Abstract
The clinical impact of FLT3-internal tandem duplications (ITDs), an adverse prognostic marker in adults aged < 60 years with primary cytogenetically normal acute myeloid leukemia (CN-AML), requires further investigation in older patients. In CN-AML patients aged ≥ 60 years treated on Cancer and Leukemia Group B frontline trials, we found that FLT3-ITD remained associ-ated with shorter disease-free survival (P < .001; hazard ratio = 2.10) and overall survival (P < .001; hazard ratio = 1.97) in multivariable analyses. This impact on shorter disease-free survival and overall survival was in patients aged 60-69 (P < .001, each) rather than in those aged ≥ 70 years. An FLT3-ITD–associated gene-expression signature revealed overexpression of FLT3, homeobox genes (MEIS1, PBX3, HOXB3), and immunotherapeutic tar-gets (WT1, CD33) and underexpression of leukemia-associated (MLLT3, TAL1) and erythropoiesis-associated (GATA3, EPOR, ANK1, HEMGN) genes. An FLT3-ITD–associated microRNA-expression signature included overexpressed miR-155 and underexpressed miR-144 and miR-451. FLT3-ITD identifies older CN-AML patients with molecular high risk and is associated with gene- and microRNA-expression signatures that provide biologic insights for novel therapeutic approaches.
Introduction
Approximately two-thirds of patients with acute myeloid leukemia (AML) are aged ≥ 60 years.1 Despite advances in therapy, their prognosis remains poor. FLT3-internal tandem duplications (ITDs) are a well documented adverse prognostic marker in younger adults (< 60 years) with primary cytogenetically normal AML (CN-AML).2 FLT3-ITD prognostic impact requires further investigation in similar older patients. Therefore, we analyzed the frequency and prognostic significance of FLT3-ITD in patients aged ≥ 60 years with primary CN-AML intensively treated on Cancer and Leukemia Group B (CALGB) frontline protocols. To gain biologic insights associated with FLT3-ITD, we also derived global gene- and microRNA-expression signatures.
Methods
We studied pretreatment marrow (n = 205) or blood (n = 38) samples having ≥ 20% blasts from 243 CN-AML patients treated on CALGB frontline protocols, which included induction with cytarabine and ≥ 1 course of cytarabine during consolidation. In addition, some patients received investigational drugs (ie, Valspodar and low-dose interleukin-2 or Genasense [for details, see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article]). All patients provided Ohio State University Institutional Review Board–approved informed consent for participation in the studies in accordance with the Declaration of Helsinki.
FLT3-ITD, FLT3-TKD, MLL–Partial Tandem Duplication, NPM1, CEBPA, WT1, IDH1, and IDH2 mutations and FLT3-ITD allelic ratio (AR) were analyzed as previously described.3-7 Genome-wide gene- and microRNA-expression profiles were generated using, respectively, Affymetrix U133 plus 2.0 (Affymetrix)4,6,8 and the OSUCCC microRNA (version 4.0) arrays.6 All original microarray data are available from ArrayExpress under accession numbers E-TABM-1032 and E-TABM-1029.
Clinical end point definitions and statistical and microarray data analyses are detailed in the supplemental Methods.
