NK cells are an important component of innate immunity. Their best-characterized function is the capability to kill virus-infected or tumor cells and to release proinflammatory cytokines.1,2 NK-cell function is primarily regulated by several activating and inhibitory receptors, some of which recognize major histocompatibility complex (MHC) class I molecules and play a fundamental role in the capability of NK cells to discriminate between normal and aberrant target cells.3-5
Human mature natural killer (NK) cells are generally divided into 2 subsets based on the relative surface density of CD56 antigen: CD56bright cells, predominant in secondary lymphoid tissues, and CD56dim cells, predominant in peripheral blood (PB). CD56bright cells have been shown to derive from CD34+ hemopoietic stem cells (HSC) via phenotypically identified stages.6,7 In turn, CD56bright PB NK cells are thought to give rise to CD56dim cells because they appear first after HSC transplantation and in cytokine-driven models of NK-cell in vitro differentiation.7-9 In addition, they have longer telomeres than CD56dim cells.9 In agreement with these concepts, recent studies revealed that the density of surface expression of CD94 or of CD62L identifies functional intermediaries between CD56bright and CD56dim human NK-cell subsets.10 These data support the notion that, in vivo, human CD56bright NK cells may undergo progressive differentiation ending with a CD94lowCD62LnegCD56dim phenotype.
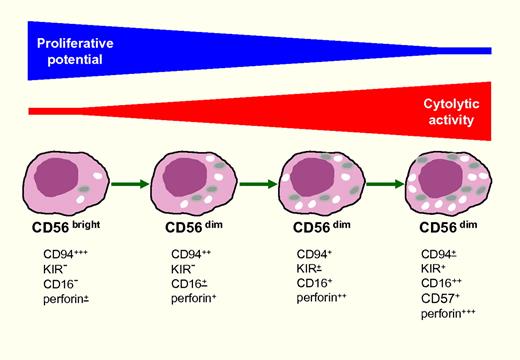
Model of human CD56dim NK-cell differentiation. Different experimental evidence supports the notion that peripheral blood CD56bright give rise to CD56dim NK cells. These 2 subsets differ in proliferative potential, cytolytic activity, and capability of secreting IFN-γ or TNF-α upon cytokine stimulation. With respect to cytokine secretion, however, recent studies revealed that CD56dim are capable of rapid secretion upon cell triggering via activating receptors (Fauriat et al12 and A. De Maria et al, personal communication). Two independent articles showed that CD56dim cells can be further fractionated into different cell subsets on the basis of their surface markers and function. As depicted in this schematic figure, the progression of CD56dim toward putative terminally differentiated NK cells is accompanied by the progressive loss of their proliferative capacity and the acquisition of more efficient cytolytic activity. Different maturational stages can be identified on the basis of the progressive down-regulation of CD94 and the expression of (1or more) KIRs and of CD16. CD57 expression is acquired at later stages and marks terminally differentiated cells with high cytolytic activity but very low proliferative potential.
Model of human CD56dim NK-cell differentiation. Different experimental evidence supports the notion that peripheral blood CD56bright give rise to CD56dim NK cells. These 2 subsets differ in proliferative potential, cytolytic activity, and capability of secreting IFN-γ or TNF-α upon cytokine stimulation. With respect to cytokine secretion, however, recent studies revealed that CD56dim are capable of rapid secretion upon cell triggering via activating receptors (Fauriat et al12 and A. De Maria et al, personal communication). Two independent articles showed that CD56dim cells can be further fractionated into different cell subsets on the basis of their surface markers and function. As depicted in this schematic figure, the progression of CD56dim toward putative terminally differentiated NK cells is accompanied by the progressive loss of their proliferative capacity and the acquisition of more efficient cytolytic activity. Different maturational stages can be identified on the basis of the progressive down-regulation of CD94 and the expression of (1or more) KIRs and of CD16. CD57 expression is acquired at later stages and marks terminally differentiated cells with high cytolytic activity but very low proliferative potential.
Notably, it was commonly accepted that CD56bright NK cells are the main source of cytokine production, while CD56dim are mostly responsible for cytolytic activity and target cell killing. However, recent studies challenged this concept (Juelke et al11 and Fauriat et al12 , and A. De Maria, personal communication). They revealed that the CD56dim subset is also a major source of proinflammatory cytokines and chemokines that are induced rapidly (within hours) after target cell recognition. In addition, analysis of the engagement of different activating receptors or adhesion molecules revealed a hierarchy among factors released upon CD56dim interaction with target cells.12 In particular, chemokine production can be induced by low levels of stimulation (eg, engagement of individual activating receptors) and occurs more rapidly than the release of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α). This requires a higher degree of ligand expression for triggering NK receptors on target cells. Taken together, these data point to CD56dim cells as important effectors not only for killing abnormal cells but also for rapid induction of inflammatory responses involving recruitment of other defensive cells and promotion of cellular resistance to infection together with initial shaping of adaptive immune responses.
In this issue of Blood, two important articles focus on the CD56dim NK-cell subset.13,14 Both studies, by the use of informative markers and receptors, further dissect this subset and provide convincing evidence that CD56dim cells change their phenotypic properties and continue to differentiate throughout their lifespan. The loss of expression of NKG2A, the acquisition of killer immunoglobulin-like receptors (KIRs)and CD57, and the change in the pattern of homing molecules allow the definition of sequential steps of cell maturation accompanied by a progressive decline of proliferating capacity with poor responsiveness to cytokine stimulation. Björkström et al13 show that the various intermediates of this maturation process are detectable in varying proportions in healthy donors while they appear in sequence in patients after HSC transplantation or in humanized mice during the process of reconstitution of the immune system. Data regarding progression from CD94+ to KIR+ NK cells were reported in previous studies in patients undergoing HSC transplantation,7 as well as in in vitro studies8,9 that analyzed the cytokine requirement to obtain mature KIR+ NK cells. However, the present study represents a major progress toward a better understanding of NK-cell complexity because it establishes correlates between the evolving surface phenotype and the loss, acquisition, or maintenance of functional capabilities, including proliferative potential and cytolytic activity. Notably, the process of CD56dim NK-cell differentiation is unidirectional. Moreover, while modulation of NKG2A may be reverted, the expression of both KIR and CD57 is not reversible. At present, it remains elusive which signals drive the differentiation of CD56dim NK cells both during normal homeostasis and during infection. Björkström et al exclude that the process may be dependent on the influence of the interaction with self-HLA class I ligands in the process of NK-cell licensing (or education).15 In this context, the expression of CD57 is similar in licensed and unlicensed NK cells and reduced cytokine responses are detectable in all CD57+ NK cells, irrespective of whether they are licensed or not. These data would imply that NK-cell licensing or their differentiation process are 2 parallel but independent events.
The study by Lopez-Vergès et al14 points primarily to the CD57 antigen to define functionally distinct subsets of CD56dim NK cells. CD57 was previously described as a marker of terminal differentiation of human CD8+ cytolytic T lymphocytes. In addition, variable proportions (30%-60%) of CD56dimCD16+ NK cells in normal adults were CD57+ while no CD57 expression was detected in CD56bright NK cells. Fetal and newborn NK cells virtually lack CD57 expression, thus suggesting that CD57 might represent a marker of senescence/terminal differentiation also in NK cells. Analysis of CD57+ and CD57− NK cells within the CD56dim NK-cell subset reveals that the CD57+ subset expresses a repertoire of NK receptors suggestive of a more differentiated phenotype. In addition, CD57+ cells display a lower cytokine-induced proliferating capability compared with CD57− cells. Similar data were obtained when cells were analyzed for the expression of CD94 or CD62L.10,11 On the other hand, CD16-mediated cell triggering induced a higher frequency of IFN-γ–producing cells among CD57+ cells while CD57+ cell responses to IL-12 or IL-18 are low. Remarkably, CD16 is acquired at late stages of peripheral blood NK-cell differentiation and the amount of surface CD16 correlates with the level of NK-cell maturation. These data are in substantial agreement with those by Björkström et al and emphasize the relevance of CD57 in the identification of highly mature, possibly terminally differentiated, NK cells.
In agreement with this concept, not only CD16 but also KIRs were predominantly expressed by the CD57+ NK-cell subset that was also characterized by partial down-regulation of NKp46 and NKp30. The higher expression of CD16 molecules in CD57+ cells is in line with the higher production of IFN-γ in response to CD16-mediated cell triggering. On the other hand, the marked down-regulation of IL-2Rα may explain the reduced ability of CD57+ cells to proliferate (or produce cytokines) in response to IL-2. It is conceivable that down-regulation of the surface expression of other key elements of cytokine receptors such as the IL-2Rβ, a subunit of the common receptor for IL-2 and IL-15, may explain why these cytokines do not efficiently activate CD57+ NK cells or restore their proliferative capability. In this context, the defective expression of cytokine receptors may explain why these cells do not produce IFN-γ in response to cytokines, while they are good IFN-γ producers after ligation of natural cytotoxicity receptors (A. De Maria, personal communication) or other activating receptors12 or CD16, as shown by Lopez-Vergès et al.14 These data support the notion that CD57+ NK cells are not exhausted, but, on the contrary, are important effector cells mediating not only target cell killing but also rapid release of proinflammatory chemokines and cytokines upon ligation of activating receptors,12 that is, in the process of NK-mediated attack to abnormal (eg, HLA-class I deficient) cells or to IgG-coated cells or pathogens. A comprehensive view of CD56dim NK-cell differentiation is shown in the figure.
Besides representing an important progress in our understanding of NK-cell biology, the phenotypic identification of CD56dim NK-cell subsets, characterized by defined functional capabilities, also has important implications in monitoring of NK cells in pathologic conditions in which these effectors may contribute to defenses or, possibly, to tissue damage. In addition, because NK cell–based therapy has acquired great relevance in the treatment of high-risk leukemias, primarily in the haploidentical HSC transplantation setting (reviewed in Moretta et al16 ), the possibility of precisely identifying NK-cell subsets endowed with particular (or prominent) functional capabilities may complement the identification of alloreactive NK cells in monitoring antileukemic NK-cell responses. In addition, information on the stimuli required to trigger NK cells at different stages of maturation may help in tailoring optimal NK-cell responses in pathologic conditions, HSC transplantation, or vaccination. Along this line, it will be important to further identify cellular or soluble stimuli (eg, cytokines, Toll-like receptor ligands) capable of inducing rapid NK-cell differentiation and acquisition of the desired effector or regulatory function.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal