these cells may skew toward an erythroid fate, gaining expression of gata1.
Identifying RA as an inhibitor of primitive hematopoiesis helps to explain the blood defects in cdx4 mutants. In zebrafish, cdx4 is first expressed along the involuting edge of the gastrula and later as a gradient along the A-P axis during somitogenesis. It regulates expansion of mesodermal cell fates during gastrulation to extend the A-P axis of the animal, and then it functions to specify the expression domains of numerous posterior hox genes.16 Expression analysis of cdx4−/− embryos shows up-regulation of raldh2 mRNA,4,17 which presumably would increase RA, thereby linking the cdx4-hox and RA signal cascades. Increased RA in cdx4−/− embryos pushes the anterior margin of the blood stripes posteriorly. In addition to shorter blood stripes, cdx4 mutants also have a reduced density of gata1+ cells within the blood stripes compared with wild-type embryos. DEAB-treated cdx4−/− embryos have longer blood stripes, but they still maintain the decreased density of gata1+ cells.
Combining the data presented here with that of previously published reports,4,16,40 we propose a model for cdx4 regulation of blood development as determined by RA and/or hox genes (Figure 7A). For blood development to proceed, (1) the posterior mesoderm must be competent, as mediated by hoxa9a and other hox genes, and (2) the blood program must be initiated, with subsequent expression of scl and gata1 in committed hematopoietic progenitor cells. In the absence of cdx4, reduced expression of posterior hox genes, such as hoxa9a, decreases the competence of the mesoderm to differentiate into erythroid precursors. In addition, loss of cdx4 expands the posterior raldh2 expression domain, allowing increased RA signaling to suppress blood specification over a greater territory, leading to shorter blood stripes. Loss of cdx4 has also been shown to delay the onset of the blood program,40 resulting in reduced and delayed expression of scl and gata1, an effect that may be mediated by both RA and posterior hox genes. This model explains the different structural patterns of gata1+ cells in embryos treated with exogenous RA and cdx4−/− embryos after overexpression of scl or hoxa9a or after treatment with DEAB (Figure 7B).
Proposed model for the regulation of blood development by cdx4 as mediated by RA and hoxa9a. (A). cdx4 directly modulates expression of numerous hox genes, including hoxa9a. cdx4 also inhibits expression of raldh2, thereby determining the extent of the RA gradient in the developing posterior mesoderm. As a result, cdx4 regulates 3 aspects of primitive blood development that are mediated by RA and/or hoxa9a: (1) specification of competent mesoderm, mediated by hoxa9a; (2) regulating the onset of the blood program, mediated by both hoxa9a and RA; and (3) setting the anterior edge of the hematopoietic stripe in the posterior mesoderm, mediated by RA. It is probable that other hox genes regulated by cdx4 have similar function, although here we report results with hoxa9a only. (B). Schematic representation of gata1+ cells in the posterior mesoderm of flat-mounted zebrafish embryos at the 10 ss. Red boxes indicate expression of gata1 in the lateral plate mesoderm flanking the paraxial mesoderm of each embryo. Solid filled boxes represent strong expression, and checked boxes represent relatively sparse expression. Numbers indicate the somite pair adjacent to the most anterior gata1+ cells. scl overexpression rescues gata1+ cells after exogenous RA treatment but not in cdx4 mutants. This can be explained by taking into account the notion that posterior hox genes may act as “competence factors” for blood cell differentiation. In cdx4 mutants, the posterior hox gene expression domains are decreased and shifted posteriorly so that even in the presence of exogenous scl, the mesoderm is unable to activate the blood program. In RA-treated embryos, the cdx-hox pathway is intact, and the posterior mesoderm remains competent to respond to scl and form blood, albeit with the anterior margin of the gata1+ cells still shifted posteriorly. Likewise, hoxa9a overexpression rescues blood development in cdx4−/− embryos, but not RA-treated embryos. Normally expressed throughout the posterior mesoderm, hoxa9a expression in cdx4−/− embryos is isolated in the very tip of the tailbud.16 Overexpression of hoxa9a in cdx4−/− embryos adequately corrects the competency of the posterior mesoderm so that gata1+ cells are expanded and appear very similar to wild-type embryos. In contrast, expression of hoxa9a is not decreased after RA treatment. Therefore the cdx4-hox pathway remains relatively intact in RA-treated embryos, so increased hoxa9a expression would not be expected to have a significant effect on erythropoiesis.
Proposed model for the regulation of blood development by cdx4 as mediated by RA and hoxa9a. (A). cdx4 directly modulates expression of numerous hox genes, including hoxa9a. cdx4 also inhibits expression of raldh2, thereby determining the extent of the RA gradient in the developing posterior mesoderm. As a result, cdx4 regulates 3 aspects of primitive blood development that are mediated by RA and/or hoxa9a: (1) specification of competent mesoderm, mediated by hoxa9a; (2) regulating the onset of the blood program, mediated by both hoxa9a and RA; and (3) setting the anterior edge of the hematopoietic stripe in the posterior mesoderm, mediated by RA. It is probable that other hox genes regulated by cdx4 have similar function, although here we report results with hoxa9a only. (B). Schematic representation of gata1+ cells in the posterior mesoderm of flat-mounted zebrafish embryos at the 10 ss. Red boxes indicate expression of gata1 in the lateral plate mesoderm flanking the paraxial mesoderm of each embryo. Solid filled boxes represent strong expression, and checked boxes represent relatively sparse expression. Numbers indicate the somite pair adjacent to the most anterior gata1+ cells. scl overexpression rescues gata1+ cells after exogenous RA treatment but not in cdx4 mutants. This can be explained by taking into account the notion that posterior hox genes may act as “competence factors” for blood cell differentiation. In cdx4 mutants, the posterior hox gene expression domains are decreased and shifted posteriorly so that even in the presence of exogenous scl, the mesoderm is unable to activate the blood program. In RA-treated embryos, the cdx-hox pathway is intact, and the posterior mesoderm remains competent to respond to scl and form blood, albeit with the anterior margin of the gata1+ cells still shifted posteriorly. Likewise, hoxa9a overexpression rescues blood development in cdx4−/− embryos, but not RA-treated embryos. Normally expressed throughout the posterior mesoderm, hoxa9a expression in cdx4−/− embryos is isolated in the very tip of the tailbud.16 Overexpression of hoxa9a in cdx4−/− embryos adequately corrects the competency of the posterior mesoderm so that gata1+ cells are expanded and appear very similar to wild-type embryos. In contrast, expression of hoxa9a is not decreased after RA treatment. Therefore the cdx4-hox pathway remains relatively intact in RA-treated embryos, so increased hoxa9a expression would not be expected to have a significant effect on erythropoiesis.
By testing DEAB exposure at specific intervals during development, our results confirmed that DEAB exposure during gastrulation was required to see the expansion of gata1+ cells. This exposure window for the RA effect on hematopoiesis in zebrafish corresponds to mouse embryos at the late primitive streak to early neural plate stage (∼ embryonic day 7.5), before somitogenesis. When the yolk sacs of these murine embryos were cultured ex vivo and plated in colony-forming assays, DEAB expanded the number of primitive erythroid progenitors, whereas RA inhibited them. This result may appear to contradict prior work by Goldie et al6 that showed normal primitive erythroid development in yolk sacs of Raldh2 null mice. However, the investigators did not evaluate erythroid colony formation in methylcellulose as we did. On the basis of our data, increased EryP-CFC and BFU-E colonies would be expected from Raldh2−/− yolk sacs, but these experiments have not been done.
Likewise, primitive erythroid progenitors were expanded when DEAB was added early during EB culture. This window between days 2 and 3 of ESC differentiation coincides with the emergence of the first hematopoietic mesoderm in EBs.31 When the DEAB was added later in the EB cultures, the progenitors were not significantly different from controls. Taken together, these data suggest a limited and early window for expansion of gata1-expressing erythroid progenitors when RA is inhibited, both in developing zebrafish and mouse embryos, as well as murine ESC cultures.
Our data establish a new connection between RA signaling and SCL that operates during early embryogenesis. Identifying such interactions may provide insight into the ability to direct hematopoietic development from ESCs.41,42 In addition, such factors that participate in hematopoiesis during embryogenesis may promote expansion or even malignant transformation of hematopoietic cells if aberrantly expressed and might thereby advance our understanding of leukemogenesis. Many genes regulating early hematopoietic development, such as scl and runx1, were first identified as oncogenes expressed in human leukemias. Our data indicate that cdx4, another known leukemic oncogene,43 regulates embryonic blood development by RA signaling in addition to hox genes. By regulating differentiation, this interaction of RA and SCL may similarly be important in adult hematopoiesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Teresa Bowman and Owen Tamplin for critical review of the manuscript and for helpful discussions. We thank Anne Koniski for assistance with yolk sac explant experiments. We thank Wendy London for help with statistical analyses.
This work was supported by National Institutes of Health grants (5K08DK074595, J.L.O.d.J.; DK09361, J.P.; and 2R01HL048801-15A2, L.I.Z.), and by the Howard Hughes Medical Institute (G.Q.D. and L.I.Z.).
National Institutes of Health
Authorship
Contribution: J.L.O.d.J., A.J.D., Y.W., J.P., P.O., and E.P. performed experiments; J.L.O.d.J., A.J.D., Y.W., J.P., G.Q.D., and L.I.Z. designed the research and analyzed the data; and J.L.O.d.J., A.J.D., and L.I.Z. wrote the manuscript.
Conflict-of-interest disclosure: L.I.Z. owns stock in FATE Inc. G.Q.D. is a member of the scientific advisory boards of MPM Capital, Epizyme, iPIerian, and Solasia. The remaining authors declare no competing financial interests.
The current affiliation for Y.W. is The Institute of Biomedical Research, Shanghai, China.
Correspondence: Leonard I. Zon, HHMI/Children's Hospital, 300 Longwood Ave, Karp 7, Boston, MA 02115; e-mail: zon@enders.tch.harvard.edu.
References
Author notes
J.L.O.d.J. and A.J.D. contributed equally to this study.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal