Abstract
Dysregulated production of hepcidin is implicated in anemia of inflammation, whereas interleukin-6 (IL-6) is a major inducer of hepcidin production. Overproduction of IL-6 is responsible for pathogenesis of multicentric Castleman disease (MCD), a rare lymphoproliferative disorder accompanied by systemic inflammatory responses and anemia. In this study, we investigated the roles of hepcidin and IL-6 in anemia of inflammation and the long-term effects of anti–IL-6 receptor antibody (tocilizumab) treatment on serum hepcidin and iron-related parameters in MCD patients. We found that tocilizumab treatment resulted in a rapid reduction of serum hepcidin-25 in 5 of 6 MCD patients. Long-term reductions, accompanied by progressive normalization of iron-related parameters and symptom improvement, were observed in 9 of 9 cases 1.5, 3, 6, and 12 months after the start of tocilizumab treatment. In in vitro experiments, IL-6–induced up-regulation of hepcidin mRNA in hepatoma cell lines was completely inhibited by tocilizumab but increased in the presence of patients' sera. Our results suggest that, although multiple factors affect serum hepcidin levels, IL-6 plays an essential role in the induction of hepcidin in MCD. This accounts for the long-term ameliorative effect of IL-6 blockage with tocilizumab on anemia by inhibiting hepcidin production in MCD patients.
Introduction
Hepcidin is an antimicrobial peptide hormone mainly synthesized in the liver and has emerged as a key regulator of body iron homeostasis.1,2 It reduces intestinal iron absorption and blocks iron release from macrophages by down-regulating the expression of the iron exporter ferroportin.3 An increase in hepcidin synthesis is implicated in anemia of inflammation (AI), also known as anemia of chronic disease,4,5 which is commonly observed in patients with chronic infections, malignancies, trauma, and chronic inflammatory disorders.6 Hepatic hepcidin production is modulated by iron homeostasis, hypoxia, erythropoiesis, and inflammatory stimuli.7,8 Interleukin-6 (IL-6), on the other hand, is a major inducer of hepcidin expression both in vivo and in vitro, and it has been demonstrated that STAT3 is the major transcription factor responsible for the up-regulation of hepcidin via the IL-6 transduction pathway during inflammation.9-11 To date, however, most of our knowledge that IL-6–induced hepcidin causes AI has come from studies of animal and cellular models, and only a few published studies have dealt directly with the role of hepcidin in clinical settings.4,8,12
Castleman disease (CD) is a relatively rare lymphoproliferative disorder with hyperplastic lymph nodes characterized histologically by follicular hyperplasia and capillary proliferation associated with endothelial hyperplasia. CD has been classified histopathologically as either hyaline-vascular or plasma cell type. The hyaline-vascular type is generally localized, and the plasma-cell type, as well as being localized, can also be diffuse (multicentric CD [MCD]) with generalized lymphadenopathy and multiple organ involvement. Enlarged lymph nodes with massive plasma cell infiltration in interfollicular regions of hyperplastic lymph follicles, accompanied by systemic chronic inflammatory findings, are typical manifestations of plasma-cell type CD.13 Dysregulated production of IL-6 from affected lymph nodes has been identified as responsible for systemic manifestations,14 such as general fatigue, fever, anemia, polyclonal hypergammaglobulinemia, hypoalbuminemia, and an increase in acute phase proteins. Anemia is a common symptom in MCD patients, characterized by hypochromic microcytic anemia with AI features, such as low levels of serum iron, hemoglobin (Hb), and transferrin, but its mechanism has not been fully elucidated. Because IL-6 is the main cytokine contributing to the pathogenesis of MCD,14 MCD is an ideal model for studying the relationship between hepcidin and IL-6 in AI. We previously demonstrated that the blockage of IL-6 by an anti–IL-6 receptor (IL-6R) antibody significantly improved the systemic manifestations of MCD, including Hb level,15,16 which indicates that excessive IL-6 production in MCD may be associated with hypochromic microcytic anemia. Recently, we reported that serum hepcidin levels were reduced within one day in 2 cases of MCD treated with tocilizumab,17 suggesting that hepcidin production in MCD is mainly induced by IL-6. However, it remains to be confirmed whether IL-6–induced hepcidin directly causes AI and whether IL-6 blockage remains effective in long-term treatment. To answer these questions, we evaluated the long-term effects of tocilizumab treatment on serum levels of hepcidin-25 (a major form of active hepcidin peptide, which is composed of 25 residues and promotes internalization and degradation of ferroportin1,18 ) and on iron-related parameters in MCD patients. Other serum factor(s) affecting IL-6–induced hepcidin expression in AI were also investigated.
Methods
Patients and clinical laboratory examinations
Subjects in this study were recruited from patients participating in a program of tocilizumab therapy for CD. Patients were considered eligible if they met our previously reported criteria for clinical and histopathologic diagnosis of plasma-cell or mixed type MCD.15 MCD patients treated with tocilizumab were divided into short-term (6 cases) and long-term (9 cases) study courses according to the patients' preference. Samples for the short-term study course were obtained before treatment and on days 1 and 14 after the treatment, and those for the long-term course before treatment and 1.5, 3, 6, and 12 months after the initiation of the treatment. None of the patients had autoimmune diseases, such as rheumatoid arthritis or Sjogren syndrome, or malignancies. Three patients in the short-term study group (cases I, II, and III), and 4 patients in the long-term study group (cases 1, 4, 5, and 7) were treated with prednisolone. Two patients (cases III and 4) were simultaneously treated with cyclophosphamide. All were seronegative for HIV and Kaposi sarcoma-associated herpesvirus/human herpesvirus 8 detection. Case 6 in the long-term study group underwent hemodialysis during the course of tocilizumab treatment. Based on our previous dose-determination studies,16 tocilizumab was infused in principle every 2 weeks at a dose of 8 mg/kg. The serum samples were separated by centrifugation 2044g and stored at −80°C until assayed for hepcidin.
Serum hepcidin-25 was quantified with a liquid chromatography-tandem mass spectrometry-based assay system as reported previously.17,19 Serum IL-6 was determined by the chemiluminescent enzyme immunoassay using a human IL-6 chemiluminescent enzyme immunoassay Fujirebio, which detects IL-6 in both free and soluble IL-6R–bound forms.20 Other serum parameters were measured with standard laboratory techniques. This study was approved by the ethics committee of Osaka University Hospital. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Reagents and cell culture
Recombinant human IL-6 was provided by Ajinomoto, and humanized anti–IL-6R antibody (tocilizumab) was produced and provided by Chugai Pharmaceutical. Recombinant human erythropoietin (rEPO) was purchased from Sigma-Aldrich. The human hepatoma cell lines PLC/PRF/5 and Hep3B were donated by the Cell Resource Center for Biomedical Research, Tohoku University. Cell lines were grown in Dulbecco modified Eagle medium supplemented with 10% heat inactivated fetal bovine serum, 2mM glutamine, and 50 U/mL penicillin-streptomycin.
RNA preparation and real-time RT-PCR
Cells were seeded at 5 × 105 cells/well in 6-well plates and stimulated with IL-6 at various concentrations under subconfluent conditions. The effect of IL-6 blockage was investigated by preincubation for 30 minutes with tocilizumab (25 μg/mL). To examine the effect of patient sera on hepcidin mRNA induction in hepatocytes, sera from MCD patients or healthy controls were added to PLC/PRF/5 cells to a final concentration of 10%. For positive control, some wells were treated with 10 ng/mL recombinant human IL-6. To test the effect of EPO on IL-6–induced hepcidin mRNA in hepatocytes, PLC/PRF/5 cells were incubated with 0.01 to 100 U/mL rEPO for 30 minutes and then treated with IL-6. After 24 hours of IL-6 treatment, total RNA was extracted with the RNeasy Total RNA Kit (QIAGEN) according to the manufacturer's instructions. cDNA was then synthesized with Moloney murine leukemia virus reverse transcriptase (Promega) in 25-μL reactions containing 2 μg of total RNA. Real-time quantitative reverse-transcribed polymerase chain reaction (RT-PCR) was performed with the GeneAmp 7000 Sequence Detection System and SYBR Green dye (Applied Biosystems) according to the manufacturer's protocol. The following PCR primer sequences were used for amplifying human hepcidin cDNA12 : forward primer, 5′-CCTGACCAGTGGCTCTGTTT-3′; and reverse primer, 5′CACATCCCACACTTTGATCG-3′. The PCR was performed for a final volume of 50 μL composing 2 × 25 μL SYBR Green PCR Master Mix (Applied Biosystems). Thermal cycle parameters were 2 minutes at 50°C and 10 minutes at 95°C, followed by 40 cycles of amplification with 15 seconds at 95°C for denaturation, 1 minute at 60°C for annealing and elongation. β2-Microglobulin was used as an internal control for correction of hepcidin expression. Experiments were performed in triplicate, and each was repeated 3 times with consistent results.
Statistical analysis
Laboratory data were expressed as mean plus or minus SD of tested samples. Statistical analysis used the paired t test. The overall effect of treatment was evaluated with the paired t test on the basis of changes from baseline level observed at different intervals after tocilizumab administration. Group comparisons were performed with the Pearson correlation test. The values of serum hepcidin-25 and iron-related parameters were log-transformed to normalize the distribution of variables before analysis. P values less than .05 were considered significant.
Results
Effects of tocilizumab on IL-6–induced hepcidin expression in hepatic cells
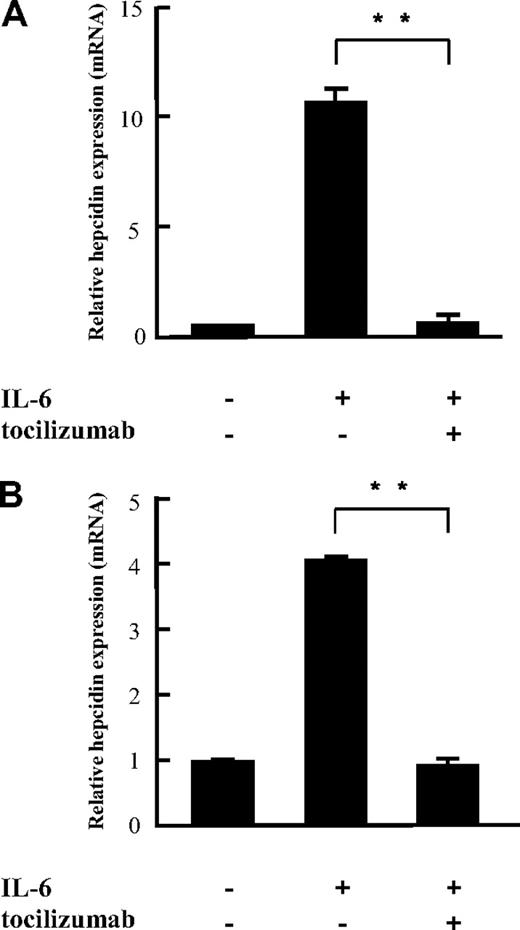
Because IL-6 has been shown to promote hepcidin expression in hepatocytes at transcription level, we investigated the effect of tocilizumab on hepcidin expression in vitro by quantifying hepcidin transcripts with real-time PCR in PLC/PRF/5 and Hep3B cells. The 2 cell lines showed similar expression patterns on IL-6 stimulation (0.1-10 ng/mL), that is, hepcidin expression increased in a dose-dependent manner in both (see Figure 5). Although 100 ng/mL IL-6 was found to produce maximal activation of hepcidin, suboptimal doses of IL-6 (10 ng/mL) were used for this study. IL-6 up-regulated hepcidin expression 10.3- and 4.2-fold in PLC/PRF/5 and Hep3B cells, respectively, compared with untreated control cells (Figure 1). IL-6–induced hepcidin expression was completely blocked by tocilizumab, indicating that IL-6 acts directly on hepcidin expression in hepatocytes and that tocilizumab is an effective inhibitor of hepcidin induction by IL-6.
Inhibitory effects of tocilizumab on IL-6–induced hepcidin mRNA expression in vitro. Tocilizumab (25 μg/mL) was incubated with PLC/PRF/5 (A) and Hep3B (B) hepatocyte cell lines for 30 minutes before IL-6 (10 ng/mL) stimulation. Hepcidin mRNA was assayed with quantitative real-time PCR 24 hours after IL-6 stimulation. The figures show mean ± SD of triplicate measurements. The same experiment was repeated at least 3 times with consistent results, and representative data are shown. **P < .01 by paired t test.
Inhibitory effects of tocilizumab on IL-6–induced hepcidin mRNA expression in vitro. Tocilizumab (25 μg/mL) was incubated with PLC/PRF/5 (A) and Hep3B (B) hepatocyte cell lines for 30 minutes before IL-6 (10 ng/mL) stimulation. Hepcidin mRNA was assayed with quantitative real-time PCR 24 hours after IL-6 stimulation. The figures show mean ± SD of triplicate measurements. The same experiment was repeated at least 3 times with consistent results, and representative data are shown. **P < .01 by paired t test.
Short-term effect of tocilizumab treatment on serum level of hepcidin-25 and iron-related parameters
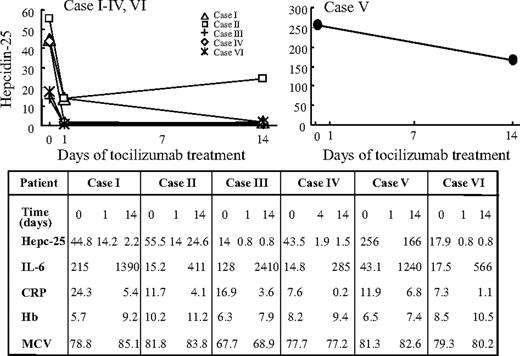
Consistent with our previously reported findings17 and those of the in vitro experiment shown in Figure 1, short-term administration of tocilizumab resulted in down-regulation of the serum hepcidin level in 6 of 6 MCD cases (Figure 2). In all these cases except case V, rapid reduction of serum hepcidin-25 was observed after the initial dose of tocilizumab, followed by a decrease in C-reactive protein (CRP) and improvement of anemia. In case V, the initial hepcidin level was extremely high (256 ng/mL) and decreased only slightly after tocilizumab administration, whereas the serum hepcidin level on day 35 remained quite high (90.5 ng/mL) even after 3 doses of tocilizumab. On the same day, an antibody against tocilizumab was detected in the serum, so that this medication was discontinued. In the other 5 cases, serum hepcidin levels dropped below the upper normal limit within 14 days. Increased blood IL-6 was observed in all patients after the start of tocilizumab treatment (Figure 2). This result was consistent with a previously reported finding20 that, as long as IL-6 signaling was completely inhibited, free serum IL-6 increased because IL-6R–mediated consumption of IL-6 was inhibited by the unavailability of tocilizumab-free IL-6R. Therefore, the increase in the free IL-6 level during tocilizumab treatment closely reflects the actual endogenous IL-6 production and an effective blocking of tocilizumab. A slight but insignificant increase in mean corpuscular volume (MCV) levels (Figure 2) was seen in all cases except case IV. To verify the effect of tocilizumab on hypochromic microcytic anemia in MCD, a long-term observation was carried out next.
Rapid decrease of serum hepcidin-25 in 6 MCD patients treated with tocilizumab during 14-day short-term course. Administration of tocilizumab resulted in down-regulation of the serum hepcidin level in 6 of 6 MCD cases. (Left panel) In all cases except case V, rapid reduction of serum hepcidin-25 was observed on days 1 or 4 after the initial dose of tocilizumab, followed by reductions in CRP and improvement in Hb levels. (Table) Hb, the serum values of hepcidin-25, CRP, IL-6, and MCV in MCD patients before and after 14 days of therapy with tocilizumab. (Right panel) In case V, the initial hepcidin level was extremely high (256 ng/mL), and it decreased only slightly after tocilizumab administration.
Rapid decrease of serum hepcidin-25 in 6 MCD patients treated with tocilizumab during 14-day short-term course. Administration of tocilizumab resulted in down-regulation of the serum hepcidin level in 6 of 6 MCD cases. (Left panel) In all cases except case V, rapid reduction of serum hepcidin-25 was observed on days 1 or 4 after the initial dose of tocilizumab, followed by reductions in CRP and improvement in Hb levels. (Table) Hb, the serum values of hepcidin-25, CRP, IL-6, and MCV in MCD patients before and after 14 days of therapy with tocilizumab. (Right panel) In case V, the initial hepcidin level was extremely high (256 ng/mL), and it decreased only slightly after tocilizumab administration.
Long-term effect of tocilizumab treatment on serum level of hepcidin-25
Long-term responses and outcomes after tocilizumab administration were investigated in 9 MCD subjects, including 2 cases (cases I and II) in the short-term course. Basal clinical characteristics of these patients before tocilizumab administration are presented in Table 1.
Basal clinical characteristics of the patients with Castleman disease
| Case no. . | Sex . | Age, y . | Hemoglobin, g/dL . | CRP, mg/dL . | RBCs, ×106/μL . | MCV, fL . | MCH, pg . | Serum Fe, μg/dL . | Serum ferritin, ng/mL . | EPO, mU/mL . | Hepc, ng/mL . | IL-6, pg/mL . | Albumin, g/dL . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 56 | 11.7 | 4.9 | 4.1 | 88 | 28 | 37 | 76 | 32.9 | 33.8 | 16.7 | 3.1 |
| 2 | Male | 60 | 9.9 | 5.4 | 4.1 | 77 | 24 | 16 | 42 | 19.8 | 37.2 | 435.8 | 2.9 |
| 3 | Female | 39 | 8.4 | 13.6 | 3.6 | 80 | 26 | 13 | 321 | 19.6 | 95.0 | 94.9 | 1.9 |
| 4 | Female | 33 | 9.8 | 5.7 | 4.0 | 77 | 24 | 18 | 80 | 44.0 | 74.5 | 30.8 | 3.2 |
| 5 | Female | 24 | 4.8 | 22.7 | 2.8 | 67 | 17 | 13 | 173 | 67.0 | 49.2 | 215.0 | 1.6 |
| 6 | Male | 73 | 12.6 | 0.8 | 4.3 | 87 | 28 | 54 | 61 | 11.7 | 89.0 | 8.7 | 3.2 |
| 7 | Female | 32 | 10.3 | 14.6 | 3.9 | 82 | 26 | 32 | 399 | 8.8 | 55.5 | 15.2 | 2.6 |
| 8 | Male | 52 | 11.9 | 1.3 | 4.3 | 83 | 27 | 50 | 24 | 18.0 | 28.6 | 26.7 | 2.6 |
| 9 | Female | 37 | 7.2 | 6.4 | 3.7 | 64 | 19 | 13 | 21 | 47.6 | 8.0 | 318.4 | 2.4 |
| Case no. . | Sex . | Age, y . | Hemoglobin, g/dL . | CRP, mg/dL . | RBCs, ×106/μL . | MCV, fL . | MCH, pg . | Serum Fe, μg/dL . | Serum ferritin, ng/mL . | EPO, mU/mL . | Hepc, ng/mL . | IL-6, pg/mL . | Albumin, g/dL . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 56 | 11.7 | 4.9 | 4.1 | 88 | 28 | 37 | 76 | 32.9 | 33.8 | 16.7 | 3.1 |
| 2 | Male | 60 | 9.9 | 5.4 | 4.1 | 77 | 24 | 16 | 42 | 19.8 | 37.2 | 435.8 | 2.9 |
| 3 | Female | 39 | 8.4 | 13.6 | 3.6 | 80 | 26 | 13 | 321 | 19.6 | 95.0 | 94.9 | 1.9 |
| 4 | Female | 33 | 9.8 | 5.7 | 4.0 | 77 | 24 | 18 | 80 | 44.0 | 74.5 | 30.8 | 3.2 |
| 5 | Female | 24 | 4.8 | 22.7 | 2.8 | 67 | 17 | 13 | 173 | 67.0 | 49.2 | 215.0 | 1.6 |
| 6 | Male | 73 | 12.6 | 0.8 | 4.3 | 87 | 28 | 54 | 61 | 11.7 | 89.0 | 8.7 | 3.2 |
| 7 | Female | 32 | 10.3 | 14.6 | 3.9 | 82 | 26 | 32 | 399 | 8.8 | 55.5 | 15.2 | 2.6 |
| 8 | Male | 52 | 11.9 | 1.3 | 4.3 | 83 | 27 | 50 | 24 | 18.0 | 28.6 | 26.7 | 2.6 |
| 9 | Female | 37 | 7.2 | 6.4 | 3.7 | 64 | 19 | 13 | 21 | 47.6 | 8.0 | 318.4 | 2.4 |
Normal values are as follows: hemoglobin, 12.0-17.0 /dL; CRP, < 0.2 mg/dL; RBCs, 3.9-5.1 × 106/μL; MCV, 84-98 fL; MCH, 27-32 pg; serum Fe, 41-127 μg/dL; serum ferritin, 2-82 ng/mL; EPO, 6-25 mU/mL; Hepc, IL-6, < 10 pg/mL; and albumin 3.6-4.7 g/dL.
MCH indicates mean corpuscular hemoglobin; and Hepc, serum hepcidin-25.
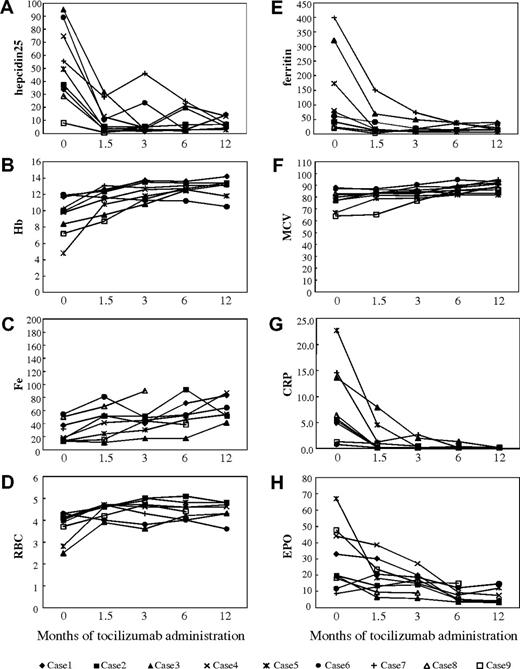
As also seen in the short-term course, the initial serum level of hepcidin-25 tended to be elevated in MCD patients before tocilizumab administration (Figure 3A) with an average of 52 ng/mL (normal range, 22 ± 12 ng/mL) and varying over a wide range (9-95 ng/mL). Long-term administration of tocilizumab resulted in a continuing decrease and eventual normalization of serum hepcidin-25 levels after 6 and 12 months for all patients (Figure 3A). The mean levels of hepcidin-25 were 10.9 at 1.5, 10.5 at 3, 10.2 at 6, and 7.7 ng/mL at 12 months after the initiation of tocilizumab treatment (P < .01; paired t test vs baseline). This long-term decrease in serum hepcidin was followed by a progressive decline in CRP and gradual improvement of Hb levels (Figure 3B,G), indicating that the effect observed during the short-term course persisted during long-term tocilizumab administration. Recurrence of hepcidin-25 level elevation was observed in case 7 at 3 months after the initiation of the treatment (Figure 3A) in conjunction with a slightly elevated CRP level (2.7 mg/dL; Figure 3G), suggesting that IL-6 was insufficiently blocked. After increasing the frequency of tocilizumab administration from every 2 weeks to once a week, both serum hepcidin-25 and CRP levels decreased to normal range after 6 and 12 months (Figure 3A,G). Case 6 was a patient undergoing hemodialysis, whose serum hepcidin was somewhat high after 3 months but decreased to normal levels 6 and 12 months after the initiation of tocilizumab therapy. Only case 9 showed a normal level of serum hepcidin and a very low level of Hb (7.2 g/dL) at baseline. Long-term administration of tocilizumab resulted in a further decrease in serum hepcidin-25 and normalization of Hb level.
Suppression of hepcidin production accompanied by improvement in inflammatory anemia as well as disease activity in 9 MCD patients treated with anti–IL-6R antibody. Long-term effects were assessed at 1.5, 3, 6, and 12 months after the start of tocilizumab therapy. Serum hepcidin-25 was quantified with a liquid chromatography-tandem mass spectrometry-based assay system. Other serum parameters were measured with standard laboratory techniques. (A) Serum level of hepcidin-25. (B) Hb. (C) Serum iron. (D) RBCs. (E) Ferritin. (F) MCV. (G) CRP. (H) EPO.
Suppression of hepcidin production accompanied by improvement in inflammatory anemia as well as disease activity in 9 MCD patients treated with anti–IL-6R antibody. Long-term effects were assessed at 1.5, 3, 6, and 12 months after the start of tocilizumab therapy. Serum hepcidin-25 was quantified with a liquid chromatography-tandem mass spectrometry-based assay system. Other serum parameters were measured with standard laboratory techniques. (A) Serum level of hepcidin-25. (B) Hb. (C) Serum iron. (D) RBCs. (E) Ferritin. (F) MCV. (G) CRP. (H) EPO.
Long-term effect of tocilizumab treatment on anemia status and disease activity
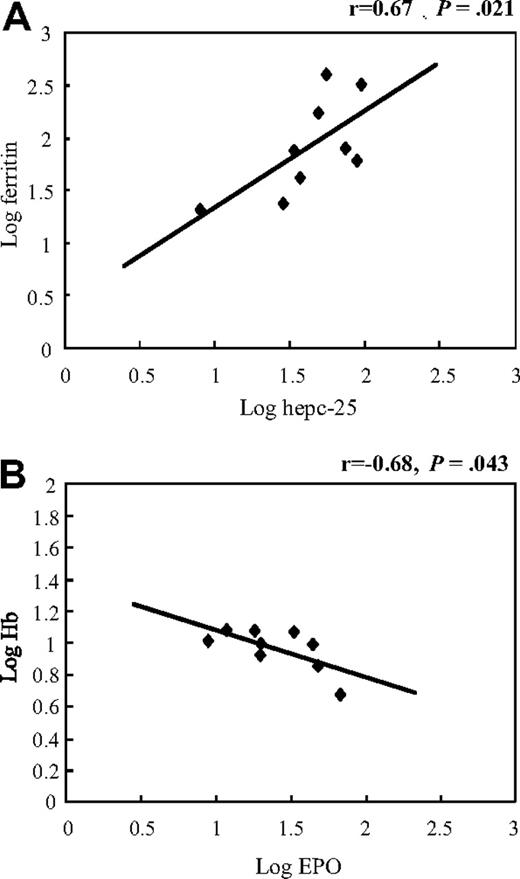
In addition to Hb, other iron metabolism-related parameters, such as serum iron (Fe), ferritin, red blood cell count (RBC), and MCV, were assayed before and after tocilizumab treatment (Figure 3B-F). The mean values before treatment were 9.5 g/dL for Hb level, 27 μg/dL for Fe, 133 ng/mL for ferritin, 3.7 × 1012/L for RBC, and 78.4 fL for MCV. Tocilizumab markedly improved anemia status of these patients because mean Hb, Fe, RBC, and MCV values increased to 11.4 g/dL, 43 μg/dL, 4.3 × 1012/L, and 81.8 fL, respectively, and ferritin decreased to 40 ng/mL at 1.5 months after initiation of treatment (P < .05; paired t test vs baseline; Figure 3B-F), and this effect continued until 12 months, with mean Hb, Fe, RBC, ferritin, and MCV values of 12.8 g/dL, 62 μg/dL, 6.2 × 1012/L, 21 ng/mL, and 88.8 fL, respectively, at the end of the study period (P < .05; Figure 3B-F). Correlation analysis disclosed that the serum hepcidin-25 level correlated significantly and positively with ferritin (r = 0.67, P = .021, Figure 4A), but not with the other parameters, such as Hb (r = 0.18, P = .63), serum iron (r = 0.17, P = .65), and EPO (r = −0.38, P = .32) before tocilizumab administration. Long-term tocilizumab treatment resulted in a progressive decrease in serum ferritin 1.5, 3, 6, and 12 months after tocilizumab with mean levels of 40, 24, 22, and 21 ng/mL, respectively.
Association between hepcidin-25 and ferritin, and between EPO and Hb in serum of MCD patients. Pearson correlation analysis of baseline values showed (A) a positive correlation between hepcidin-25 and ferritin and (B) an inverse association between EPO and Hb.
Association between hepcidin-25 and ferritin, and between EPO and Hb in serum of MCD patients. Pearson correlation analysis of baseline values showed (A) a positive correlation between hepcidin-25 and ferritin and (B) an inverse association between EPO and Hb.
Correlation analysis showed an inverse relationship between baseline levels of EPO and Hb in MCD patients (r = −0.68, P = .043, Figure 4B). Long-term tocilizumab treatment resulted in a progressive decrease in mean serum EPO from 29 mU/mL at baseline to 20 mU/mL at 1.5, 16 at 3, 7.8 at 6, and 7.1 at 12 months after the initiation of tocilizumab administration.
Long-term administration of tocilizumab also resulted in systemic improvement in disease symptoms, such as diminished fatigue, alleviation of lymphadenopathy and fever, managed chronic inflammation, and increased serum albumin and body weight in all patients.
Effect of patient sera and human EPO on IL-6–induced hepcidin expression
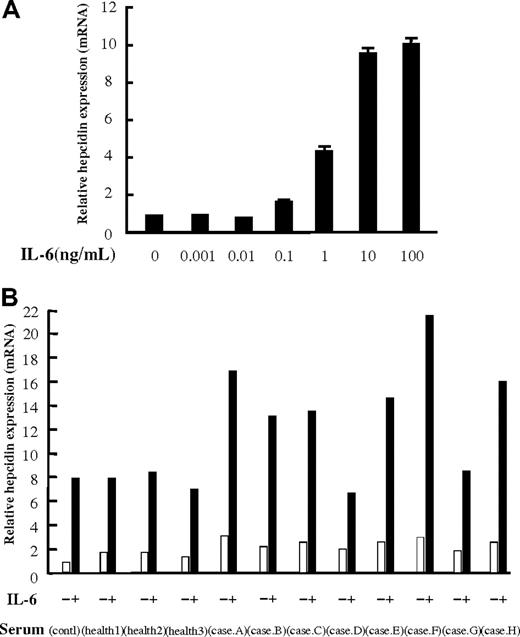
To clarify whether MCD patient sera contain humoral factors other than IL-6, which affect hepcidin expression, we performed the following experiments. First, to identify the minimal concentration of IL-6, which can induce hepcidin expression in vitro, we treated human hepatoma PLC/PRF/5 cells with various concentrations of IL-6. IL-6 at 0.1 to 10 ng/mL, but not at 0.001 to 0.01 ng/mL, increased hepcidin expression in a dose-dependent manner (Figure 5A). Some of our MCD cases showed initial IL-6 levels higher than 0.1 ng/mL (Table 1; Figure 2; cases 2, 5, 9, I, and III). Next we tested the effect of patients' sera on hepcidin expression in PLC/PRF/5 cells. The cells were treated with 10% sera from 8 MCD patients before the start of tocilizumab administration or from 3 healthy controls. As expected, the 3 healthy control sera had no effect on IL-6–induced hepcidin expression compared with that of the sera with the addition of 10% fetal calf serum (Figure 5B). Sera from 6 of these patients (cases A, B, C, E, F, and H) plus IL-6 (10 ng/mL) enhanced the induction of hepcidin expression (13- to 17-fold), compared with the effect of IL-6 alone (8-fold; Figure 5B), whereas sera from patients D and G plus IL-6 had no such effect, showing only a 6- or 8-fold enhancement of hepcidin induction.
Effect of patient sera on IL-6–induced hepcidin expression in PLC/PRF/5 cells. (A) Dose required for response of hepcidin mRNA expression to IL-6 was determined in PLC/PRF/5 cells grown in medium containing IL-6 at final concentrations of 0.001, 0.01, 0.1, 1, 10, and 100 ng/mL. IL-6 at 0.1 to 10 ng/mL, but not at 0.001 to 0.01 ng/mL, increases the expression of hepcidin in a dose-dependent manner. (B) Hepcidin mRNA expression in PLC/PRF/5 cells treated with culture medium containing 10% of either fetal calf serum, serum from healthy volunteers (n = 3), or serum from MCD patients (n = 8). Cells stimulated with IL-6 (10 ng/mL) showed an approximately 8-fold increase in hepcidin mRNA expression compared with nonstimulated controls. Serum from patients A, B, C, E, F, and H enhanced IL-6–induced hepcidin expression (13- to 22-fold), whereas serum from patients D and G and serum from 3 healthy volunteers had no such effect on IL-6–induced hepcidin expression (6- to 8-fold increase).
Effect of patient sera on IL-6–induced hepcidin expression in PLC/PRF/5 cells. (A) Dose required for response of hepcidin mRNA expression to IL-6 was determined in PLC/PRF/5 cells grown in medium containing IL-6 at final concentrations of 0.001, 0.01, 0.1, 1, 10, and 100 ng/mL. IL-6 at 0.1 to 10 ng/mL, but not at 0.001 to 0.01 ng/mL, increases the expression of hepcidin in a dose-dependent manner. (B) Hepcidin mRNA expression in PLC/PRF/5 cells treated with culture medium containing 10% of either fetal calf serum, serum from healthy volunteers (n = 3), or serum from MCD patients (n = 8). Cells stimulated with IL-6 (10 ng/mL) showed an approximately 8-fold increase in hepcidin mRNA expression compared with nonstimulated controls. Serum from patients A, B, C, E, F, and H enhanced IL-6–induced hepcidin expression (13- to 22-fold), whereas serum from patients D and G and serum from 3 healthy volunteers had no such effect on IL-6–induced hepcidin expression (6- to 8-fold increase).
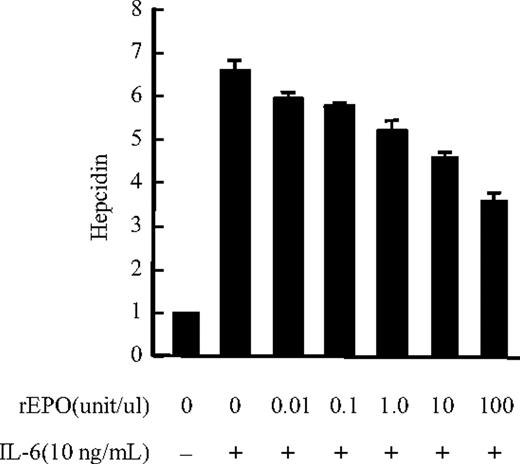
To test whether hepcidin production is affected by erythropoietic activity in AI patients, the effect of rEPO on IL-6–induced hepcidin expression was analyzed in human hepatoma PLC/PRF/5 cells, and we found that IL-6–induced hepcidin mRNA expression was down-regulated by rEPO in a dose-dependent manner (from 0.1 to 100 U/mL) in these cells (Figure 6), whereas 100 U/mL rEPO led to maximal inhibition (50% reduction) of IL-6–induced hepcidin expression.
Effect of human EPO on IL-6–induced hepcidin expression in PLC/PRF/5 cells. IL-6–induced hepcidin mRNA expression in PLC/PRF/5 cells was suppressed by rEPO. An approximately 6-fold increase in hepcidin mRNA expression was observed in cells stimulated with IL-6 (10 ng/mL) compared with nonstimulated controls. rEPO suppressed IL-6–induced hepcidin expression in a dose-dependent manner (0.1-100 U/mL), whereas 100 U/mL rEPO led to maximal inhibition of IL-6–induced hepcidin mRNA expression (50% reduction).
Effect of human EPO on IL-6–induced hepcidin expression in PLC/PRF/5 cells. IL-6–induced hepcidin mRNA expression in PLC/PRF/5 cells was suppressed by rEPO. An approximately 6-fold increase in hepcidin mRNA expression was observed in cells stimulated with IL-6 (10 ng/mL) compared with nonstimulated controls. rEPO suppressed IL-6–induced hepcidin expression in a dose-dependent manner (0.1-100 U/mL), whereas 100 U/mL rEPO led to maximal inhibition of IL-6–induced hepcidin mRNA expression (50% reduction).
Discussion
Anemia in association with MCD is considered a prototype of AI, and its pathogenesis appears to be multifactorial. We previously reported a reduction of serum hepcidin-25 in 2 cases of MCD within a single day after treatment with tocilizumab, indicating that IL-6 may play an important role in hepcidin production in MCD.17 However, this study covered only 2 cases over a very short time period, so that any direct association between hepcidin and anemia in MCD patients remained to be demonstrated. In the current study, on the other hand, we included more cases with a much longer observation period of up to 12 months. Consistent with in vitro results (Figure 1) and those previously reported,17 administration of tocilizumab resulted in a rapid decline in hepcidin levels in 6 of 6 MCD cases during a short-term treatment course (Figure 2). Although serum hepcidin-25 rapidly decreased to normal levels in most subjects (except for case V) within one day, CRP, Hb, and MCV in most cases did not attain normalization on day 14 after tocilizumab administration (Figure 2). Long-term treatment was therefore considered necessary for further evaluation of the effect of tocilizumab on AI in MCD patients.
We found that the reduction in serum hepcidin-25 levels was maintained during the long-term course of tocilizumab administration (Figure 3A), which further confirmed that IL-6 is involved in hepcidin production in AI. In addition, tocilizumab therapy not only resulted in reduced hepcidin levels but also progressively improved or normalized iron-related parameters, such as Hb, serum Fe, RBC, ferritin, and MCV levels (Figure 3B-F). The suppression of hepcidin production as a result of tocilizumab treatment, accompanied by improvement of iron metabolism parameters, indicates that excessive hepcidin production induced by elevated IL-6 contributed to AI in the MCD patients. Because the hepcidin-25 levels at baseline did not correlate with prednisolone use and decreased significantly in all patients after tocilizumab treatment, we concluded that hepcidin-25 levels in our patients were not affected by corticosteroids or immunosuppressants.
In case V enrolled in the short-term course, the initial hepcidin level was extremely high (256 ng/mL) and remained considerably elevated (90.5 ng/mL) on day 35 even after 3 doses of tocilizumab. As an antibody against tocilizumab was detected in this patient, the medication was discontinued because the antibody reduced the IL-6 inhibition activity of tocilizumab. This finding further supports the direct involvement of IL-6 in hepcidin production, so that monitoring of serum hepcidin may be useful for predicting the efficacy of treatment for MCD cases, although further evaluation of this concept is necessary. Because IL-6 is a major CRP inducer both in vitro and in vivo,21 the CRP level in serum has been used for monitoring the efficacy of tocilizumab treatment.15,16 In the study presented here, 8 of 9 patients enrolled in the long-term course showed normal CRP levels (< 0.2 mg/dL) after 3 months of treatment, whereas only case 7 displayed a slightly elevated level at that time (Figure 3G), which suggests that IL-6 blockage was insufficient. It is worth noting that an elevated serum hepcidin-25 level was also observed after 1.5 and 3 months of treatment in the same patient (Figure 3A), whereas serum ferritin also showed incomplete reduction (Figure 3E). By increasing the tocilizumab dose to 8 mg/kg weekly, serum hepcidin, ferritin, and CRP of case 7 improved to normal range after 6 and 12 months (Figure 3A,E,G). These results make it clear that IL-6 is responsible for hepcidin production in MCD patients with anemia and provide further evidence that serum hepcidin level can be used as an indicator of the efficacy of tocilizumab therapy.
Serum ferritin levels in our MCD cases before tocilizumab administration showed a significant correlation with serum hepcidin-25 levels (Figure 4A). Initiation of tocilizumab treatment resulted in down-regulation of serum ferritin accompanied by a decrease in serum hepcidin levels (Figure 3E). Ferritin is a cellular storage protein for iron, and an elevated ferritin level generally reflects excessive iron storage as commonly observed in AI. It can thus be speculated that excessive hepcidin leads to an increase in iron storage and diminishes the amount of serum iron available for Hb synthesis and erythrocyte production, which leads in turn to hypochromic microcytic anemia in MCD. Our results are consistent with those of previously published studies8,22 and confirm that hepcidin shows a strongly positive correlation with serum ferritin concentration.
The initial serum levels of hepcidin-25 before tocilizumab administration were generally elevated but varied widely (9-250 ng/mL; Figures 2, 3A), whereas serum IL-6 levels were markedly elevated in most of our MCD cases before tocilizumab administration (Table 1). Moreover, serum hepcidin-25 correlated well with ferritin (Figure 4A) but not with IL-6, so that serum hepcidin-25 levels did not seem to be controlled by the level of serum IL-6 alone. We therefore hypothesized that other serum factors, besides IL-6, have an effect on serum hepcidin-25 levels. Anemia has been proved to be a negative regulator for hepcidin production.7 Our analysis indicated that cases I and III (short-term treatment) and case 9 (long-term treatment) have severe anemia with respective Hb levels of 5.7, 6.3, and 7.2 g/dL in conjunction with low levels of serum hepcidin-25 before tocilizumab administration. These findings suggest that very severe anemia probably counteracts the effect of IL-6 on hepcidin production. In addition, recently published studies have indicated that EPO negatively regulates hepcidin either in vivo or in vitro through indirect action and that this action requires an increase in erythropoiesis and possibly release of some mediators by bone marrow.23-25 The tendency of serum EPO and Hb to show an inverse relationship (Figure 4B), as observed in our MCD patients, supports this viewpoint. Our in vitro study demonstrated that human EPO inhibits IL-6–induced hepcidin mRNA expression in hepatocytes (Figure 6), indicating that IL-6–induced hepcidin production may be directly down-regulated by EPO. This result also explains why hepcidin-25 showed no significant correlation with serum IL-6 levels.
In addition to EPO, growth differentiation factor-15 and twisted gastrulation-1 also have been reported to mediate hepcidin suppression in response to augmented hematopoietic activity.26-29 The most recent evidence suggests that SMAD7 and TMPRSS6 (a protease that cleaves hemojuvelin) suppress hepcidin expression in response to iron-controlled regulation of hepcidin.30,31 It is thus quite possible that other negative regulators down-regulated the serum hepcidin level in our patients.
Recently, several investigators have made a case for the existence of a serum-associated factor other than growth differentiation factor-15 that regulates hepcidin synthesis, mainly based on findings from animal experiments and in vitro experiments using patient sera.32,33 To investigate whether such putative factors affecting IL-6–induced hepcidin production are present in serum, we examined the effect on hepcidin expression of the addition of our patients' sera to PLC/PRF/5 cells. Interestingly, the addition of 10% serum from cases A, B, C, E, F, and H enhanced IL-6–induced hepcidin expression, whereas serum from cases D and G did not (Figure 5). As IL-6 concentrations in our MCD patient sera varied widely from 10 to 400 pg/mL and the minimum concentration of recombinant IL-6 required for inducing hepcidin expression in PLC/PRF/5 was 100 pg/mL, it seems unlikely that hepcidin expression could be induced by 10% patient serum. We therefore suggest that enhancing factor(s) were present in our MCD patients' sera, which were involved in IL-6–induced hepcidin expression by acting on the IL-6 signal pathway.
Bone morphogenetic proteins (BMPs) are a group of cytokines of the transforming growth factor-β family. In addition to proinflammatory cytokines, the BMP-SMAD pathway is another major signaling pathway to activate hepcidin expression.34 BMP6 is critical for the activation of this signaling cascade,35,36 whereas hemojuvelin is a coreceptor for BMP signaling and as critical as BMP6 for hepcidin expression.37 To the best of our knowledge, however, there have been no reports about involvement of transforming growth factor-β in CD, and further investigation is needed to determine whether the BMP pathway contributes to IL-6–induced hepcidin expression in MCD patients.
In conclusion, these findings and speculations suggest that, in addition to IL-6, other factors, such as anemic status, erythropoietic activity, or as yet unknown factors in serum, could influence hepcidin production by either cooperating with or counteracting IL-6. The proposed model describing this mechanism is shown in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This seems to imply that the mechanisms for serum hepcidin level regulation may be complicated. Nevertheless, although other factors may be involved in hepcidin production, it is clear that IL-6 plays an essential role in hepcidin regulation, so that IL-6 blockage may well constitute a promising molecular targeting therapy for AI. Our clinical results with IL-6 blockage therapy suggest that the pathogenic cascade causing anemia associated with MCD started from hepcidin induction by IL-6, which then leads to anemia.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr M. Nishino and Dr M. Mizutani (Matsusaka Hospital), Dr H. Fujihara (Osaka City Juso Hospital), Dr F. Matsuyama (Kurashiki Central Hospital), and Dr M. Hino (Osaka City University Hospital) for providing outstanding treatment and helpful advice, and Ms K. Umetani for secretarial assistance.
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Osaka Foundation for Promotion of Clinical Immunology, and the Amyloidosis Research Committee for the research on Intractable Disease from the Ministry of Health, Labor and Welfare, Japan.
National Institutes of Health
Authorship
Contribution: S.-N.J.S. designed and performed the research, analyzed the data, and wrote the paper; N.T., H.K., T.I., and T.N. performed the research and analyzed the data; and K.Y. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kazuyuki Yoshizaki, Center for Advanced Science and Innovation, Osaka University, 2-1 Yamada-Oka, Suita, Osaka 565-0871, Japan; e-mail: kyoshi@casi.osaka-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal