Abstract
Hepcidin is the principal iron-regulatory hormone and a pathogenic factor in anemia of inflammation. Patients with multiple myeloma (MM) frequently present with anemia. We showed that MM patients had increased serum hepcidin, which inversely correlated with hemoglobin, suggesting that hepcidin contributes to MM-related anemia. Searching for hepcidin-inducing cytokines in MM, we quantified the stimulation of hepcidin promoter-luciferase activity in HuH7 cells by MM sera. MM sera activated the hepcidin promoter significantly more than did normal sera. We then examined the role of bone morphogenetic proteins (BMPs) and interleukin-6 (IL-6), the major transcriptional regulators of hepcidin. Mutations in both BMP-responsive elements abrogated the activation dramatically, while mutations in the IL-6–responsive signal transducer and activator of transcription 3-binding site (STAT3-BS) had only a minor effect. Cotreatment with anti–BMP-2/4 or noggin-Fc blocked the promoter induction with all MM sera, anti–IL-6 blocked it with a minority of sera, whereas anti–BMP-4, -6, or -9 antibodies had no effect. BMP-2–immunodepleted MM sera had decreased promoter stimulatory capacity, and BMP-2 concentrations in MM sera were significantly higher than in normal sera. Our results demonstrate that BMP-2 is a major mediator of the hepcidin stimulatory activity of MM sera.
Introduction
Multiple myeloma (MM) is a malignant plasma cell disorder that accounts for approximately 10% of all hematologic cancers.1 The disease is characterized by monoclonal proliferation of plasma cells together with overproduction of a monoclonal antibody,2 often accompanied by anemia, hypercalcemia, renal insufficiency, or bone lesions.3
Approximately 97% of MM patients develop anemia during the course of their illness, and 70% are anemic at diagnosis. The anemia is usually normocytic/normochromic,4 serum-iron levels are normal to low, serum ferritin is high, and hemosiderin is prominent in bone marrow macrophages.5 This suggests than iron release from reticuloendothelial macrophages is impaired, consistent with anemia of inflammation.6
The main mediator of anemia of inflammation is the iron-regulatory hormone, hepcidin. Hepcidin is produced by hepatocytes and acts on the iron exporter, ferroportin. Binding of hepcidin to ferroportin induces the internalization and degradation of ferroportin, thereby preventing cellular iron efflux and causing retention of iron, mainly inside enterocytes, macrophages, and hepatocytes.7 Pathologic induction of hepcidin by inflammation causes hypoferremia, restricting the iron supply for erythropoiesis and, eventually, causing anemia. The interleukin-6 (IL-6)–hepcidin axis was shown to be important for inflammation-related hypoferremia.8 However, in chronic inflammation, IL-6–independent pathways may also induce hepcidin mRNA.9
Recently, 2 studies described the involvement of hepcidin in anemia of MM in humans. We reported that patients with stage III MM (n = 44) at diagnosis had higher urinary hepcidin levels than normal controls. Furthermore, in the subset of myeloma patients with normal renal function, urinary hepcidin was inversely correlated with hemoglobin level at diagnosis.6 After a serum hepcidin assay was developed, we showed that these patients, as expected, had elevated serum hepcidin, compared with healthy individuals.10 In a study evaluating 34 MM patients at diagnosis or recurrence, Katodritou et al similarly demonstrated that patients' serum hepcidin levels were elevated and inversely correlated with hemoglobin concentrations.11 Using Hep3B cells in vitro, we showed that treatment with MM patients' sera induced hepcidin mRNA expression more than healthy sera, and that with some samples, hepcidin induction was abrogated by neutralizing anti–IL-6 antibodies.6 However, for other sera, the antibodies had no effect, suggesting that additional serum factors can induce hepcidin expression.
Hepcidin expression is predominantly regulated on the transcriptional level. Two families of cytokines are known to be major regulators of hepcidin: the IL-6–like family and the bone morphogenetic protein (BMP) family. BMPs and IL-6 act on the human hepcidin promoter through BMP-responsive elements (BREs) and the signal transducer and activator of transcription 3 (STAT3)–binding site (STAT3-BS), respectively.12-15 IL-616 and BMPs17,18 have been reported to be produced in MM, and we surmised that they could also be involved in the pathogenesis of myeloma-related anemia. We used serum samples from myeloma patients to identify circulating substances that contribute to anemia by mediating the induction of hepcidin. We characterized these substances by interfering with their activity, either on the promoter level by mutating the specific response elements in the hepcidin promoter or by blocking the cytokine/receptor interaction.
Methods
Cell culture
HuH7 human hepatoma cells were cultured in Dulbecco modified Eagle medium (DMEM; Gibco Invitrogen), containing 10% fetal bovine serum (FBS; HyClone Thermo Fisher Scientific), 10 μg/mL ciprofloxacin, and 50 μg/mL gentamycin, at 37°C in 5% CO2. HuH7 cells were purchased from ATCC Standards and maintained and stored in our laboratory.
Generation of hepcidin promoter-luciferase constructs
The entire sequence of the human hepcidin (HAMP) promoter, consisting of 2997 base pairs (bp) upstream of the hepcidin translational start site, was amplified, and cloned into pGL4.17 firefly luciferase reporter vector (Promega). This construct was used as a template for site-directed mutagenesis of STAT3-BS sequence and BRE1 and 2 (Figure 2). Mutations were introduced using the Quikchange XL Site-Directed Mutagenesis kit, using the manufacturer-recommended cycling parameters (Agilent-Stratagene). The primers listed in Table 1 were designed with the Quikchange Primer Design Program (Agilent-Stratagene; http://www.stratagene.com). The mutated plasmid was transformed into XL10-Gold Ultracompetent cells (Stratagene) and isolated using PureLink Quick Plasmid Miniprep Kit (Invitrogen). The mutations were verified by direct sequencing (Laragen). The sequencing primers are listed in Table 2. Plasmids with confirmed mutations were amplified and isolated using Endofree Plasmid Maxi Kit (QIAGEN). Isolated plasmids were evaluated for purity and size with spectrophotometry and gel electrophoresis, respectively. These plasmids were used as templates for the second round of site-directed mutagenesis to construct plasmids carrying 2 or 3 mutations. We generated a total of 8 different constructs: 1 wild-type HAMP promoter construct, 3 constructs with single mutations, and 4 constructs with different combination of the mutations.
Primers for site-directed mutagenesis
| Site . | Sense 5′- . | Antisense 5′- . |
|---|---|---|
| STAT3-BS | cgccaccaccttcttggaattgagacagagcaaa | tttgctctgtctcaattccaagaaggtggtggcg |
| BRE-1 | cccgccttttcggtgccaccaccttct | agaaggtggtggcaccgaaaaggcggg |
| BRE-2 | caccaaggctctggtgcctgtgctgtgac | gtcacagcacaggcaccagagccttggtg |
| Site . | Sense 5′- . | Antisense 5′- . |
|---|---|---|
| STAT3-BS | cgccaccaccttcttggaattgagacagagcaaa | tttgctctgtctcaattccaagaaggtggtggcg |
| BRE-1 | cccgccttttcggtgccaccaccttct | agaaggtggtggcaccgaaaaggcggg |
| BRE-2 | caccaaggctctggtgcctgtgctgtgac | gtcacagcacaggcaccagagccttggtg |
Primers for direct sequencing of mutated regions in plasmids
| Mutation site . | Forward 5′- . | Reverse 5′- . |
|---|---|---|
| BRE-2 | ggtctccgtgtcaacagttcctgaaa | aagatggcctcagatgggaatagc |
| STAT3-BS/BRE-1 | ccagaacctatgcacgtgtg | cgtgccgtctgtctggct |
| Mutation site . | Forward 5′- . | Reverse 5′- . |
|---|---|---|
| BRE-2 | ggtctccgtgtcaacagttcctgaaa | aagatggcctcagatgggaatagc |
| STAT3-BS/BRE-1 | ccagaacctatgcacgtgtg | cgtgccgtctgtctggct |
Transfection and dual luciferase assay
Reverse transfection of the human hepatocyte cell line, HuH7, was performed with lipofectamine 2000 (Invitrogen) as a transfection reagent. Briefly, the mixture of plasmids and lipofectamine in Optimem I reduced serum medium (Gibco Invitrogen) was prepared and aliquoted in a 96-well plate. To each well, 2 plasmids were added, a test hepcidin promoter construct and the reference, thymidine kinase–Renilla luciferase plasmid (pTK-RL; Promega). HuH7 cells were trypsinized, resuspended in Optimem, and added to the transfection mix in the 96-well plate. After 16 hours of incubation at 37°C, the complexes were removed and replaced by Optimem media containing recombinant human cytokines (IL-6, BMP-2, -4, -6, and -9; R&D Systems) or human serum. Cells were incubated at 37°C for 6 hours. We ensured that treatments did not alter firefly Renilla values, indicating that cells did not significantly increase in number during treatment, which could potentially confound the experiment. After treatment, cells were lysed using 1× in passive lysis buffer (Promega), and bioluminescence signal was measured using Veritas Microplate Luminometer with dual auto injectors (Turner Biosytems). The firefly luciferase (reporter) signal was measured by adding Luciferase Assay Reagent II, followed by the addition of Stop&Glo substrate to measure Renilla luciferase bioluminescence. The firefly/Renilla luciferase ratio was used to normalize for transfection efficiency. Results were expressed as fold change over untreated cells by dividing the firefly/Renilla ratio of treated cells by the firefly/Renilla ratio of untreated cells.
Collection of human samples
All blood samples were obtained after informed consent in accordance with the Declaration of Helsinki, as approved by the appropriate institutional review boards. Sera samples used in this study were from Durie-Salmon stage III19 myeloma patients from Veterans Administration (VA) hospitals in Los Angeles, Pittsburgh, San Antonio, and Chicago,6 (n = 13) as well as from stage I, II, or III myeloma patients from Theagenion Cancer Center, Thessaloniki, Greece (n = 12).11 All MM samples were from patients who were newly diagnosed and had not received any cytotoxic chemotherapy. Control sera (n = 15) were obtained from L.T.-H. and University of California Los Angeles.
Serum hepcidin assay
Serum hepcidin levels of the sera samples used in this study were measured by a competitive ELISA as described and validated by Ganz et al.10 Briefly, 96-well plates were coated with anti–human hepcidin antibody. Serum samples were added in 1:20 dilution in Tris [tris(hydroxymethyl)aminomethane]-buffered saline containing 0.05% Tween-20 (TBS-T), with 10 ng/mL biotinylated hepcidin-25 as a tracer. Standard curves were prepared by the serial 2-fold dilution of synthetic hepcidin in TBS-T containing the tracer. After washing, the assay was developed with streptavidin-peroxidase and tetramethyl benzidine, color development was stopped by sulfuric acid, and the plate was read at 450 nm on a DTX 880 microplate reader (Beckman Coulter). Standard curves were fitted with 12-point fit using GraphPad Prism Version 4 (GraphPad Software). The fitted curve was then used to convert sample absorbance readings to hepcidin concentrations.
Neutralization experiments
Mouse antibodies against human IL-6, IL-6R, BMP-2/4, -4, -6, -9, as well as BMP antagonist noggin-Fc chimera were all obtained from R&D Systems. For experiments, the concentration of all antibodies and noggin-Fc was 1 μg/mL, except for anti–BMP-2/4 and anti–BMP-6, which were used at 5 μg/mL. Isotype control (IC) was an unrelated goat immunoglobulin (IgG) antibody and was used at a concentration of 1.8 μg/mL. The cytokine inhibitors were added to the wells before adding experimental treatment such as patient sera or recombinant human cytokines.
Immunodepletion of serum
To selectively immunodeplete BMP-2 from sera, we used the Pierce Direct BMP-2 IP kit (Thermo Fisher Scientific). Briefly, 5 μg of anti–BMP-2/4 antibody or isotype control was coupled to a spin column containing Aminolink Plus Coupling Resin. After 2 hours incubation with shaking at room temperature, the resin was washed and stored in Coupling Buffer for less than a week before use. Efficiency of coupling was determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). For immunodepletion, the coupling buffer was removed and 150 μL of serum was incubated on the spin column overnight with continuous shaking at 4°C. The next morning, immunodepleted serum was collected and used to treat transfected cells.
ELISA for BMP-2
To measure the amount of BMP-2 present in the serum, we used the Quantikine BMP-2 immunoassay (R&D Systems). Microplates were precoated with a monoclonal antibody specific for BMP-2. Standards and samples were incubated in the microplate for 2 hours at room temperature while being shaken at 500 rpm. After washing away any unbound substances, a horseradish peroxidase (HRP)–linked monoclonal antibody specific for BMP-2 was added to the wells and incubated for 2 hours at room temperature with continuous shaking. After washing, the assay was developed with tetramethyl benzidine, the color development was stopped by an acid solution, and the plate was read at 450 nm on a microplate spectrophotometer (Spectramax 190; Molecular Devices). A correction measurement at 540 nm (γ2) was subtracted to correct for plate imperfections. Values were calculated based on the standard curve and expressed as picograms per millilter.
Statistical analysis
The Student t test was used for compare 2 groups of normally distributed data. The Mann Whitney rank-sum test was used to compare data that were not normally distributed. To compare multiple groups, 1-way analysis of variance (ANOVA) was used to address statistical significance. Correlations between the various measured parameters were calculated by Pearson correlation. A P value < .05 was considered as statistically significant.
Results
Serum hepcidin levels negatively correlated with hemoglobin concentrations in MM patients
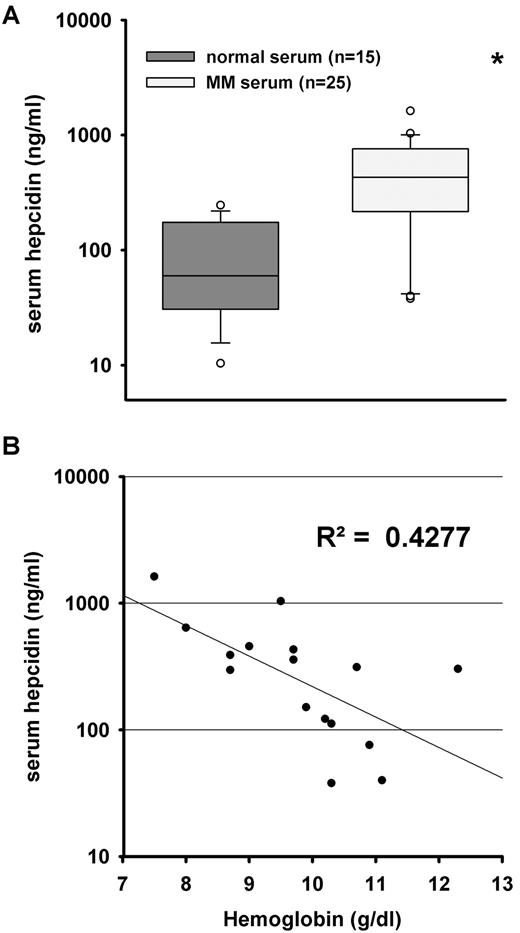
In this study, we used MM patient sera from VA hospitals in Los Angeles, Pittsburgh, San Antonio, and Chicago, and from Theagenion Cancer Center, Thessaloniki, Greece (n = 25). The hematologic parameters were previously measured.6,10,11 These patients all had a normocytic/normochromic anemia. For the US patients (n = 13), mean hemoglobin level was 10.0 g/dL (range 7.5-12). Their mean hematocrit level was 30 (range 22.7-37.3). Their mean reticulocyte percent was 0.8% (no patient had a reticulocyte count > 2.5%). The mean serum iron level was 61.5 mg/dL (range 26-175) and serum transferrin was 248 mg/dL (range 143-365). As our normal range of serum iron and transferrin was 60-170 and 240-400, respectively, the mean iron and transferrin levels are both at the lower end of normal, consistent with the anemia of “chronic disease.” In addition, the serum ferritin levels were markedly increased (mean 552 ng/mL, with normal range of 22-322 ng/mL), also consistent with this classification of anemia. The Greek patients (n = 12) had a similar clinical profile, but their laboratory test values were not normally distributed: median hemoglobin 10.1 (range 6.1-10.9), transferrin saturation 21.1% (range 9.7-53.4), and ferritin 291 (range 206-1997). Of the 25 patients, 16 had normal renal function, indicated by serum creatinine levels of 1.4 mg/dL or lower. MM patients had significantly higher serum hepcidin levels compared with age-matched controls (n = 15; Figure 1A). We analyzed the relationship between hepcidin and hemoglobin in this cohort to detect the possible effect of hepcidin on anemia distinct from the known confounding effects of renal failure. As shown, hepcidin serum levels inversely correlated with hemoglobin concentrations in patients with normal renal function (Figure 1B; R2 = 0.4277; P = .006), again indicating a possible contribution of increased hepcidin to the pathogenesis of MM anemia.
Correlation between serum hepcidin levels and hemoglobin concentrations. We used 25 MM patient serum samples for which hemoglobin content and serum hepcidin concentration were previously measured.6,10,11 (A) Serum hepcidin levels in MM patients (n = 25) were significantly higher than in healthy individuals (n = 15). Boxes represent median and 25-75 percentiles, and whiskers represent 10 and 90 percentiles. Circles are the outliers. Statistical significance was determined with the Mann-Whitney rank-sum test; *P < .01, compared with normal serum. (B) Patients with normal renal function (n = 16) were included to investigate the relationship between serum hepcidin levels and hemoglobin concentrations. Hemoglobin concentrations and serum hepcidin inversely correlate (R2 = 0.4277, P = .006, Pearson correlation).
Correlation between serum hepcidin levels and hemoglobin concentrations. We used 25 MM patient serum samples for which hemoglobin content and serum hepcidin concentration were previously measured.6,10,11 (A) Serum hepcidin levels in MM patients (n = 25) were significantly higher than in healthy individuals (n = 15). Boxes represent median and 25-75 percentiles, and whiskers represent 10 and 90 percentiles. Circles are the outliers. Statistical significance was determined with the Mann-Whitney rank-sum test; *P < .01, compared with normal serum. (B) Patients with normal renal function (n = 16) were included to investigate the relationship between serum hepcidin levels and hemoglobin concentrations. Hemoglobin concentrations and serum hepcidin inversely correlate (R2 = 0.4277, P = .006, Pearson correlation).
Development of an in vitro system for analysis of hepcidin transcriptional regulation
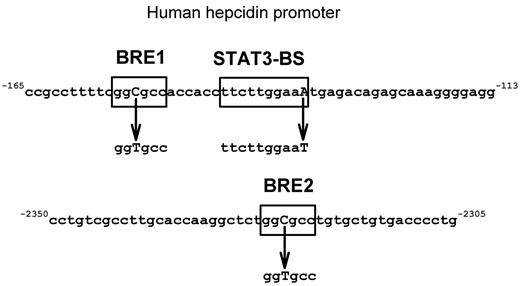
The HuH7 cell line is of human hepatic origin, fast-growing, and readily transfectable. We transfected the cells with WT or mutant HAMP promoter-luciferase constructs containing the full-length human hepcidin promoter (2997 bp). Using site-directed mutagenesis, the promoter was mutated at promoter sequences thought to be involved in signaling by IL-6 and BMPs (Figure 2). We validated the assay by testing known inducers of hepcidin expression and their effect on mutated hepcidin-promoter constructs. We then used the assay to screen MM patient sera for their ability to induce HAMP promoter activity and to identify serum cytokines responsible for hepcidin induction.
Scheme of mutations introduced in HAMP promoter. Boxed areas are the putative transcription factor binding sites for BMP and IL-6 pathways. Mutated nucleotides are shown in bold capitals. Mutations in BRE1 and -2 were previously described in Island et al33 and the STAT3-BS mutation in Truksa et al.15
Scheme of mutations introduced in HAMP promoter. Boxed areas are the putative transcription factor binding sites for BMP and IL-6 pathways. Mutated nucleotides are shown in bold capitals. Mutations in BRE1 and -2 were previously described in Island et al33 and the STAT3-BS mutation in Truksa et al.15
WT HAMP promoter was induced by IL-6, BMP-2, -4, -6, and -9 in a dose-dependent manner
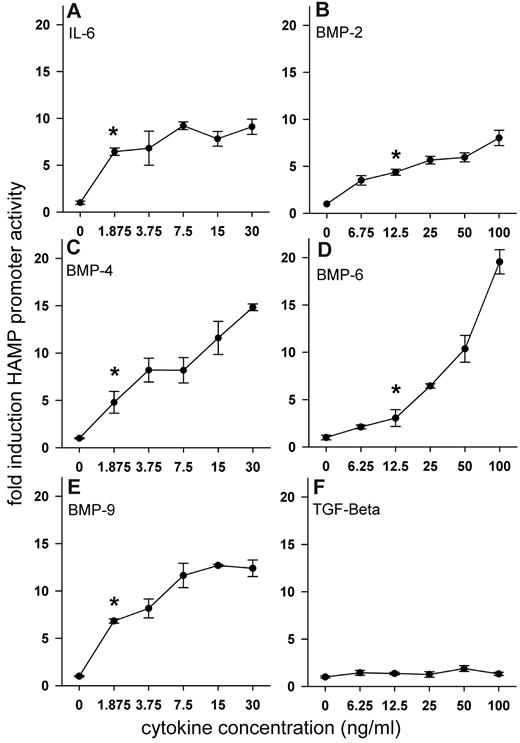
We tested a set of cytokines known to induce hepcidin in vitro, including IL-6 and several members of the TGF-β superfamily, to determine to what extent they induce hepcidin promoter activity in our system. HuH7 cells were transfected with WT-HAMP promoter-luciferase construct and treated with increasing doses of recombinant human IL-6, BMP-2, -4, -6, -9, and TGF-β1 (Figure 3A-F). BMP-4, -9, and IL-6 were the most potent inducers of HAMP promoter activity and achieved statistically significant induction at concentrations less than 2 ng/mL. BMP-2 and -6 significantly induced promoter activity at 6.75 and 12.5 ng/mL, respectively. TGF-β1 had no effect on HAMP promoter activity, even at very high doses.
Dose-dependent effect of recombinant human cytokines on HAMP promoter activity. HuH7 cells were cotransfected with WT-HAMP promoter-firefly luciferase construct and pTK-RL construct and treated with increasing doses of recombinant human cytokines. After treatment, cells were lysed and luciferase activity was measured. Results for individual cytokines (A-F) are expressed as fold induction over untreated control (firefly/renilla ratio of the experimental condition divided by the firefly/renilla ratio of untreated control). Dots and error bars represent mean ± SD of at least 3 independent experiments executed in duplicate. Statistical significance was determined with the Mann-Whitney rank-sum test, and *P < .05 compared with untreated control.
Dose-dependent effect of recombinant human cytokines on HAMP promoter activity. HuH7 cells were cotransfected with WT-HAMP promoter-firefly luciferase construct and pTK-RL construct and treated with increasing doses of recombinant human cytokines. After treatment, cells were lysed and luciferase activity was measured. Results for individual cytokines (A-F) are expressed as fold induction over untreated control (firefly/renilla ratio of the experimental condition divided by the firefly/renilla ratio of untreated control). Dots and error bars represent mean ± SD of at least 3 independent experiments executed in duplicate. Statistical significance was determined with the Mann-Whitney rank-sum test, and *P < .05 compared with untreated control.
Mutations in the STAT3-BS and BREs of HAMP promoter abrogated the response to IL-6 and BMPs, respectively
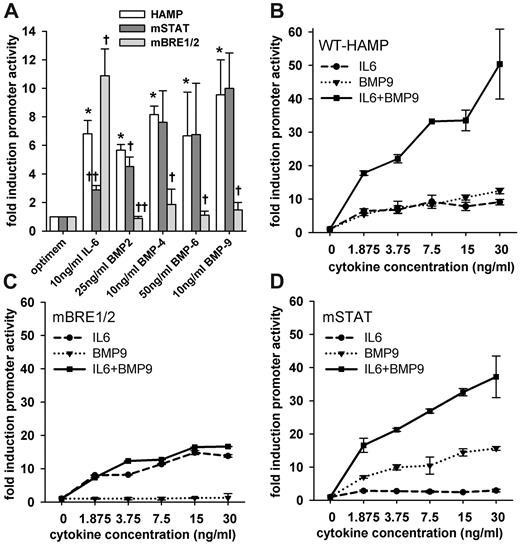
To determine which promoter elements were responsible for the inducing effect of IL-6 and BMPs on HAMP promoter, we subjected the WT-HAMP promoter to site-directed mutagenesis as described in the Methods. In total, 7 mutant constructs were generated: 3 constructs with single mutations in STAT3-BS or BREs and 4 constructs with different combinations of mutations. We selected the combination of constructs that could differentiate between IL-6 and BMP signaling. The single mutation in STAT3-BS (mSTAT construct) and the double mutations in BRE1 and 2 (mBRE1/2 construct) disrupted the signaling by IL-6 or BMPs, respectively. The triple-mutated construct (mAll3 construct) disrupted signaling by both IL-6 and BMPs (data not shown). The response of our mSTAT and mBRE1/2 mutant constructs to IL-6 and BMP-2, -4, -6, and -9 in HuH7 cells is shown in Figure 4. Due to differences in the potency of the cytokines, we chose the doses to obtain similar promoter activation after stimulation by each cytokine. BMP-2 and -6 were less potent; therefore, we used higher concentrations to match the response to IL-6, BMP-4, and -9. As expected, the induction of luminescence by IL-6 was disrupted by the mutation in STAT3-BS. Surprisingly, mutations in both BREs resulted in even higher promoter activation by IL-6. The induction by BMPs was disrupted by mutations in the BRE1/2, but not by STAT3-BS, mutation (Figure 4A). Of note, the baseline promoter activity (firefly:renilla ratio) was decreased, compared with WT, when either STAT3-BS or BRE1/2 were mutated, compared with WT-HAMP (data not shown), possibly because these sites may be involved in basal hepcidin transcription. Nevertheless, the ability to respond (ie, fold induction) was not decreased in the mutated constructs (eg, mBRE1/2 construct was still induced by IL-6).
Effect of mutations in STAT3-BS or BREs on HAMP promoter response to cytokines. HuH7 cells were transfected with WT-HAMP, mSTAT, or mBRE1/2 promoter-firefly luciferase construct together with pTK-RL construct and treated with the indicated concentration of recombinant human cytokines. (A) IL-6 signaling was abrogated by the mutation in the STAT3-BS, but not by the mutation in both BREs. In contrast, BMP signaling was disrupted by the mutation in both BREs, but not by the STAT3-BS mutation. Bars represent mean ± SD of at least 3 independent experiments executed in duplicate. Statistical significance was determined with the Student t test or Mann-Whitney rank-sum test. *P < .05, compared with untreated control (optimem/WT-HAMP); †P < .05; and ††P < .001, compared with the WT-HAMP construct within the same cytokine group. (B) IL-6 and BMP-9 act synergistically on the HAMP promoter. (C) The mutations in BREs abrogated the synergy, while IL-6 induction was preserved. (D) The mutation of the STAT3-BS did not abrogate the synergy or the BMP-9 induction. Dots and error bars represent mean ± SD. Results are expressed as fold induction over untreated control.
Effect of mutations in STAT3-BS or BREs on HAMP promoter response to cytokines. HuH7 cells were transfected with WT-HAMP, mSTAT, or mBRE1/2 promoter-firefly luciferase construct together with pTK-RL construct and treated with the indicated concentration of recombinant human cytokines. (A) IL-6 signaling was abrogated by the mutation in the STAT3-BS, but not by the mutation in both BREs. In contrast, BMP signaling was disrupted by the mutation in both BREs, but not by the STAT3-BS mutation. Bars represent mean ± SD of at least 3 independent experiments executed in duplicate. Statistical significance was determined with the Student t test or Mann-Whitney rank-sum test. *P < .05, compared with untreated control (optimem/WT-HAMP); †P < .05; and ††P < .001, compared with the WT-HAMP construct within the same cytokine group. (B) IL-6 and BMP-9 act synergistically on the HAMP promoter. (C) The mutations in BREs abrogated the synergy, while IL-6 induction was preserved. (D) The mutation of the STAT3-BS did not abrogate the synergy or the BMP-9 induction. Dots and error bars represent mean ± SD. Results are expressed as fold induction over untreated control.
IL-6 and BMP-9 induced WT HAMP promoter activity in a synergistic manner
Of the multiple cytokines that contribute to the stimulation of hepcidin in MM, some may act synergistically. To examine this possibility, we transfected HuH7 cells with WT-HAMP promoter-luciferase construct and treated them with combinations of different concentrations of IL-6 and BMP-9. We chose these 2 cytokines because of the similarity of their dose-dependent induction of HAMP promoter activity (Figure 3A,E). The combination treatment confirmed the synergistic action of IL-6 and BMP-9 even at very low concentrations (Figure 4B). Treatment with 1.875 ng/mL IL-6 by itself or 1.875 ng/mL BMP-9 by itself caused a 4- and 5-fold increase in luminescence, respectively. Adding the 2 cytokines together resulted in an 18-fold increase in luminescence. Increasing concentrations of IL-6 or BMP-9 by themselves only slightly increased hepcidin promoter activity (7- and 12-fold, respectively, at 30 ng/mL concentration); however, combined treatment, again, showed a synergistic induction of hepcidin promoter activity (50-fold increase). Of note, synergy was also observed between IL-6 and BMP-2 (data not shown). Our findings suggest crosstalk for hepcidin regulation between the SMAD and JAK/STAT signaling pathways.
Next, we evaluated the ability of the mutations in the STAT3-BS and BRE1/2 to distinguish the inducing and synergistic effect of IL-6 and BMP signaling on HAMP promoter activity. When both BREs were mutated, the response to BMP-9 was completely abrogated. The BRE1/2 mutation also abrogated the synergism between IL-6 and BMPs (Figure 4C). When the STAT3-BS was mutated, the response of IL-6 was blunted, but the response to BMP-9 was not affected. The synergism between BMP-9 and IL-6 also remained, but their synergistic effect on the STAT3-BS mutant promoter was slightly lower than on the WT-HAMP promoter (Figure 4D). Adding both cytokines together induced promoter activity by 16-fold at 1.875 ng/mL of each cytokine, whereas promoter activity was induced by 7- and 3-fold when BMP-9 and IL-6 were added individually.
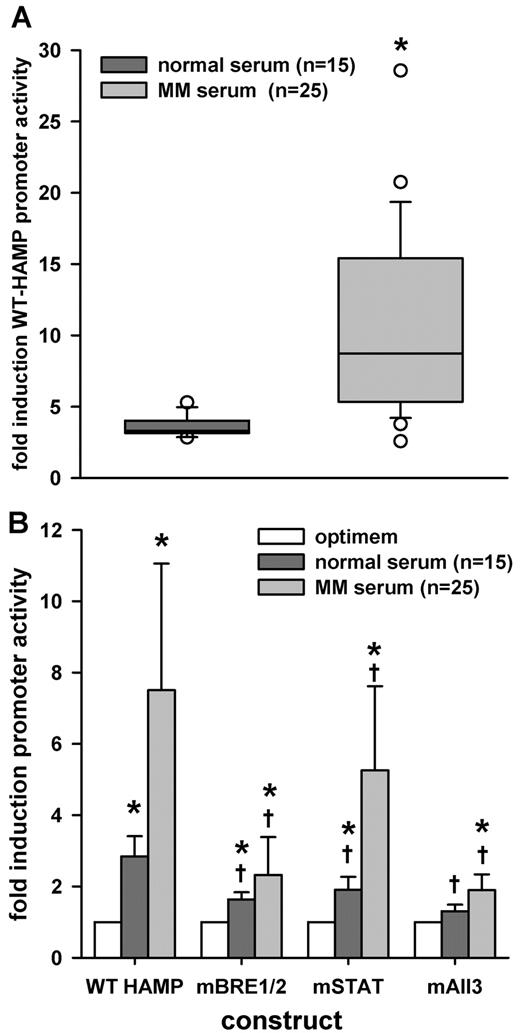
MM patient sera induced WT HAMP promoter activity
Our previous experiments indicated that the in vitro system could detect the presence of hepcidin-inducing cytokines, differentiate BMP from other signaling pathways with the use of the mBRE1/2 construct, and, using the mAll3 construct, detect the presence of additional factors besides IL-6 and BMPs. Next, we tested whether the sera from MM patients would induce WT-HAMP promoter activity. We transfected HuH7 cells with the WT-HAMP construct and treated cells for 6 hours with sera at 10% concentration dissolved in Optimem medium. We compared MM patient sera (n = 25) to normal human sera from healthy donors (n = 15). MM patient sera significantly induced hepcidin promoter activity, in comparison to normal sera (Figure 5A). The correlation coefficient comparing hepcidin concentrations in the 40 sera and hepcidin promoter activity after treatment with those sera was 0.377 (P = .017, Pearson product moment correlation). It is not surprising that the correlation was not higher, considering that not all hepcidin regulatory factors circulate in the blood, and that immortalized hepatic cell lines may not contain the full hepatocyte repertoire of receptors and transduction pathways.
Effect of MM patient sera on HAMP promoter activity. HuH7 cells were cotransfected with WT-HAMP, mSTAT, mBRE1/2, or mAll3 promoter-firefly luciferase construct, together with pTK-RL construct, and treated with 10% serum in Optimem. After 6 hours, cells were lysed and luciferase activity was measured. (A) Treatment with MM patient sera caused a significantly higher induction of WT-HAMP promoter activity, compared with sera from healthy individuals. Results are expressed as fold induction over untreated control. Statistical significance was determined with the Mann-Whitney rank-sum test. Boxes represent median and 25-75 percentiles, and whiskers represent 10 and 90 percentiles. Circles are outliers; *P < .05, compared with normal sera. (B) Mutation of BREs abrogated induction by MM sera dramatically, while the STAT3-BS mutation had a statistically less significant effect. Results are expressed as fold induction over untreated control (construct/optimem). Bars represent mean ± SD. Statistical significance was determined with 1-way ANOVA to compare the same sera for the different mutations. To compare MM patient sera with normal sera within the same construct, the Mann-Whitney rank-sum test was used; *P < .05, compared with untreated control (construct/optimem), and †P < .05, compared with WT-HAMP promoter activity for the same sera.
Effect of MM patient sera on HAMP promoter activity. HuH7 cells were cotransfected with WT-HAMP, mSTAT, mBRE1/2, or mAll3 promoter-firefly luciferase construct, together with pTK-RL construct, and treated with 10% serum in Optimem. After 6 hours, cells were lysed and luciferase activity was measured. (A) Treatment with MM patient sera caused a significantly higher induction of WT-HAMP promoter activity, compared with sera from healthy individuals. Results are expressed as fold induction over untreated control. Statistical significance was determined with the Mann-Whitney rank-sum test. Boxes represent median and 25-75 percentiles, and whiskers represent 10 and 90 percentiles. Circles are outliers; *P < .05, compared with normal sera. (B) Mutation of BREs abrogated induction by MM sera dramatically, while the STAT3-BS mutation had a statistically less significant effect. Results are expressed as fold induction over untreated control (construct/optimem). Bars represent mean ± SD. Statistical significance was determined with 1-way ANOVA to compare the same sera for the different mutations. To compare MM patient sera with normal sera within the same construct, the Mann-Whitney rank-sum test was used; *P < .05, compared with untreated control (construct/optimem), and †P < .05, compared with WT-HAMP promoter activity for the same sera.
Next, we compared the effect of mutations in the STAT3-BS and BREs in the HAMP promoter on the response to normal and MM patient sera. We transfected HuH7 cells, followed by treatment with 10% serum in Optimem medium. Both normal (n = 15) and MM patient (n = 25) sera induced WT-HAMP promoter activity, compared with Optimem-only control, but MM sera caused significantly greater induction, compared with normal sera, for all constructs (P < .05; Figure 5B). The mutations in the HAMP promoter decreased the response to serum significantly (Figure 5B). With normal sera, both BRE1/2 and STAT3-BS mutations similarly reduced the serum effects and the response to normal sera was completely abrogated with the use of mAll3 construct, suggesting that the hepcidin-inducing activity of normal sera depends on both BMPs and IL-6. With MM sera, BRE1/2 mutation caused a consistently greater decrease in response than the STAT3-BS mutation. The response was never completely abrogated, not even with the mALL3 construct. Remarkably, we observed that serum from 5 patients contained factors signaling through both BREs and STAT-3BS, while the other 20 contained factors using the BREs (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Anti–BMP-2/4 and noggin-Fc abrogated the stimulatory effect of MM patient sera
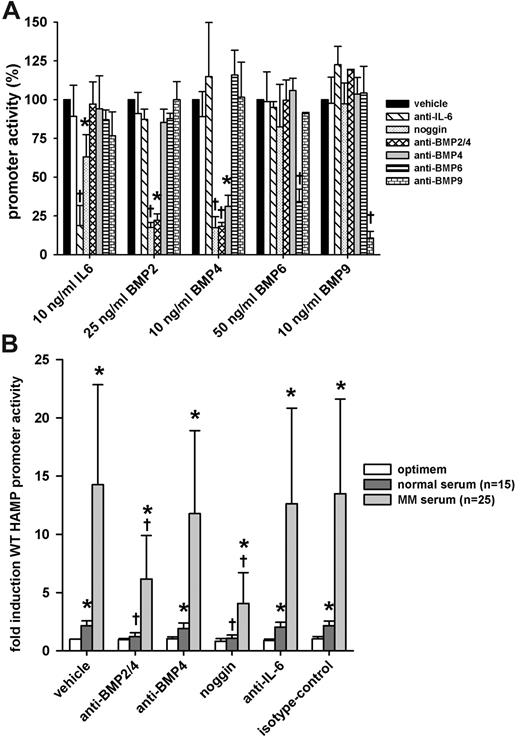
Using cytokine inhibitors, we further explored the contribution of hepcidin promoter-stimulating cytokines from MM sera. We first confirmed the specificity and potency of neutralizing antibodies and inhibitors by assessing their ability to neutralize recombinant human cytokines. We transfected HuH7 cells with WT-HAMP promoter-luciferase construct and treated them with human recombinant IL-6, BMP-2, -4, -6, and -9 with or without cytokine inhibitors. A combination of anti–IL-6 antibody and anti–IL-6 receptor antibody was used to interfere with IL-6 signaling. We also used noggin, a natural extracellular BMP antagonist, which binds several BMPs, some with high affinity (eg, BMP-2 and -4), others with lower affinity (eg, BMP-7).20 Anti–BMP-2/4, anti–BMP-4, anti–BMP-6, and anti–BMP-9 were used to neutralize their respective targets. Isotype control was used to assess any nonspecific effects of the treatment with cytokine inhibitors. We chose the dose of cytokine inhibitors so as to decrease promoter activation to approximately the same level for each cytokine. Anti–BMP-2/4 and anti-BMP6 were less effective in neutralizing their antigen and thus were used at higher concentrations than anti–IL-6, anti–BMP-4, and -9. In the absence of sera, the cytokine inhibitors by themselves had no effect on WT-HAMP promoter activity (data not shown). As expected, anti–IL-6/anti–IL-6 receptor-neutralizing antibodies abrogated the promoter response to IL-6, but not to any BMPs. Interestingly, noggin-Fc showed slight, but significant, inhibitory effects on IL-6–mediated HAMP promoter stimulation. Anti–BMP-6 and -9 abrogated the promoter induction by BMP-6 and -9, respectively. Anti–BMP-2/4 and noggin/Fc completely neutralized the effect of BMP-2 and -4, whereas anti–BMP-4 specifically affected BMP-4–, but not BMP-2–mediated, stimulation (Figure 6A).
Effect of cytokine inhibitors on the induction of HAMP promoter activity by MM patient sera. HuH7 cells were cotransfected with WT-HAMP promoter-firefly luciferase construct and pTK-RL construct. Next, cells were treated with indicated doses of cytokines or 10% serum with or without cytokine inhibitors. After treatment, cells were lysed and luciferase activity was measured. (A) Specificity array of the cytokine inhibitors. All results are expressed as percentage promoter activity. Bars represent mean ± SD of at least 3 independent experiments executed in duplicate. Statistical significance was determined with the Student t test or Mann-Whitney rank-sum test; *P < .05, and †P < .001, compared with the cytokine-only group (cytokine/vehicle). (B) The effect of cytokine inhibitors on the induction of the WT-HAMP promoter by MM or healthy sera. Anti–BMP-2/4 and noggin-Fc significantly reversed the stimulatory effect of MM sera. Results are expressed as fold induction over untreated control (vehicle/optimem). Bars represent mean ± SD. Statistical significance was determined with 1-way ANOVA to compare the same sera for the different cytokine inhibitors. To compare MM patient sera with normal sera within the same group, the Mann-Whitney rank-sum test was used; *P < .05, compared with untreated control (vehicle/optimem), and †P < .05, compared with serum only group (vehicle/serum).
Effect of cytokine inhibitors on the induction of HAMP promoter activity by MM patient sera. HuH7 cells were cotransfected with WT-HAMP promoter-firefly luciferase construct and pTK-RL construct. Next, cells were treated with indicated doses of cytokines or 10% serum with or without cytokine inhibitors. After treatment, cells were lysed and luciferase activity was measured. (A) Specificity array of the cytokine inhibitors. All results are expressed as percentage promoter activity. Bars represent mean ± SD of at least 3 independent experiments executed in duplicate. Statistical significance was determined with the Student t test or Mann-Whitney rank-sum test; *P < .05, and †P < .001, compared with the cytokine-only group (cytokine/vehicle). (B) The effect of cytokine inhibitors on the induction of the WT-HAMP promoter by MM or healthy sera. Anti–BMP-2/4 and noggin-Fc significantly reversed the stimulatory effect of MM sera. Results are expressed as fold induction over untreated control (vehicle/optimem). Bars represent mean ± SD. Statistical significance was determined with 1-way ANOVA to compare the same sera for the different cytokine inhibitors. To compare MM patient sera with normal sera within the same group, the Mann-Whitney rank-sum test was used; *P < .05, compared with untreated control (vehicle/optimem), and †P < .05, compared with serum only group (vehicle/serum).
Next, we tested the ability of the cytokine inhibitors to abrogate the observed induction of HAMP promoter activity when cells were treated with MM patient sera. HuH7 cells were transfected with WT-HAMP promoter-luciferase construct and treated with MM patient sera with or without cytokine inhibitors. After treatment, cells were lysed and luciferase activity was measured. Both MM and normal sera significantly induced promoter activity; however, the effect of MM sera was significantly greater than that of normal sera (P < .05; Figure 6B). Anti–BMP-2/4 and noggin-Fc reduced the response to both normal and MM sera significantly, with more pronounced decrease observed with MM sera (Figure 6B). Anti–BMP-6 and anti–BMP-9 showed no significant neutralizing activity (data not shown). Compared with anti–BMP-2/4, anti–BMP-4 antibody had no significant effect, indicating that BMP-2 is likely responsible for most of the hepcidin-inducing activity. For MM patient sera, besides BMP-2 and, to a lesser extent, BMP-4, another unidentified factor appears to play a role in HAMP promoter induction, as there was still some residual promoter activity even in the presence of anti–BMP-2/4 and noggin-Fc. Interestingly, in the same 5 patients whose sera induced STAT-3BS–dependent responses, IL-6 antibodies (as well as anti–BMP-2/4 antibodies) abrogated the serum-mediated stimulation of hepcidin promoter activity (supplemental Figure 1B).
BMP-2 concentrations were higher in MM sera, compared with sera from healthy individuals
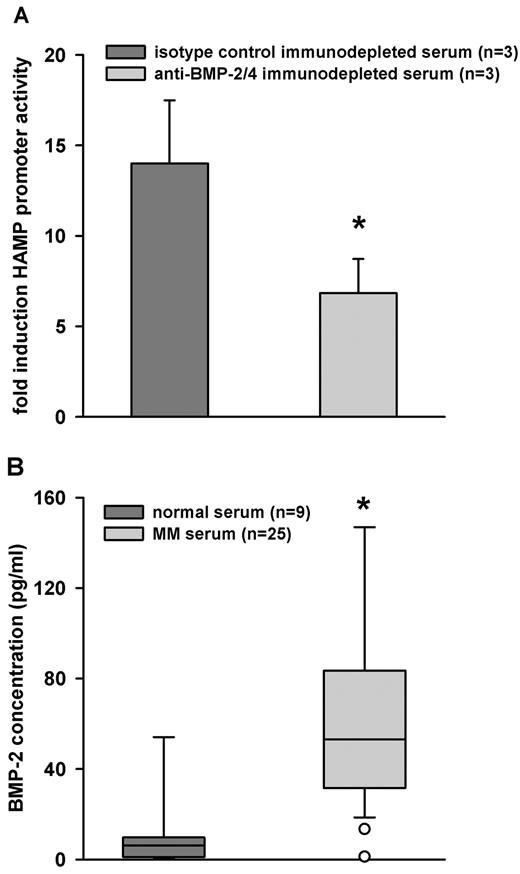
To confirm the neutralization experiments and to ascertain whether the BMP-2 activity was due to the cytokine presence in MM sera or to its secretion by HuH7 cells, we used anti–BMP-2/4 to deplete any BMP-2 from the MM sera. The depleted sera were used to treat HuH7 cells transfected with WT-HAMP promoter-luciferase construct. Sera depleted for BMP-2 had blunted stimulatory activity, compared with sera depleted with isotype control (Figure 7A). Finally, we measured BMP-2 levels in MM and healthy sera. As expected, we found significantly higher concentrations of BMP-2 in MM patient sera (n = 25), compared with sera from healthy individuals (n = 9; Figure 7B). We did not observe a correlation between serum BMP-2 and hepcidin levels of MM patients (data not shown). This was not surprising, because hepcidin is also affected by other factors, including erythropoiesis, iron intake and stores, and renal function. It would require a much larger sample to isolate the effects of BMP-2 on this background.
BMP-2 is present in MM sera. (A) MM sera (n = 3) were immunodepleted with anti–BMP-2/4 or isotype-control antibody. Depleted sera were used to treat HuH7 cells that had been transfected with WT-HAMP promoter-luciferase construct. After treatment, cells were lysed and luciferase activity was measured. Bars represent mean ± SD. Statistical significance was determined with the Student t test; *P < .001. (B) ELISA assay was used to measure BMP-2 values in normal (n = 9) and MM sera (n = 23). As expected, MM sera contained higher amounts of BMP-2, then normal sera. Bars represent mean ± SD. The Mann-Whitney rank-sum test was used to determine statistical significance, and *P < .001.
BMP-2 is present in MM sera. (A) MM sera (n = 3) were immunodepleted with anti–BMP-2/4 or isotype-control antibody. Depleted sera were used to treat HuH7 cells that had been transfected with WT-HAMP promoter-luciferase construct. After treatment, cells were lysed and luciferase activity was measured. Bars represent mean ± SD. Statistical significance was determined with the Student t test; *P < .001. (B) ELISA assay was used to measure BMP-2 values in normal (n = 9) and MM sera (n = 23). As expected, MM sera contained higher amounts of BMP-2, then normal sera. Bars represent mean ± SD. The Mann-Whitney rank-sum test was used to determine statistical significance, and *P < .001.
Discussion
Patients with MM often present with iron-restricted anemia, and overproduction of hepcidin likely contributes to the anemia.6,10 The myeloma-related stimulators of hepcidin have not been systematically characterized. Several BMPs and IL-6 have been identified as important regulators of hepcidin.8,12,21,22 We therefore considered these cytokines as possible candidates responsible for the hepcidin induction in MM patients. With our cellular reporter system, we quantified hepcidin promoter activity in response to patient sera.
We introduced mutations in the hepcidin promoter construct, which were based on previous hepcidin promoter mapping studies that identified hepcidin promoter elements involved in the response to BMPs and IL-6. Apparently, murine and human hepcidin promoters are regulated differently by IL-6. The STAT3-BS is important for human hepcidin promoter response to IL-6,12,14 but is not critical for mouse hepcidin promoter response to IL-6.15 Using the human promoter, we observed a significant, but incomplete, loss of promoter response to IL-6 when STAT3-BS was mutated. This was possibly due to our point mutation allowing partial STAT3 binding, as the introduction of a different mutation in the same STAT3-BS had more deleterious effects on IL-6–mediated induction.15 For BMP signaling, 2 BREs are involved in both the murine and human hepcidin promoter response.13,23,24 In agreement with other studies, we successfully disrupted signaling by BMPs by mutating both BREs. However, unlike in another study in which BREs in human hepcidin promoter were apparently necessary for response to IL-6,23 we observed that BREs were not involved in IL-6 responsiveness. This is consistent with observations in the murine hepcidin promoter, where it was shown that IL-6 induces murine hepcidin promoter independently from the BREs.13
Our novel observation that IL-6 and BMPs stimulate hepcidin promoter activity in a synergistic manner points to a crosstalk between the 2 signaling pathways. The BRE elements are important for the synergy while the STAT3-BS is less important. In pathologic conditions, where both types of cytokines are elevated, hepcidin might be more induced compared with conditions where only 1 type of cytokine is elevated. Two unrelated studies described a possible mechanism behind the synergy between SMAD- and STAT-mediated signaling involving the transcriptional coactivator, p300, as a bridge between SMAD and STAT on the promoter level.25,26 In the hepcidin promoter, the STAT3-BS and proximal BRE are close enough to allow bridging by p300. Even the distal BRE could be involved, if chromatin looping brought the transcription factor binding sites into close proximity. Further investigations are needed to elucidate the role of p300 or other synergistic mechanisms in hepcidin regulation. As serum levels of IL-616 and BMP-2 (this study) are elevated in patients with MM, synergistic up-regulation of hepcidin may be occurring.
Previously, we observed that serum hepcidin and urinary hepcidin are elevated in newly diagnosed MM patients with anemia, compared with healthy controls.6,10,11 The hepcidin levels correlated negatively with hemoglobin, indicating that hepcidin could be causal in the anemia of MM. In addition, our cellular reporter system also displayed higher hepcidin promoter activity after treatment with serum of MM patients, compared with serum from healthy individuals. This suggested that 1 or more hepcidin-inducing factors is present in MM patient sera.
Subsequently, we narrowed down the putative hepcidin inducers from MM sera using agonist-selective mutations of the HAMP promoter. We showed that BMPs are likely involved because of the observed abrogation of the stimulatory effect of MM sera by the mutation of BREs in the hepcidin promoter-luciferase construct. This was consistent for all patients. In addition, mutation of the STAT3-BS resulted in a blunted response in 5 patients, suggesting that IL-6–like cytokines also contribute to the observed induction. Of note, even with the mAll3 construct, the stimulation of promoter activity by MM sera was not completely abrogated, suggesting that MM sera contain factor(s) acting through other, yet unidentified elements in the HAMP promoter. Alternatively, if the point mutation in STAT3-BS was not sufficient to disrupt signaling by IL-6 or IL-6–like cytokines, the triple-mutated construct may have been subject to residual signaling driven by STAT3-BS.
Our promoter mutagenesis studies highlighted an important role of BMPs in hepcidin regulation in MM. We next tried to identify the specific BMPs using selective cytokine inhibitors. In addition, we used the same approach to clarify the role of IL-6 in MM sera. All of the cytokine inhibitors were potent and specific inhibitors of their targets. Interestingly, noggin (a BMP antagonist) and anti–BMP-2/4 antibody showed a slight neutralizing effect on IL-6–mediated promoter induction. HuH7 cells produce BMP-2 and -4 in culture,27 which could synergize with exogenous IL-6. Noggin-Fc and anti–BMP-2/4 may neutralize the effect of synergizing autocrine BMPs and thereby decrease the effect of IL-6. When neutralizing molecules were added to HuH7 cells together with MM sera, anti–BMP-2/4 or noggin-Fc blocked the induction of the hepcidin promoter by sera from all patients, indicating an important role of BMP-2–mediated hepcidin regulation in MM. Consistent with the above-mentioned results, anti–IL-6 antibodies abrogated the induction observed by the same 5 MM sera samples that showed abrogation by STAT3-BS mutation. This is in agreement with a previous study that showed that IL-6 contributes in some patients, while not in others.6 Interestingly, like in vitro, the presence of IL-6 and BMP2 together in the serum may act synergistically on hepcidin expression in vivo.
An important limitation of our reporter system is that the responsiveness of immortalized cells to different stimuli may differ from the response of hepatocytes in vivo, and we may have missed some stimulators or inhibitors using this system. Hepcidin production in vivo is controlled by several in vivo modulators, including inflammation, erythropoiesis, and iron stores, and these have not only systemic effects, but also complex local effects on hepatocytes and other liver cells. Our cellular system, however, only represented the responsiveness of HuH7 cells to stable circulating substances in MM patient sera. Of the tested cytokines, our system could detect several BMPs and IL-6, but not TGF-β1, in agreement with a previous report that HuH7 cells lack TGF-β receptor 2,28 which might explain the unresponsiveness to TGF-β.
Notably, we demonstrated that BMP-2 levels are increased in patients with MM, in comparison to control subjects. This is, to our knowledge, the first observation that a BMP is secreted into serum in MM patients to higher than normal levels and may have systemic effects. Indeed, mice treated with exogenous BMP-2 manifested increased hepcidin expression and lower serum-iron levels,29 indicating that circulating BMP-2 can affect systemic iron homeostasis. Previously, it has been demonstrated that BMPs, in particular BMP-4 and -6, are elevated in BM micromilieu.18 Moreover, BMP-6 was shown to be expressed by MM cells.17 Whether BMP-2 is expressed by tumor cells or in the BM micromilieu is unknown. BMP-2 might be produced in the BM microenvironment during bone repair, where it would induce bone and cartilage in vivo.30 Alternatively, proinflammatory cytokines, such as TNF-α, a known pathogenic factor in MM31 may induce BMP-2 expression in chondrocytes.32
In summary, many MM patients present with anemia caused or exacerbated by hepcidin induction. Our results indicate that BMP-2 is a major mediator of hepcidin-stimulatory capacity in MM sera, and this pathway may contribute to the anemia of MM. A subset of patients had additional IL-6 activity that may act synergistically with BMP-2 on hepcidin induction. Compounds directed against BMP-2 or its signaling pathway may lower hepcidin and ameliorate anemia in MM.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was funded by National Institutes of Health grant RO1 DK 065029 (T.G. and E.N.), an Interdisciplinary Seed Grant from the UCLA-Jonsson Comprehensive Cancer Center (E.N., A.L., and T.G.), and by research support from the VA, Los Angeles, CA (A.L.).
National Institutes of Health
Authorship
Contribution: K.M. executed the cloning and the experiments, analyzed the data, and wrote the manuscript; G.D.R., A.H., F.E., C.F., N.C., E.K., and L.T.-H. contributed to patient samples; S.R. and K.V. analyzed the data and revised the manuscript; and A.L., E.N., and T.G. designed the research, contributed to sera samples, analyzed the data, and revised the manuscript.
Conflict-of-interest disclosure: E.N. and T.G. are part-owners and officers of Intrinsic LifeSciences, the company that developed and performed the human serum hepcidin assay used in this study. The remaining authors declare no competing financial interests.
Correspondence: Tomas Ganz, Department of Medicine, David Geffen School of Medicine, 10833 Le Conte Ave, CHS 37-055, Los Angeles, CA 90095-1690; e-mail: tganz@mednet.ucla.edu.