Results and discussion
Clinical and molecular associations and prognostic impact of FLT3-ITD
Of the 243 patients, 72 (30%) had FLT3-ITD, 18 (7%) had FLT3-TKD, and 6 (2%) had both. With the prognostic significance of FLT3-TKD in younger patients currently uncertain, for subsequent analyses we compared patients harboring FLT3-ITD alone with those with FLT3 wild-type (FLT3-WT). FLT3-ITD patients had higher white blood cell counts (P < .001) and percentages of blood (P < .001) and marrow (P < .001) blasts, more often extramedullary involvement (P = .04), higher percentages of NPM1 (P < .001) and WT1 (P = .001) mutations, and lower percentages of IDH2 mutations (P = .02; Table 1). These FLT3 mutation percentages and associated pretreatment characteristics are similar to those reported in younger CN-AML.9,10
Comparisons of clinical characteristics, molecular features, and outcomes of older primary CN-AML patients with and without FLT3-ITD
| Characteristic . | FLT3-ITD (n = 72) . | FLT3-WT (n = 147) . | P . |
|---|---|---|---|
| Age, y | .92 | ||
| Median | 68 | 69 | |
| Range | 60-81 | 60-83 | |
| Age group, n (%) | .67 | ||
| 60-69 y | 41 (57) | 78 (53) | |
| ≥ 70 y | 31 (43) | 69 (47) | |
| Sex, no. of males (%) | 36 (50) | 75 (51) | 1.00 |
| Race, n (%) | .81 | ||
| White | 66 (92) | 129 (90) | |
| Nonwhite | 6 (8) | 14 (10) | |
| Protocol,* n (%) | |||
| 8525 | 11 (15) | 10 (7) | |
| 8923 | 9 (13) | 9 (6) | |
| 9420 | 3 (4) | 3 (2) | |
| 9720 | 27 (38) | 75 (51) | |
| 10201 | 22 (31) | 50 (34) | |
| Hemoglobin, g/dL | .35 | ||
| Median | 9.7 | 9.4 | |
| Range | 6.0-15.0 | 6.0-13.1 | |
| Platelet count, ×109/L | .40 | ||
| Median | 60 | 72 | |
| Range | 4-850 | 5-510 | |
| WBC, ×109/L | < .001 | ||
| Median | 47.1 | 10.4 | |
| Range | 0.9-450.0 | 0.9-198.0 | |
| Blood blasts, % | < .001 | ||
| Median | 70 | 24 | |
| Range | 0-99 | 0-96 | |
| Bone marrow blasts, % | < .001 | ||
| Median | 82 | 57 | |
| Range | 7-97 | 7-96 | |
| Centrally reviewed FAB classification, n (%) | |||
| M0 | 0 (0) | 4 (5) | |
| M1 | 17 (35) | 11 (13) | |
| M2 | 9 (18) | 31 (36) | |
| M4 | 12 (24) | 22 (26) | |
| M5 | 11 (22) | 12 (14) | |
| M6 | 0 (0) | 5 (6) | |
| Extramedullary involvement, n (%) | 20 (29) | 23 (16) | .04† |
| NPM1, n (%) | < .001 | ||
| Mutated | 53 (74) | 68 (46) | |
| Wild-type | 19 (26) | 79 (54) | |
| WT1, n (%) | .001 | ||
| Mutated | 11 (15) | 4 (3) | |
| Wild-type | 61 (85) | 143 (97) | |
| CEBPA, n (%) | .53 | ||
| Mutated | 11 (15) | 18 (12) | |
| Wild-type | 61 (85) | 129 (88) | |
| MLL-PTD, n (%) | .50 | ||
| Positive | 2 (4) | 8 (7) | |
| Negative | 53 (96) | 104 (93) | |
| IDH1, n (%) | .25 | ||
| Mutated | 5 (9) | 21 (17) | |
| Wild-type | 50 (91) | 102 (83) | |
| IDH2, n (%) | .02 | ||
| Mutated, R140 IDH2 | 8 (15) | 29 (24) | (IDH2-mutated vs IDH2-WT) |
| Mutated, R172 IDH2 | 0 (0) | 10 (8) | |
| Wild-type | 47 (85) | 84 (68) | |
| Outcome for all patients, no. | 72 | 147 | |
| Complete remission rate, n (%) | 48 (67) | 103 (70) | .64 |
| Disease-free survival | .007 | ||
| Median, y | 0.5 | 1.0 | |
| Disease-free at 3 y, % (95% CI) | 10 (4-21) | 18 (12-26) | |
| Overall survival | < .001 | ||
| Median, y | 0.8 | 1.4 | |
| Alive at 3 y, % (95% CI) | 14 (7-23) | 23 (16-30) | |
| Outcome for patients aged 60-69 y, no. | 41 | 78 | |
| Complete remission rate, no. (%) | 29 (71) | 58 (75) | .67 |
| Disease-free survival | < .001 | ||
| Median, y | 0.4 | 1.0 | |
| Disease-free at 3 y, % (95% CI) | 7 (1-20) | 19 (10-30) | |
| Overall survival | < .001 | ||
| Median, y | 0.6 | 1.4 | |
| Alive at 3 y, % (95% CI) | 10 (3–21) | 26 (16–36) | |
| Outcome for patients age ≥ 70 y, no. | 31 | 69 | |
| Complete remission rate, no. (%) | 19 (61) | 45 (65) | .82 |
| Disease-free survival | .94 | ||
| Median, y | 0.9 | 1.0 | |
| Disease-free at 3 y, % (95% CI) | 16 (4-35) | 18 (8-30) | |
| Overall survival | .71 | ||
| Median, y | 0.9 | 1.3 | |
| Alive at 3 y, % (95% CI) | 19 (8-35) | 20 (11-30) |
| Characteristic . | FLT3-ITD (n = 72) . | FLT3-WT (n = 147) . | P . |
|---|---|---|---|
| Age, y | .92 | ||
| Median | 68 | 69 | |
| Range | 60-81 | 60-83 | |
| Age group, n (%) | .67 | ||
| 60-69 y | 41 (57) | 78 (53) | |
| ≥ 70 y | 31 (43) | 69 (47) | |
| Sex, no. of males (%) | 36 (50) | 75 (51) | 1.00 |
| Race, n (%) | .81 | ||
| White | 66 (92) | 129 (90) | |
| Nonwhite | 6 (8) | 14 (10) | |
| Protocol,* n (%) | |||
| 8525 | 11 (15) | 10 (7) | |
| 8923 | 9 (13) | 9 (6) | |
| 9420 | 3 (4) | 3 (2) | |
| 9720 | 27 (38) | 75 (51) | |
| 10201 | 22 (31) | 50 (34) | |
| Hemoglobin, g/dL | .35 | ||
| Median | 9.7 | 9.4 | |
| Range | 6.0-15.0 | 6.0-13.1 | |
| Platelet count, ×109/L | .40 | ||
| Median | 60 | 72 | |
| Range | 4-850 | 5-510 | |
| WBC, ×109/L | < .001 | ||
| Median | 47.1 | 10.4 | |
| Range | 0.9-450.0 | 0.9-198.0 | |
| Blood blasts, % | < .001 | ||
| Median | 70 | 24 | |
| Range | 0-99 | 0-96 | |
| Bone marrow blasts, % | < .001 | ||
| Median | 82 | 57 | |
| Range | 7-97 | 7-96 | |
| Centrally reviewed FAB classification, n (%) | |||
| M0 | 0 (0) | 4 (5) | |
| M1 | 17 (35) | 11 (13) | |
| M2 | 9 (18) | 31 (36) | |
| M4 | 12 (24) | 22 (26) | |
| M5 | 11 (22) | 12 (14) | |
| M6 | 0 (0) | 5 (6) | |
| Extramedullary involvement, n (%) | 20 (29) | 23 (16) | .04† |
| NPM1, n (%) | < .001 | ||
| Mutated | 53 (74) | 68 (46) | |
| Wild-type | 19 (26) | 79 (54) | |
| WT1, n (%) | .001 | ||
| Mutated | 11 (15) | 4 (3) | |
| Wild-type | 61 (85) | 143 (97) | |
| CEBPA, n (%) | .53 | ||
| Mutated | 11 (15) | 18 (12) | |
| Wild-type | 61 (85) | 129 (88) | |
| MLL-PTD, n (%) | .50 | ||
| Positive | 2 (4) | 8 (7) | |
| Negative | 53 (96) | 104 (93) | |
| IDH1, n (%) | .25 | ||
| Mutated | 5 (9) | 21 (17) | |
| Wild-type | 50 (91) | 102 (83) | |
| IDH2, n (%) | .02 | ||
| Mutated, R140 IDH2 | 8 (15) | 29 (24) | (IDH2-mutated vs IDH2-WT) |
| Mutated, R172 IDH2 | 0 (0) | 10 (8) | |
| Wild-type | 47 (85) | 84 (68) | |
| Outcome for all patients, no. | 72 | 147 | |
| Complete remission rate, n (%) | 48 (67) | 103 (70) | .64 |
| Disease-free survival | .007 | ||
| Median, y | 0.5 | 1.0 | |
| Disease-free at 3 y, % (95% CI) | 10 (4-21) | 18 (12-26) | |
| Overall survival | < .001 | ||
| Median, y | 0.8 | 1.4 | |
| Alive at 3 y, % (95% CI) | 14 (7-23) | 23 (16-30) | |
| Outcome for patients aged 60-69 y, no. | 41 | 78 | |
| Complete remission rate, no. (%) | 29 (71) | 58 (75) | .67 |
| Disease-free survival | < .001 | ||
| Median, y | 0.4 | 1.0 | |
| Disease-free at 3 y, % (95% CI) | 7 (1-20) | 19 (10-30) | |
| Overall survival | < .001 | ||
| Median, y | 0.6 | 1.4 | |
| Alive at 3 y, % (95% CI) | 10 (3–21) | 26 (16–36) | |
| Outcome for patients age ≥ 70 y, no. | 31 | 69 | |
| Complete remission rate, no. (%) | 19 (61) | 45 (65) | .82 |
| Disease-free survival | .94 | ||
| Median, y | 0.9 | 1.0 | |
| Disease-free at 3 y, % (95% CI) | 16 (4-35) | 18 (8-30) | |
| Overall survival | .71 | ||
| Median, y | 0.9 | 1.3 | |
| Alive at 3 y, % (95% CI) | 19 (8-35) | 20 (11-30) |
WBC indicates white blood count; FAB, French-American-British classification; FLT3-ITD, internal tandem duplication of the FLT3 gene; WT, wild-type; and MLL-PTD, partial tandem duplication of the MLL gene.
Cancer and Leukemia Group B frontline treatment protocol received. See supplemental material for details.
FLT3-ITD patients tended to have more extramedullary involvement, particularly in terms of gum hypertrophy and lymphadenopathy.
Median follow-up was 3.8 years (range, 2.3-11.6) for patients alive.
FLT3-ITD indicates internal tandem duplication of the FLT3 gene; WT, wild-type; and CI, confidence interval.
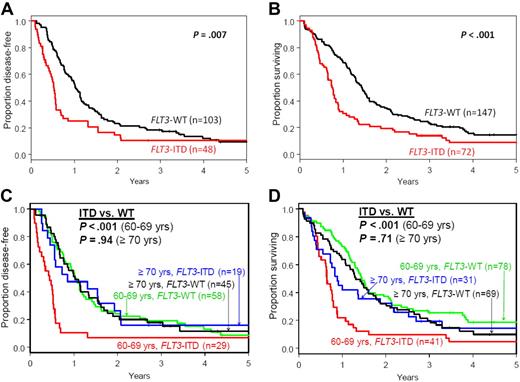
Compared with FLT3-WT patients, FLT3-ITD patients had similar complete remission rates (P = .64) and shorter disease-free survival (DFS; P = .007) and overall survival (OS; P < .001; Table 1, Figure 1A-B). No impact of treatment protocol on outcome was observed (see supplemental text). In multivariable analyses, FLT3-ITD remained associated with shorter DFS (P < .001; hazard ratio [HR] = 2.10), after adjustment for NPM1 mutations, white blood cell counts, and hemoglobin levels, and with shorter OS (P < .001; HR = 1.97), after adjustment for NPM1 mutations (supplemental Table 1). FLT3-ITD compared with FLT3-WT patients had approximately twice the risk of relapse or death. Our results differ from a recent analysis evaluating a relatively small subset of primary CN-AML patients aged > 60 years (n = 70) where FLT3-ITD (n = 18) did not impact OS.11
Kaplan-Meier survival plots of older patients with cytogenetically normal, primary acute myeloid leukemia according to FLT3 mutational status. (A) Disease-free and (B) overall survival of all patients aged ≥ 60 years. (C) Disease-free and (D) overall survival of patients aged 60-69 years and those aged ≥ 70 years.
Kaplan-Meier survival plots of older patients with cytogenetically normal, primary acute myeloid leukemia according to FLT3 mutational status. (A) Disease-free and (B) overall survival of all patients aged ≥ 60 years. (C) Disease-free and (D) overall survival of patients aged 60-69 years and those aged ≥ 70 years.
In younger CN-AML, a high FLT3-ITD:WT AR is associated with inferior DFS and OS compared with low-AR and FLT3-WT patients, who have similar outcomes.3,9,12 In our study, a high AR (> 0.615, median AR value for FLT3-ITD–positive patients) was associated with worse DFS and OS compared with FLT3-WT patients (supplemental Figure 2 and supplemental Table 2). There was no difference in outcomes between high-AR and low-AR patients, who tended to have worse outcomes than FLT3-WT patients. Therefore, regardless of the AR, FLT3-ITD patients seemingly have worse outcomes than FLT3-WT in the older CN-AML population.
We recently reported a difference in impact of NPM1 mutations in patients aged 60-69 versus patients aged ≥ 70 years.6 Thus, we evaluated the impact of FLT3-ITD in these age subgroups. FLT3-ITD patients aged 60-69 years had shorter DFS (P < .001) and OS (P < .001) than FLT3-WT patients (Table 1 and Figure 1C-D). Moreover, in this age subgroup, FLT3-ITD was independently associated with shorter DFS (P < .001; HR = 2.94) and OS (P < .001; HR = 2.79) in multivariable models (supplemental Table 3). In contrast, FLT3-ITD did not impact DFS (P = .94) or OS (P = .71) in patients aged ≥ 70 years (Table 1 and Figure 1C-D). Reasons for these age-related differences in outcome are unknown. Allelic ratio may have a role; patients aged 60-69 years tended to more often have high AR than patients ≥ 70 years (67% vs 33%, P = .14).
FLT3-ITD–associated global gene and microRNA expression in older primary CN-AML
Only 1 FLT3-ITD–associated gene- and no FLT3-ITD–associated microRNA-expression signatures have been reported in younger CN-AML.8,13-15 No such signatures have been reported exclusively for older primary CN-AML patients.
A global gene-expression signature, comprising 4494 probe-sets (2898 genes), was derived by comparing microarrays of FLT3-ITD patients (n = 38) with those of FLT3-WT patients (n = 79; supplemental Figure 3A and supplemental Table 4). Nearly two-thirds of the probe sets were up-regulated in association with FLT3-ITD, and the signature appears to provide interesting biologic and therapeutic insights.
Among up-regulated genes associated with FLT3-ITD with biologic and/or clinical significance were FLT3 itself; the homeobox gene, MEIS1, described to induce FLT3 expression16 ; homeobox genes HOXB3 and PBX3, a MEIS1 cofactor; IGFBP2, whose encoded protein plays a key role in AKT pathway activation17 ; WT1, a potential immunotherapeutic target18 ; SOCS2, recently implicated in promoting sensitivity to FLT3 inhibitors19 ; and CD33, whose overexpression concurrent with FLT3-ITD and absence of P-glycoprotein sensitizes leukemia stem/progenitor cells in vitro to the immunotherapeutic agent gemtuzumab ozogamicin.20
Ten of the up-regulated genes identified in our older CN-AML signature (ie, CFH, SOCS2, STON2, SCHIP1, PBX3, MYO1B, DAPK1, FAM38B, IL2RA, and GOLGA8A) were also up-regulated in a recently described 20-gene classifier associated with FLT3-ITD in younger CN-AML patients.15 Among the down-regulated genes were leukemia-associated MLLT3 and TAL1 and erythropoiesis-associated GATA3, EPOR, ANK1, and HEMGN. PDE4B, underexpressed in older FLT3-ITD–positive CN-AML, was also underexpressed in association with FLT3-ITD in the aforementioned 20-gene classifier.15
Gene ontology analysis revealed overrepresentation of genes involved in cellular metabolic processes (supplemental Table 5). Emerging data underscore the potential importance of altered metabolic pathways in leukemogenesis, eg, somatic mutations in the IDH1 and IDH2 genes encoding isocitrate dehydrogenases in CN-AML.7,21 Notably, IDH1 overexpression was associated with FLT3-ITD (supplemental Figure 3A).
For the first time in primary CN-AML, an FLT3-ITD–associated microRNA-expression signature, comprising 32 differentially expressed probes, was derived (supplemental Figure 3B and supplemental Table 6). The most overexpressed (2.8-fold) microRNA was miR-155, previously associated with FLT3-ITD14,22 and one of the first microRNAs with demonstrated transforming ability in vivo.14 Our older FLT3-ITD patients also had increased miR-125b-2*. It is unknown whether this microRNA has a different function than the sense-strand–derived miR-125b that was reported to antagonize growth inhibitory signals in ETV6/RUNX1 acute lymphoblastic leukemia23 and whose overexpression was associated with IDH2 mutations.7 Among the underexpressed microRNAs were miR-144 and miR-451, normally up-regulated during erythropoiesis.24 Little is known about miR-488 and miR-486-5p, the most underexpressed microRNAs associated with FLT3-ITD; however, multiple cancers underexpress miR-486-5p.25
Because the adverse prognostic impact of FLT3-ITD was observed within the 60- to 69-year-old age group, but not in older patients, we compared gene and microRNA expression in FLT3-ITD–positive patients aged 60-69 with those of patients aged ≥ 70 years. There was no significant interaction between age groups and FLT3-ITD status on genome-wide gene (global test, P = .82) or microRNA expression (global test, P = .57).
Although our results require confirmation, they show FLT3-ITD negatively impacts the outcomes of 60- to 69-year-old patients, who could be considered for more intensive therapies, similar to those for younger patients. Increasing the “cut-off” point for AML treatment stratification from 60 to 70 years is also implicated by our results. Finally, FLT3-ITD in older CN-AML associates with gene- and microRNA-expression signatures that provide biologic insights, which may lead to novel therapeutic approaches.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Donna Bucci of the CALGB Leukemia Tissue Bank and The Ohio State University Comprehensive Cancer Center's Nucleic Acid and Microarray Shared Resources for technical support. We thank CALGB-participating institutions, medical professionals, and AML patients for their valuable involvement with this study.
This work was supported in part by National Cancer Institute (Bethesda, MD) grants CA140158, CA101140, CA114725, CA16058, CA77658, CA089341, and CA129657, The Coleman Leukemia Research Foundation (to C.D.B.) and the Deutsche Krebshilfe–Dr Mildred Scheel Cancer Foundation (to H.B.). The research for CALGB 9665, 8461, 10201, 9720, 9420, 8923, 8525, and 20202 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Monica M. Bertagnolli, MD, Chairman) and to the CALGB Statistical Center (Stephen George, PhD; CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
National Institutes of Health
Authorship
Contribution: S.P.W., G.M., M.D.R., K. Maharry, D.M., K.B.H., K. Mrózek, and C.D.B. contributed to the design and analysis of this study; S.P.W., G.M., K. Maharry, M.D.R., K. Mrózek, and C.D.B. contributed to the writing of this manuscript and all authors agreed on the final version; S.P.W., H.B., Y.-Z.W., S.S., K.H.M., and J.W. carried out laboratory-based research; K. Maharry, M.D.R., D.M., and K.B.H. performed statistical analyses; and B.L.P., M.R.B., T.H.C., J.E.K., M.W., J.O.M., R.M.S., A.J.C., R.A.L., M.A.C., G.M., and C.D.B. were involved directly or indirectly in care of patients and/or sample procurement.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of participating Cancer and Leukemia Group B institutions, principal investigators, and cytogeneticists, please see the supplemental Appendix.
Correspondence: Michael A. Caligiuri, The Ohio State University, Comprehensive Cancer Center, The James Cancer Hospital, Suite 519, 300 W 10th Ave, Columbus, OH 43210; e-mail: michael.caligiuri@osumc.edu; or Clara D. Bloomfield, The Ohio State University, Comprehensive Cancer Center, 1216 James Cancer Hospital, 300 W 10th Ave, Columbus, OH 43210; e-mail: clara.bloomfield@osumc.edu; or Guido Marcucci, The Ohio State University, Comprehensive Cancer Center, 809C Biomedical Research Tower, 460 W 12th Ave, Columbus, OH 43210; e-mail: guido.marcucci@osumc.edu.