Abstract
Apoptosis of short-lived plasma cells after a few days of intense immunoglobulin secretion is critical for maintaining a controlled humoral immune response. The mechanisms that regulate this process are poorly understood. Here we report that the key apoptotic caspases, caspase-3 and caspase-9, become resistant to activation by apoptotic stimuli when B cells differentiate into short-lived plasma cells. As a consequence, apoptosis of most short-lived plasma cells in vitro and in vivo is effector caspase-independent. We also show that a triaspartic acid repeat that normally prevents activation of caspase-3 becomes stabilized in short-lived plasma cells and myeloma cell lines. The block on caspase activation occurs before the accumulation of intracellular immunoglobulins and a progressive rise in secretory stress in the endoplasmic reticulum (ER). Plasma cells show increased susceptibility to ER stress–induced apoptosis and activate the ER-associated caspase-12, which is required specifically for nuclear apoptotic events. In nonlymphoid cells that cannot activate effector caspases, programmed cell death is delayed in response to ER stress. These observations suggest that the block on activation of key apoptotic caspases has evolved in short-lived plasma cells to prolong survival under conditions of ER stress resulting from high-level immunoglobulin secretion.
Introduction
Plasma cells are terminally differentiated B lymphocytes that provide both immediate and prolonged protection from infections by producing large amounts of antibodies. The immune response produces short-lived and long-lived plasma cells. After antigen encounter, short-lived plasma cells are rapidly formed in secondary lymphoid organs, where they undergo apoptosis after a few days of intensive antibody secretion.1 In contrast, a proportion of plasma cells survive for prolonged periods to maintain long-term humoral immunity.2 While the longevity of long-lived plasma cells has been shown to be related to survival signals provided by bone marrow niches,3 the mechanisms that regulate apoptosis of short-lived plasma cells are poorly understood but have considerable importance for controlling the magnitude of the humoral immune responses. Abnormal survival of short-lived and long-lived plasma cells in mice has been linked to the development of autoimmune diseases, which are characterized by the production of autoreactive antibodies. Mice that lack the proapoptotic molecule Bim accumulate plasma cells and succumb to autoimmune kidney disease.4 The spleens of mice prone to the autoimmune disease systemic lupus erythematosus also contain increased numbers of short-lived and long-lived plasma cells.5 In humans, resistance to programmed cell death is thought to contribute to the survival of transformed plasma cells in multiple myeloma.6,7
Programmed cell death normally occurs by apoptosis, a tightly regulated process that is critical for the development and maintenance of tissues.8 The apoptotic program is executed by a family of proteases known as caspases, which dismantle the dying cell in an orderly way by cleaving a large number of cellular substrates.9 Extrinsic and intrinsic signaling pathways lead to caspase activation. In the extrinsic pathway, extracellular ligands bind to specific cell surface receptors called death receptors. This leads to the recruitment of cytosolic adaptor proteins and activation of caspase-8, which is known as an initiator caspase and activates downstream caspases.9 The intrinsic pathway is activated by intracellular damage to organelles or DNA. It begins when proapoptotic members of the Bcl-2 family of proteins cause permeabilization of the outer mitochondrial membrane. This results in release of intermembrane space proteins including cytochrome c and formation of a multiprotein complex, the apoptosome. Dimerization of caspase-9 in the apoptosome leads to its activation, and active caspase-9 in turn cleaves and thereby activates downstream caspases, which are known as effector casapses.10 Among the effector caspases, caspase-3 acts as the major executioner of apoptosis, cleaving hundreds of protein substrates.9 The importance of caspases in the regulation of programmed cell death has been shown by studies in mice deficient in caspase-3 and caspase-9.11-13 Given the key role of caspases in the control of cell death, it is not surprising that aberrant regulation of caspases has been linked to several diseases, including various cancers.14
Despite the important role of caspases in apoptosis, caspase-independent cell death can occur. Maintenance of the mitochondrial transmembrane potential ΔψM is critical for mitochondrial function and cell survival. During caspase-dependent apoptosis, effector caspases contribute to the loss of ΔψM by cleaving a subunit of complex I of the electron transport chain.15 Cells in which caspase activation is inhibited also undergo mitochondrial outer membrane permeabilization and mitochondrial release of cytochrome c in response to apoptotic stimuli but remain viable and regenerate mitochondria. However, if the apoptotic stimulus persists, cells ultimately lose their ΔψM and undergo delayed cell death.16,17 The mechanisms that give rise to caspase-independent cell death are poorly understood.18,19
Endoplasmic reticulum (ER) stress is one of the signals that can trigger apoptosis in eukaryotic cells. Most secreted and transmembrane proteins fold and mature in the lumen of the ER. When the folding capacity of the ER is exceeded, unfolded and misfolded proteins accumulate and cause ER stress. ER stress elicits an adaptive program termed the unfolded protein response (UPR), which comprises a network of intracellular signal transduction pathways that act to re-establish homeostasis in the ER.20 Failure of the UPR leads to excessive ER stress and apoptosis. ER stress–induced apoptosis normally requires the intrinsic pathway of apoptosis and depends on caspase-3 and caspase-9.21
Plasma cells secrete antibodies at rates of up to 10 000 moleculesper second per cell.22 To cope with this secretory challenge, terminal differentiation of B cells into plasma cells requires activation of the UPR and expansion of the ER.23-25 Intriguingly, the capacity of the proteasome to degrade misfolded proteins decreases during plasma cell differentiation, at a time when antibody secretion peaks.26 In order to reduce the load of unfolded proteins, the UPR normally leads to activation of RNA-activated PKR-like ER kinase, which mediates inhibition of protein translation.20 However, activation of RNA-activated protein kinase-like ER kinase does not occur during plasma cell differentiation.27 It has therefore been suggested that plasma cell death could be linked to ER stress caused by high levels of antibody synthesis.28
Here we report that key apoptotic caspases become resistant to activation by apoptotic stimuli when B cells differentiate into short-lived plasma cells. Moreover, we find that ER stress progressively increases during plasma cell differentiation in parallel with a decrease in the cells' ability to cope with it and that activation of the ER-associated and inflammatory caspase, caspase-12, is required specifically for nuclear apoptotic events in short-lived plasma cells. Our results suggest that an active block on activation of key apoptotic caspases prolongs plasma cell survival under conditions of ER stress but allows for apoptosis at a later stage, thereby providing a finely tuned mechanism that makes an important contribution to the regulation of the life-span of short-lived plasma cells.
Methods
Cell culture and reagents
Plasma cells were differentiated in vitro as previously described, using interleukin-5 (IL-5), IL-6, IL-10, and lipopolysaccharide (LPS).29 After 7 days in culture, CD138+ B200− postmitotic immunoglobulin M (IgM)-secreting plasma cells were purified using magnetic beads (Dynal). The plasma cells were further cultured in fresh medium with the above cytokines but without LPS. The murine lymphoma line I.29μ+ was induced to undergo plasmacytic differentiation with LPS as previously described.26,30 Activation of B cells was carried out by treating live splenocytes with LPS (10 μg/mL). Primary splenic plasma cells were obtained by immunizing mice intraperitoneally with LPS (60 μg). All mice experiments were approved by a United Kingdom Home Office Project License. After 8-10 days, CD138+ plasma cells were isolated using magnetic beads and cultured with IL-5, IL-6, and IL-10. Primary resting B cells were isolated from spleens as described previously.31 The human myeloma lines OPM-2, KMS-11, U266, and RPMI8226 were maintained in RPMI1640 supplemented with 10% fetal calf serum, the mouse myeloma line MPC11 was maintained in the same medium but with 10% horse serum. Mouse embryonic fibroblasts (MEFs) heterozyogous or double-deficient for caspase-3 and caspase-7 have been described previously.11 Tunicamycin (Calbiochem), PAC-1 (Cayman), etoposide (Sigma-Aldrich), zVADfmk (Caspase Inhibitor I; Calbiochem), and zATADfmk (Caspase-12 Inhibitor; Biovision) were dissolved in dimethyl sulfoxide (DMSO), arsenic trioxide (Sigma-Aldrich) was dissolved at a concentration of 0.33M in 1M NaOH, heated to 95°C for 5 minutes, and then stored at −20°C.
Analysis of apoptotic cell membrane alterations
Exposure of phosphatidylserine on the outer leaflet of the cell membrane and loss of cell membrane integrity were assessed by staining with annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (BD Pharmingen), respectively, and subsequent analysis on a FACScan flow cytometer using CellQuest software version 5.2.1 (Becton Dickinson).
Mitochondrial transmembrane potential ΔψM
Loss of Δψm during apoptosis was analyzed using the MitoCapture Apoptosis Detection Kit (Calbiochem) according to the manufacturer's instructions. Cells were analyzed on a FACScan flow cytometer using CellQuest software Version 5.2.1 (Becton Dickinson).
Immunoblotting
Whole cell lysates were prepared using 50mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.5, 50mM NaF, 5mM NaPyrophosphate, 1mM EDTA (ethylenediaminetetraacetic acid), 1mM dithiotreitol (DTT), 10% glycerol, 1% Triton, and Complete EDTA-free Protease Inhibitor Cocktail (Roche). Cytosolic extracts were prepared by permeabilizing cells in 0.025% digitonin dissolved in HMKEE buffer (20mM HEPES, pH 7.2, 5mM MgCl2, 10mM KCl, 1mM EDTA, and 1mM EGTA [ethyleneglycoltetraacetic acid]) with 250mM sucrose and protease inhibitor, and recovery of the supernatant after centrifugation at 12 000g for 5 minutes. Primary antibodies were: anti-BiP, anti-caspase-12, anti-caspase-3, anti-active caspase-3, anti-Mst1, anti-cytochrome c, anti-Caspase-7, anti-mouse-Caspase-9 (1:1000; all from Cell Signaling), anti-GRP94 (1:2500; Abcam), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:2500; Santa Cruz Biotechnology). The Licor system (Licor Biosciences) was used to quantify IgM/GAPDH ratios in extracts from in vitro–differentiated plasma cells and I.29μ+ cells.
Caspase-12 activity assay
The CaspGLOW Fluorescein Caspase-12 Staining kit (Biovision) was used according to the manufacturer's instructions, and cells were analyzed on a FACScan flow cytometer.
Immunohistochemistry
Tissue obtained for routine hematopathologic diagnosis was fixed in 10% formalin and processed and embedded in paraffin wax according to standard procedures. Consecutive sections (4 μm) were stained with hematoxylin/eosin and with Giemsa for morphologic diagnosis. For immunohistochemical studies, the standard avidin-biotin-peroxidase technique was performed using an antibody against active caspase-3 (1:400; Cell Signaling). A total of 10 high-power fields were analyzed per sample.
Apoptotic chromatin condensation and TUNEL assay
Cells were fixed with 4% paraformaldehyde for 10 minutes and permeabilized with 0.4% Triton X for 10 minutes, followed by incubation with fluorescein-12-UTP(a) and recombinant terminal deoxynucleotidyl transferase using the DeadEnd Fluorometric TUNEL System (Promega). Slides were mounted with 10 μg/mL DAPI (Sigma-Aldrich) in Vectashield (Vector Laboratories) and viewed on a Leica SP5 confocal microscope using Leica Confocal software Version 2.2. Cells were counted in several randomly chosen fields per experiment at 63× magnification.
Statistical analysis
Means were compared by paired or unpaired 2-tailed t tests, and P values less than .05 were considered significant.
Results
Activation of key apoptotic caspases is blocked in plasma cells
To study the cellular events governing apoptosis of short-lived plasma cells, it was necessary to obtain sufficient numbers of plasma cells and to be able to follow them in culture while apoptosis was taking place. To achieve this, we used 2 previously described in vitro differentiation systems for much of the analysis and confirmed our major observations using ex vivo–isolated primary mouse plasma cells. The first system uses I.29μ+ cells, which are murine lymphoma cells that express only membrane-bound Ig. Upon stimulation with LPS, the I.29μ+ cells undergo plasmacytic differentiation within 2 to 3 days.26,30 On day 3 after LPS stimulation, the cells also begin to die, and by day 4 around half of them are apoptotic (Figure 1A). The second system uses a method for in vitro differentiation and purification of CD138+B220− postmitotic IgM-secreting plasma cells that was recently described by us.29 When these fully differentiated plasma cells are maintained in culture after isolation, approximately 60% undergo spontaneous apoptosis after 2 days (Figure 1A). Similarly, approximately 40% of ex vivo–isolated primary splenic plasma cells undergo apoptosis after 1 day in culture (Figure 1A). The systems therefore closely resemble the situation in vivo, where the majority of short-lived plasma cells rapidly undergo apoptosis in situ in secondary lymphoid organs.1
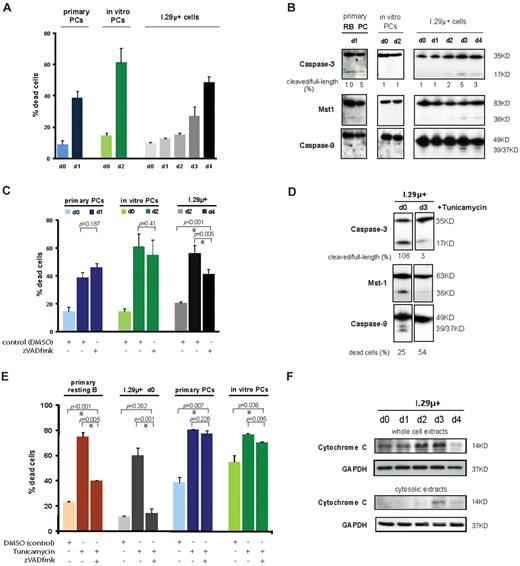
Plasma cell apoptosis is largely caspase-independent. (A) Apoptosis of ex vivo–isolated and in vitro–differentiated plasma cells and I.29μ+ cells. The proportion of dead cells was determined by staining with annexinV-FITC and propidium iodide. Primary and in vitro–differentiated plasma cells were analyzed 1 day and 2 days after isolation, respectively. I.29μ+ cells were analyzed before (d0) and one to 4 days after stimulation of plasmacytic differentiation with LPS. Mean and SEM of 2 independent experiments are shown. Statistically significant differences between means are indicated by an asterisk. (B) Immunoblotting on whole cell extracts prepared at the same time points as described in (A). Extracts from primary splenic resting B cells (RB) were prepared after one day in culture following ex vivo isolation. (C) Primary ex vivo–isolated and in vitro–differentiated plasma cells were treated with zVADfmk (100μM) or DMSO immediately after isolation for 24 and 48 hours, respectively. I.29μ+ cells were treated from day 2 to 4 after LPS stimulation. The proportion of apoptotic cells was determined as in panel A. (D) Immunoblotting with antibodies against the indicated proteins after treatment with tunicamycin (1 μg/mL) for 14 hours. Images are from the same blot. For comparison with untreated (control) cells see panel B. (E) Ex vivo–isolated resting B and plasma cells, undifferentiated I.29μ+ cells, and in vitro–differentiated plasma cells were treated with tunicamycin (1 μg/mL) and zVADfmk (100μM) or DMSO (control) for 14 hours, and the proportion of apoptotic cells determined as in panel A. Note that tunicamycin was used at 5 μg/mL in resting B cells, and treatment was extended to 24 hours in I.29μ+ cells to achieve comparable rates of apoptosis. (F) Analysis of mitochondrial outer membrane permeabilization during plasma cell apoptosis by immunoblotting of cytosolic and whole cell extracts from undifferentiated (d0) and plasmacytic (d3) I.29μ+ cells with antibodies against cytochome C and GAPDH (control).
Plasma cell apoptosis is largely caspase-independent. (A) Apoptosis of ex vivo–isolated and in vitro–differentiated plasma cells and I.29μ+ cells. The proportion of dead cells was determined by staining with annexinV-FITC and propidium iodide. Primary and in vitro–differentiated plasma cells were analyzed 1 day and 2 days after isolation, respectively. I.29μ+ cells were analyzed before (d0) and one to 4 days after stimulation of plasmacytic differentiation with LPS. Mean and SEM of 2 independent experiments are shown. Statistically significant differences between means are indicated by an asterisk. (B) Immunoblotting on whole cell extracts prepared at the same time points as described in (A). Extracts from primary splenic resting B cells (RB) were prepared after one day in culture following ex vivo isolation. (C) Primary ex vivo–isolated and in vitro–differentiated plasma cells were treated with zVADfmk (100μM) or DMSO immediately after isolation for 24 and 48 hours, respectively. I.29μ+ cells were treated from day 2 to 4 after LPS stimulation. The proportion of apoptotic cells was determined as in panel A. (D) Immunoblotting with antibodies against the indicated proteins after treatment with tunicamycin (1 μg/mL) for 14 hours. Images are from the same blot. For comparison with untreated (control) cells see panel B. (E) Ex vivo–isolated resting B and plasma cells, undifferentiated I.29μ+ cells, and in vitro–differentiated plasma cells were treated with tunicamycin (1 μg/mL) and zVADfmk (100μM) or DMSO (control) for 14 hours, and the proportion of apoptotic cells determined as in panel A. Note that tunicamycin was used at 5 μg/mL in resting B cells, and treatment was extended to 24 hours in I.29μ+ cells to achieve comparable rates of apoptosis. (F) Analysis of mitochondrial outer membrane permeabilization during plasma cell apoptosis by immunoblotting of cytosolic and whole cell extracts from undifferentiated (d0) and plasmacytic (d3) I.29μ+ cells with antibodies against cytochome C and GAPDH (control).
To investigate whether caspases are activated during programmed plasma cell death, we first carried out immunoblotting with antibodies against individual key caspases. Cleavage products of these caspases, which would indicate activation, were not detected in extracts from in vitro–differentiated plasma cells undergoing apoptosis (Figure 1B). The lack of functional caspase activity was confirmed by immunoblotting with an antibody against mammalian sterile twenty-like kinase 1 (Mst1). In the apoptotic pathway, Mst1 is involved in apoptotic chromatin compaction and cleaved by active caspase-3.32 Only minimal amounts of the cleavage products of caspases and of Mst1 were observed in plasmacytic I.29μ+ cells and primary ex vivo–isolated plasma cells undergoing apoptosis (Figure 1B). The level of cleavage of caspases and of Mst1 that was observed in primary resting B cells was higher than in primary plasma cells, despite the substantially lower rate of apoptosis in the B-cell cultures (< 20%) compared with the plasma cell cultures (40%; Figure 1B).
The minimal amounts of caspase cleavage products detected in extracts from apoptotic plasma cells prompted us to test at the functional level whether programmed plasma cell death occurs independently of caspase activation. To achieve this, cells were treated with the pan-caspase inhibitor zVADfmk, which blocks activation of caspases irreversibly.18 Addition of zVADfmk to the plasma cell cultures had no effect on the apoptosis rate of in vitro–differentiated and primary ex vivo–isolated plasma cells, indicating that the plasma cell death was caspase-independent (Figure 1C). The observations indicate that apoptotic caspases are neither activated in the majority of short-lived plasma cells when they die nor are they required for the execution of apoptosis. In the differentiated plasmacytic I.29μ+ cells, treatment with the zVADfmk had a limited effect on apoptosis (Figure 1C). This is consistent with the low-level activation of caspases in I.29μ+ cells observed on immunoblotting (Figure 1B) and could be explained by the fact that LPS-stimulated I.29μ+ cells acquire most key characteristics of plasma cells but are not fully postmitotic.30
To determine whether there is a specific block on caspase activation in plasma cells, we analyzed extracts from undifferentiated and plasmacytic I.29μ+ cells that were induced to undergo apoptosis with tunicamycin. The amount of cleavage products of caspases and Mst1 increased substantially in the undifferentiated cells in response to tunicamycin treatment (Figure 1D). In the plasmacytic cells, no increase in caspase cleavage compared with background levels was observed, despite the higher rate of apoptosis of these cells (Figure 1D). A time-course analysis of differentiating I.29μ+ cells showed that resistance of caspase-3 and Mst1 to tunicamycin occurred on day 2 after LPS stimulation (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Interestingly, caspase-9 was at least partly resistant to activation from day 1. This finding merits further studies because it suggests that the additional ER stress that is induced by tunicamycin could accelerate the block on activation of caspase-9.
To further investigate whether caspases are functional in plasma cells, we tested whether tunicamycin-induced cell death could be blocked by the pan-caspase inhibitor zVADfmk in B cells and plasma cells. While zVADfmk largely or completely blocked tunicamycin-induced apoptosis of primary resting B cells and undifferentiated I.29μ+ cells, it did not reduce tunicamycin-induced death in primary ex vivo–isolated and in vitro–differentiated plasma cells (Figure 1E). Together, these observations show that activation of key apoptotic caspases is subject to a near complete block during differentiation of B cells into short-lived plasma cells.
To test whether mitochondrial outer membrane permeabilization (which occurs upstream of caspase-9 and caspase-3 activation in the apoptotic pathway) takes place during programmed plasma cell death, immunoblotting was performed on cytosolic extracts. We found that cytochrome c was released into the cytosol when plasmacytic I.29μ+ cells underwent apoptosis, indicating that mitochondrial outer membrane permeabilization does occur during programmed plasma cell death (Figure 1F).
Plasma cell differentiation leads to stabilization of a triaspartic acid repeat that prevents caspase-3 activation
Activation of caspase-3 is normally prevented by a triaspartic acid repeat located near the caspase-3 cleavage site. During apoptosis, the repeat is thought to undergo a conformational change that allows access to the cleavage site and thereby permits cleavage-dependent activation of caspase-3 by itself and caspase-9.33,34 To determine whether the repeat becomes resistant to this change (ie, stabilized), we used a small molecule, PAC-1, known to specifically target the repeat and cause activation of caspase-3. Treatment of a variety of cells with PAC-1 has been shown to induce cleavage of caspase-3 into its active subunits, thereby triggering apoptosis.34,35
When undifferentiated I.29μ+ cells were treated with PAC-1, substantial cleavage of caspase-3 and its target Mst1 was observed, whereas no such effect was seen in differentiated plasmacytic I.29μ+ cells (Figure 2A and supplemental Figure 1A). Caspase-3 has been shown to disrupt mitochondrial function,11,15 and consistent with this, we found that PAC-1 induced apoptotic loss of ΔψM, mitochondrial release of cytochrome c, and cleavage of caspase-9 downstream of the mitochondria in undifferentiated I.29μ+ cells. However, this effect was not observed in differentiated plasmacytic I.29μ+ cells (Figure 2A-B and supplemental Figure 1B). Furthermore, treatment with PAC-1 resulted in substantial cleavage of caspase-3 in LPS-activated splenic B cells, but not in in vitro–differentiated plasma cells (Figure 2D). PAC-1 also induced apoptosis in primary resting B cells but not in primary plasma cells (Figure 2C). An additional significant observation was that PAC-1 failed to induce cleavage of caspase-3 in 2 human myeloma cell lines and induced only very weak cleavage in 2 others (Figure 2E), despite its known effectiveness in a variety of tumor cell lines.35 These observations provide evidence that the triaspartic repeat that maintains caspase-3 in its inactive form is stabilized in murine plasma cells and a proportion of human myeloma lines.
Stabilization of a safety-catch in caspase-3 in plasma cells. (A) Immunoblotting on whole cell extracts prepared from undifferentiated (d0) and plasmacytic (d3) I.29μ+ cells that had been treated for 9 hours with 10μM PAC-1 or DMSO (control). Images are from the same blot. (B) Fold increase in apoptotic loss of ΔψM in undifferentiated (d0) and plasmacytic (d3) I.29μ+ cells after treatment with PAC-1 for 9 hours (mean and SEM of 2 independent experiments). P values refer to the comparison between PAC-1 treated and control cells, and statistically significant differences between means are indicated by an asterisk. (C) Apoptosis (positive staining with annexinV-FITC and/or propidium iodide) after 14 hours of treatment with PAC-1 in primary resting B and plasma cells. To account for different baseline viabilities, relative cell death (percent dead cells/untreated control) was expressed as the difference in cell death in the absence (A) and presence (P) of a drug, normalized by the cell death achievable in the control cells [(P - A)/(100 - A) × 100]. Mean and SEM of 2 independent experiments. (D) Immunoblotting of whole cell extracts from splenic B cells 3 days after in vitro activation with LPS and freshly isolated in vitro–differentiated plasma cells. (E) Immunoblotting of whole cell extracts from the indicated human (KMS-11, RPMI8226, U266, OPM-2) and mouse (MPC-11) myeloma cell lines that had been treated with PAC-1 for 14 hours.
Stabilization of a safety-catch in caspase-3 in plasma cells. (A) Immunoblotting on whole cell extracts prepared from undifferentiated (d0) and plasmacytic (d3) I.29μ+ cells that had been treated for 9 hours with 10μM PAC-1 or DMSO (control). Images are from the same blot. (B) Fold increase in apoptotic loss of ΔψM in undifferentiated (d0) and plasmacytic (d3) I.29μ+ cells after treatment with PAC-1 for 9 hours (mean and SEM of 2 independent experiments). P values refer to the comparison between PAC-1 treated and control cells, and statistically significant differences between means are indicated by an asterisk. (C) Apoptosis (positive staining with annexinV-FITC and/or propidium iodide) after 14 hours of treatment with PAC-1 in primary resting B and plasma cells. To account for different baseline viabilities, relative cell death (percent dead cells/untreated control) was expressed as the difference in cell death in the absence (A) and presence (P) of a drug, normalized by the cell death achievable in the control cells [(P - A)/(100 - A) × 100]. Mean and SEM of 2 independent experiments. (D) Immunoblotting of whole cell extracts from splenic B cells 3 days after in vitro activation with LPS and freshly isolated in vitro–differentiated plasma cells. (E) Immunoblotting of whole cell extracts from the indicated human (KMS-11, RPMI8226, U266, OPM-2) and mouse (MPC-11) myeloma cell lines that had been treated with PAC-1 for 14 hours.
Apoptosis of inflammatory and transformed plasma cells in human lymphoid tissue occurs independently of caspase-3 activation
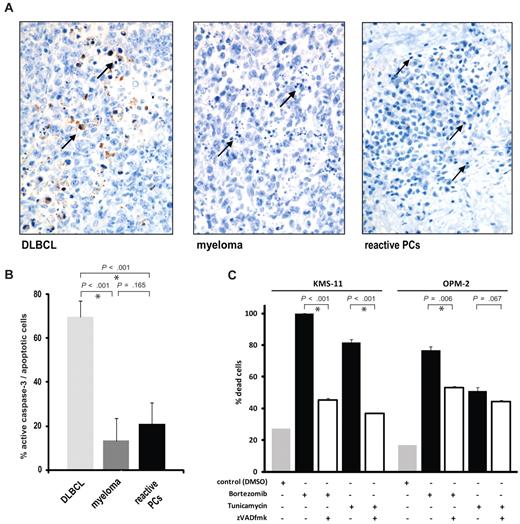
We next set out to examine whether key caspases are activated in human plasmacytic cells in vivo by staining human lymphoid tissue sections with an antibody that reacts specifically with active caspase-3. We investigated 8 cases of reactive plasmacytosis, representing physiologic accumulations of nontransformed polyclonal plasma cells as part of an acute inflammatory response, and 7 cases of extramedullary myeloma (transformed monoclonal plasma cells). As a control, 8 cases of diffuse large B cell lymphoma (DLBCL), a tumor of transformed germinal center B cells, were also analyzed. The samples were chosen because the tissue infiltrates were highly homogenous and contained a sufficient number of apoptotic cells for analysis. In DLBCL, 70% of apoptotic cells showed reactivity with the antibody against active caspase-3. In myeloma and reactive plasmacytosis, respectively, only 13% and 21% of the apoptotic cells showed detectable caspase-3 activity (Figure 3A-B).
The majority of human short-lived plasma cells undergo apoptosis without activation of caspase-3. (A-B) Immunohistochemical analysis of human lymph nodes infiltrated by diffuse large B cell lymphoma (DLBCL, n = 8), myeloma (n = 7), and reactive plasmacytosis (n = 8) using an antibody that reacts specifically with active caspase-3. The bar chart shows mean percentages and SEM of apoptotic cells staining positive for active caspase-3 relative to total apoptotic cells. Asterisks indicate statistical significance. Sample tissue sections show active caspase-3 (brown staining), and arrows indicate examples of apoptotic cells with typical condensed and/or fragmented nuclei. (C) Caspase-dependent and -independent cell death in response to apoptotic stimuli in human myeloma cell lines. The myeloma lines KMS-11 and OPM-2 were treated with bortezomib (20nM), tunicamycin (5 μg/mL), and zVADfmk (100μM) for 48 hours as indicated, and cell death was assessed by staining with annexinV-FITC and propidium iodide (mean and SEM of 2 independent experiments).
The majority of human short-lived plasma cells undergo apoptosis without activation of caspase-3. (A-B) Immunohistochemical analysis of human lymph nodes infiltrated by diffuse large B cell lymphoma (DLBCL, n = 8), myeloma (n = 7), and reactive plasmacytosis (n = 8) using an antibody that reacts specifically with active caspase-3. The bar chart shows mean percentages and SEM of apoptotic cells staining positive for active caspase-3 relative to total apoptotic cells. Asterisks indicate statistical significance. Sample tissue sections show active caspase-3 (brown staining), and arrows indicate examples of apoptotic cells with typical condensed and/or fragmented nuclei. (C) Caspase-dependent and -independent cell death in response to apoptotic stimuli in human myeloma cell lines. The myeloma lines KMS-11 and OPM-2 were treated with bortezomib (20nM), tunicamycin (5 μg/mL), and zVADfmk (100μM) for 48 hours as indicated, and cell death was assessed by staining with annexinV-FITC and propidium iodide (mean and SEM of 2 independent experiments).
Because caspase-3 activity was undetectable in most human myeloma cells undergoing spontaneous apoptosis in vivo, we next tested the extent to which human myeloma cell lines undergo caspase-independent cell death in response to pharmacologic inducers of apoptosis. Myeloma lines were treated with the proteasome inhibitor, bortezomib, which is routinely used for the treatment of myeloma, and tunicamycin in the presence of the pan-caspase inhibitor zVADfmk. Apoptosis of KMS-11 cells in response to bortezomib and tunicamycin was almost completely blocked by zVADfmk and is therefore largely caspase-dependent (Figure 3C). In contrast, cell death of OPM-2 cells was only partly inhibited by zVADfmk after treatment with bortezomib, and tunicamycin-induced apoptosis of OPM-2 cells was unaffected by inhibition of caspases and therefore caspase-independent (Figure 3C). This observation shows that the 2 myeloma lines analyzed differed substantially in their use of caspase-independent pathways.
These findings substantiate our observations in the murine systems and extend them to primary human cells, providing further evidence that apoptosis of short-lived plasma cells is largely caspase-independent. They also suggest that a block on activation of key apoptotic caspases can occur in a proportion of human myelomas. Further investigations of patient samples are warranted to determine whether a clinically relevant block on caspase activation affects treatment outcome.
Plasma cell death is linked to excessive ER stress
Large-scale secretion of antibodies in plasma cells that have completed differentiation would be expected to result in substantial ER stress. This is likely to be exacerbated by the reduction in proteasomal capacity that has been reported in these cells.26 It is well established that ER stress is a potent inducer of caspase activation and apoptosis.11,21 Therefore, we set out to examine whether the block on caspase activation in plasma cells acts to prevent or delay apoptosis that would otherwise be triggered by the increasing ER stress generated by high levels of Ig synthesis.
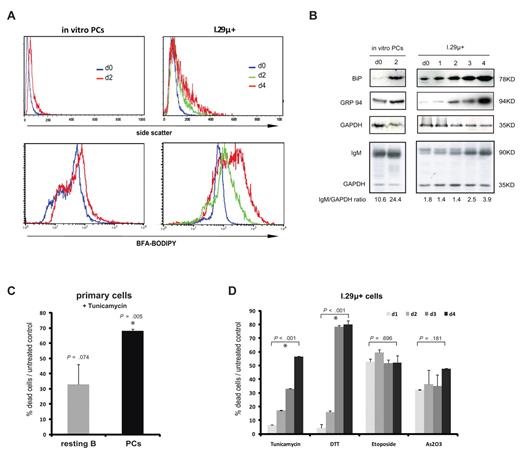
We first tested whether the secretory apparatus continues to expand in the in vitro–differentiated plasma cells used in the analysis. Increased light scattering of cells measured by flow cytometry indicates a higher organelle density and correlates well with the amount of ER present in B cells. We observed the expected increase in light scattering during plasmacytic differentiation of I.29μ+ cells in the first 2 days after LPS stimulation and a considerable further increase until day 4, when apoptosis took place (Figure 4A). This result was confirmed by direct staining of the secretory apparatus with Brefeldin A (BFA)-BODIPY (Figure 4A). Similarly, light scattering and staining with BFA-BODIPY increased between the time of purification of in vitro–differentiated plasma cells and the time when they underwent apoptosis (Figure 4A).
Plasma cell apoptosis is linked to excessive ER stress. (A) Side scatter analysis of organelle density (top panel) and staining of the secretory apparatus with BFA-BODILY (bottom panel) in in vitro–differentiated plasma cells and I.29μ+ cells. Plasma cells were analyzed immediately after purification (d0) and after 2 days in culture. I.29μ+ cells were analyzed before (d0) and 1 to 4 days after LPS stimulation. annexinV-FITC negative cells only were analyzed in the side scatter analysis to exclude nonspecific effects on light scattering in dying cells. Data are representative of 3 independent analyses (2 in in vitro–differentiated plasma cells). (B) Analysis of ER chaperones BiP and GRP94, IgM, and GAPDH (control). Immunoblotting was carried out on whole cell extracts prepared from in vitro–differentiated plasma cells at the time of purification (d0) and 2 days later, and I.29μ+ cells before (d0) and 1 to 4 days after LPS stimulation. (C-D) The normalized proportion of dead cells/untreated control (see Figure 2C) after treatment with the indicated inducers of apoptosis was determined by staining with annexin V–FITC and propidium iodide (mean and SEM of 2 independent experiments). (C) Primary ex vivo–isolated resting B and plasma cells were analyzed after treatment with 1 μg/mL tunicamycin for 14 hours. Viability of untreated controls is identical to Figure 2C. P values refer to the comparison of treated with control cells, and asterisks indicate statistical significance. (D) I.29μ+ cells 1 to 4 days after LPS stimulation were treated with tunicamycin as in panel D, DTT (1mM for 4 hours), etoposide (1 μg/mL for 16 hours), or arsenic trioxide As2O3 (1μM for 24 hours). Death rates of untreated controls are equivalent to those shown in Figure 1A and P values refer to the comparison between relative death rates on day 1 and day 4.
Plasma cell apoptosis is linked to excessive ER stress. (A) Side scatter analysis of organelle density (top panel) and staining of the secretory apparatus with BFA-BODILY (bottom panel) in in vitro–differentiated plasma cells and I.29μ+ cells. Plasma cells were analyzed immediately after purification (d0) and after 2 days in culture. I.29μ+ cells were analyzed before (d0) and 1 to 4 days after LPS stimulation. annexinV-FITC negative cells only were analyzed in the side scatter analysis to exclude nonspecific effects on light scattering in dying cells. Data are representative of 3 independent analyses (2 in in vitro–differentiated plasma cells). (B) Analysis of ER chaperones BiP and GRP94, IgM, and GAPDH (control). Immunoblotting was carried out on whole cell extracts prepared from in vitro–differentiated plasma cells at the time of purification (d0) and 2 days later, and I.29μ+ cells before (d0) and 1 to 4 days after LPS stimulation. (C-D) The normalized proportion of dead cells/untreated control (see Figure 2C) after treatment with the indicated inducers of apoptosis was determined by staining with annexin V–FITC and propidium iodide (mean and SEM of 2 independent experiments). (C) Primary ex vivo–isolated resting B and plasma cells were analyzed after treatment with 1 μg/mL tunicamycin for 14 hours. Viability of untreated controls is identical to Figure 2C. P values refer to the comparison of treated with control cells, and asterisks indicate statistical significance. (D) I.29μ+ cells 1 to 4 days after LPS stimulation were treated with tunicamycin as in panel D, DTT (1mM for 4 hours), etoposide (1 μg/mL for 16 hours), or arsenic trioxide As2O3 (1μM for 24 hours). Death rates of untreated controls are equivalent to those shown in Figure 1A and P values refer to the comparison between relative death rates on day 1 and day 4.
Expression of the major ER chaperones, glucose-regulated protein 94 (GRP94) and binding protein (BiP) is known to be up-regulated in response to ER stress.20 Therefore, we measured the levels of these proteins in extracts from LPS-stimulated I.29μ+ cells and in vitro–differentiated plasma cells. The levels of GRP94 and particularly BiP were found to increase from the time of purification of in vitro–differentiated plasma cells to the time when they underwent apoptosis (Figure 4B). In I.29μ+ cells, BiP protein levels increased during LPS-induced plasmacytic differentiation but continued to rise when differentiation was complete and apoptosis occurred (Figure 4B). Levels of GRP94 remained largely unchanged in the first 2 days after LPS-stimulation of I.29μ+ cells and only increased substantially when apoptosis started (Figure 4B). The increase in the levels of ER stress proteins was paralleled by the accumulation of intracellular IgM in both I.29μ+ cells and to an even greater degree in the in vitro–differentiated plasma cells (Figure 4B). The strong up-regulation of BiP and GRP94 in relation to the relatively mild increase in Ig levels could be due to a disproportional accumulation of misfolded proteins that is related to the impaired proteasomal capacity of plasma cells reported by Cenci et al.26
These results indicate that persistent high-level antibody secretion in plasma cells leads to secretory overload and substantial ER stress. Inhibition of key apoptotic caspases in plasma cells could act to block apoptotic signaling that is triggered by ER stress, which would allow the plasma cell to continue to secrete Ig. The resulting reprieve for the plasma cell would, however, be temporary. Overwhelming ER stress generated by continuing Ig synthesis would ultimately precipitate cell death via caspase-independent pathways, thereby creating an inbuilt limitation to the lifespan of the cell and the amount of Ig that it is able to secrete.
This model proposes that the fully differentiated short-lived plasma cell is finely balanced between the propensity to ER stress–induced apoptosis and the inhibition of cell death that is conferred by the block on activation of key apoptotic caspases. According to this model, any additional ER stress should enhance caspase-independent apoptotic signaling and thereby accelerate cell death, despite the block on activation of the executioner caspases. To test whether this is the case, the effect of the ER stress inducer tunicamycin on cell viability was analyzed. The results of this analysis showed that tunicamycin induced substantial apoptosis of primary ex vivo–isolated plasma cells, but had a limited effect on the viability of primary resting B cells (Figure 4C). We therefore further tested whether plasmacytic differentiation changes the susceptibility to ER stress–induced apoptosis by pharmacologic induction of ER stress in differentiating I.29μ+ cells. LPS-stimulated I.29μ+ cells were treated with the pharmacologic ER stressors tunicamycin and DTT on each day for 4 days of differentiation, and the proportion of cells that were killed by tunicamycin or DTT was determined. The results show that plasmacytic differentiation caused a progressive and substantial increase in susceptibility to ER stress–induced cell death (Figure 4D). In contrast, the susceptibility to apoptosis caused by etoposide (DNA damage) or arsenic trioxide (oxidative damage) remained unchanged with plasmacytic differentiation (Figure 4D).
Nuclear apoptotic events in plasma cells depend on caspase-12 activity
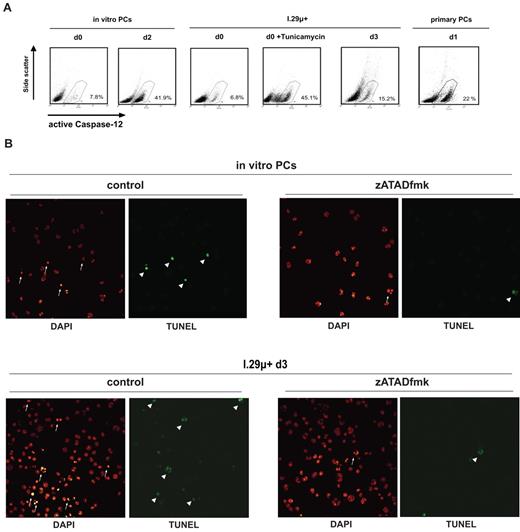
Activation of the inflammatory caspase, caspase-12, has been linked specifically to ER stress–mediated apoptosis in a variety of systems. It has therefore been suggested to function as an initiator caspase for ER stress–induced cell death, although a mechanism for caspase-12 involvement in apoptosis has not yet been identified.36-39 Caspase-12 activation leads to autoprocessing at D319 within the sequence ATAD.40 To test whether plasma cell apoptosis is associated with caspase-12 activation, cells were incubated with the FITC-conjugated cell permeable ATAD peptide, FITC-ATADfmk, which binds to active caspase-12. Treatment with FITC-ATADfmk detected active caspase-12 in in vitro–differentiated plasma cells undergoing apoptosis (Figure 5A). Furthermore, active caspase-12 was found in plasmacytic I.29μ+ cells undergoing programmed cell death, undifferentiated I.29μ+ cells treated with the ER stress inducer tunicamycin, and in ex vivo–isolated primary plasma cells undergoing apoptosis (Figure 5A). In contrast, activation of caspase-12 was not observed in freshly isolated nonapoptotic in vitro–differentiated plasma cells and undifferentiated I.29μ+ cells (Figure 5A).
Caspase-12 mediates nuclear apoptotic events during programmed plasma cell death. (A) Analysis of caspase-12 activation by staining with FITC-ATADfmk and flow cytometry. In vitro–differentiated plasma cells were analyzed at the time of purification (d0) and 2 days later. Undifferentiated (d0) I.29μ+ cells were analyzed before and after treatment with tunicamycin (1 μg/mL for 14 hours) and 3 days after LPS stimulation. Primary splenic plasma cells were analyzed 1 day after ex vivo isolation. Numbers indicate percentages of cells with positive staining from a representative experiment. (B) Freshly isolated in vitro–differentiated plasma cells and plasmacytic (d3) I.29μ+ cells were treated with the caspase-12 inhibitor zATADfmk (10μM) for 24 hours and stained with DAPI and for TUNEL-positive DNA fragmentation. Arrows and arrowheads indicate examples of nuclei with apoptotic chromatin condensation and TUNEL-positive DNA breaks, which were reduced significantly by treatment with zATADfmk (supplemental Figure 2D), in representative fields.
Caspase-12 mediates nuclear apoptotic events during programmed plasma cell death. (A) Analysis of caspase-12 activation by staining with FITC-ATADfmk and flow cytometry. In vitro–differentiated plasma cells were analyzed at the time of purification (d0) and 2 days later. Undifferentiated (d0) I.29μ+ cells were analyzed before and after treatment with tunicamycin (1 μg/mL for 14 hours) and 3 days after LPS stimulation. Primary splenic plasma cells were analyzed 1 day after ex vivo isolation. Numbers indicate percentages of cells with positive staining from a representative experiment. (B) Freshly isolated in vitro–differentiated plasma cells and plasmacytic (d3) I.29μ+ cells were treated with the caspase-12 inhibitor zATADfmk (10μM) for 24 hours and stained with DAPI and for TUNEL-positive DNA fragmentation. Arrows and arrowheads indicate examples of nuclei with apoptotic chromatin condensation and TUNEL-positive DNA breaks, which were reduced significantly by treatment with zATADfmk (supplemental Figure 2D), in representative fields.
We next investigated if caspase-12 has a functional role in plasma cell apoptosis using the caspase-12 inhibitor, zATADfmk, which we found to be specific (supplemental Figure 2A-B). The zATADfmk did not block apoptotic cell membrane changes as measured by annexinV-FITC and propidium iodide staining, nor did it have an impact on loss of the mitochondrial transmembrane potential ΔψM in in vitro–differentiated plasma cells and plasmacytic I.29μ+ cells (data not shown). Because caspase-12 has been suggested to contribute specifically to nuclear apoptotic events,41 we also investigated the impact of caspase-12 inhibition on chromatin condensation and TUNEL-positive DNA breaks. The zATADfmk largely blocked typical apoptotic nuclear condensation and DNA breaks in in vitro–differentiated plasma cells and plasmacytic I.29μ+ cells (Figure 5B). In contrast, nuclear apoptotic events in response to tunicamycin were not blocked by inhibition of caspase-12 in undifferentiated I.29μ+ cells, which can activate classical apoptotic caspases (supplemental Figure 2C). Thus, caspase-12 activity is required for nuclear apoptotic events specifically in plasma cells, thereby directly linking ER stress with programmed plasma cell death.
Absence of active apoptotic effector caspases delays ER stress–induced apoptosis in nonlymphoid cells
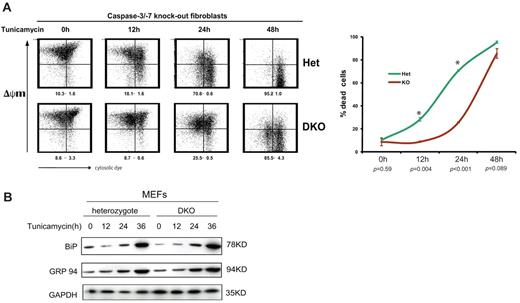
As a further test of the model, we used double-knockout (DKO) MEFs that lack caspase-3 and caspase-7 to examine the effects of ER stress on cell survival in the absence of effector caspases. Masud and colleagues have recently shown that the DKO MEFs are largely resistant to apoptosis executed through the intrinsic pathway, whereas cells containing one allele of caspase-3 and caspase-7 (heterozygotes) undergo programmed cell death normally.21 Consistent with these findings, we found that only a small proportion of DKO MEFs showed apoptotic loss of ΔψM after 24 hours of tunicamycin-induced ER stress, whereas loss of ΔψM was observed in most heterozygote MEFs during the same time period (Figure 6A). However, when ER stress persisted for longer, apoptotic loss of ΔψM ultimately occurred not only in heterozygote MEFs but also in DKO MEFs (Figure 6A). As shown by immunoblotting for the major ER chaperones BiP and GRP94, the increase in ER stress was the same in heterozygote and DKO MEFs (Figure 6B) and was reminiscent of, albeit faster than, the rise in ER stress seen during plasmacytic differentiation of I.29μ+ cells (Figure 4B). These observations therefore demonstrate that nonlymphoid cells that cannot activate key effector caspases can also tolerate progressively increasing levels of ER stress for longer periods before undergoing programmed cell death. They suggest that a fundamental property of the cellular response to ER stress is used to contribute to the regulation of the life-span of short-lived plasma cells.
Lack of active key apoptotic caspases delays ER stress–induced cell death. Caspase-3 and caspase-7 DKO and heterozygote MEFs were treated with tunicamycin (1 μg/mL) for the indicated times, and apoptotic loss of mitochondrial membrane potential ΔψM was analyzed by staining with MitoCapture and flow cytometry. (A) Representative plots are shown and the number of cells that have lost their ΔψM is indicated (mean and SEM of 2 independent experiments). The graph shows the number of apoptotic DKO and heterozygote MEFs. P values refer to the comparison of mean apoptosis rates between DKO and heterozygote MEFs, and asterisks indicate significant differences. (B) Immunoblotting with antibodies against BiP, GRP94, and GAPDH (control) carried out on extracts from DKO and heterozygote MEFs after treatment with tunicamycin (1 μg/mL) for the indicated times.
Lack of active key apoptotic caspases delays ER stress–induced cell death. Caspase-3 and caspase-7 DKO and heterozygote MEFs were treated with tunicamycin (1 μg/mL) for the indicated times, and apoptotic loss of mitochondrial membrane potential ΔψM was analyzed by staining with MitoCapture and flow cytometry. (A) Representative plots are shown and the number of cells that have lost their ΔψM is indicated (mean and SEM of 2 independent experiments). The graph shows the number of apoptotic DKO and heterozygote MEFs. P values refer to the comparison of mean apoptosis rates between DKO and heterozygote MEFs, and asterisks indicate significant differences. (B) Immunoblotting with antibodies against BiP, GRP94, and GAPDH (control) carried out on extracts from DKO and heterozygote MEFs after treatment with tunicamycin (1 μg/mL) for the indicated times.
Discussion
Most plasma cells that are generated in the immediate immune response are short-lived and undergo apoptosis in secondary lymphoid organs after a few days, indicating the existence of mechanisms that maintain a controlled early humoral immune response by triggering apoptosis after a short period of antibody secretion. Surprisingly, our results show that activation of caspase-9 and caspase-3 is blocked in short-lived plasma cells, and that apoptosis of short-lived plasma cells in vitro and in vivo is largely independent of the key apoptotic caspases. These observations suggest that short-lived plasma cells have evolved specialized mechanisms for regulating the timing of apoptosis.
A second intriguing finding of our study is that short-lived plasma cells are particularly susceptible to apoptosis induced by agents such as tunicamycin that induce ER stress. This is unexpected since plasma cells are equipped with an extensive secretory apparatus, show a strong UPR response, and up-regulate ER chaperones such as GRP94 and BiP. Furthermore, activation of ER-associated and inflammatory caspase-12 during programmed plasma cell death is required for nuclear apoptotic changes. These findings indicate that the cellular ER stress response becomes inadequate to protect short-lived plasma cells from initiating apoptotic signaling in response to secretory overload. The block on activation of the key apoptotic caspases would have the effect of inhibiting ER stress–initiated plasma cell death. Our results using caspase-3/caspase-7 DKO fibroblasts demonstrate that cell survival under conditions of ER stress in the absence of functional classical effector caspases is a temporary phenomenon and that persistent ER stress precipitates delayed apoptosis through pathways that do not involve key apoptotic caspases. Additional support for the idea that ER stress is involved in plasma cell death comes from the observation that mice lacking the proapoptotic protein Bim, which has been shown to be a key mediator of ER stress–induced apoptosis, accumulate plasma cells, and develop autoimmune kidney disease.4,42 Our findings also provide an explanation for the observation that the proapoptotic protein Bax becomes activated at the ER in plasma cells.43
Based on the results of this study, we propose a model in which the combined effects of ER stress and inhibition of key apoptotic caspases play an important role in regulating the timing of programmed cell death in short-lived plasma cells. According to this model, synthesis of large amounts of antibodies leads to the accumulation of misfolded polypeptides and progressively increasing ER stress. However, apoptotic signaling is delayed by the block on activation of key caspases, which allows antibody secretion to continue despite the secretory overload. When ER stress becomes excessive, plasma cell apoptosis is eventually executed by effector caspase-independent signaling pathways, thereby ending the early humoral immune response for a specific antibody. It will be important to investigate whether inhibition of apoptotic capases contributes to the prolonged survival of long-lived plasma cells. Furthermore, it is conceivable that the longevity of these cells is, at least in part, promoted by a lower level of secretory stress. Further investigations should be aimed at elucidating the interplay between ER stress and apoptotic signaling in short-lived and long-lived plasma cells in vivo, and its potential impact on normal and pathologic immune responses. Although our results provide evidence that intrinsic mechanisms play an important role in determining the life-span of short-lived plasma cells, it is likely that extrinsic signals provided by the microenvironment of lymphoid organs also contribute to the regulation of plasma cell survival and death.
The precise mechanisms that block activation of caspase-3 and caspase-9 in short-lived plasma cells have still to be determined. Nitrosylation of cytochrome c on its heme group is important for activation of caspases and induction of apoptosis, and this process is sensitive to the redox state of the cell.44 Ig chains enter the ER in a reduced state and undergo oxidative folding by ER resident enzymes, which causes redox stress in plasma cells.45 Changes in the redox balance in plasma cells provide a possible mechanism for reducing nitrosylation of cytochrome c and therefore its ability to activate caspase-9.
The results of this study demonstrate a specific role for caspase-12 in nuclear apoptotic events in murine short-lived plasma cells. However, plasma cell death itself, as defined by a breakdown of cell membrane integrity and mitochondrial function, did not depend on caspase-12 activity. It is important to note that nuclear condensation and fragmentation, although characteristic features of apoptosis, are neither required for the execution of programmed cell death nor for phagocytosis of apoptotic cells.46,47 Because caspase-12 has been associated specifically with ER stress in several studies,36-39 our findings on caspase-12 provide further evidence for a functional link between ER stress and apoptosis of short-lived plasma cells. However, they also support the view that caspase-12 is not universally required for the execution of apoptosis.48 Given that functional human caspase-12 is expressed in only 20% of humans of African descent,49 it will be interesting to investigate if caspase-12 or its putative ortholog, caspase-4, play a role in apoptosis of plasma cells or myeloma cells in humans.
Our findings in human myeloma cells in vivo and in human myeloma cell lines suggest that inhibition of apoptotic caspases can also occur in some myelomas. Pharmacologic approaches that interfere with the mechanisms that mediate inhibition of caspases or enhance caspase-independent apoptotic signaling could provide novel strategies for the treatment of plasma cell-related diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Richard Flavell for the caspase-3 and caspase-7 DKO and heterozygote MEFs, Simone Cenci for I.29μ+ cells, Junia Melo for OPM-2 cells, Anastasios Karadimitris for KMS-11 and UMS266 cells, and Andrew Georgiou for technical advice. We also thank Simone Cenci, Heinz Sill, Eric Lam, and Anne-Marie Moody for stimulating discussions.
This work was funded by a Kay Kendall Leukaemia Fund Research Fellowship to H.W.A. and by the Medical Research Council United Kingdom. H.W.A. was also supported by Leukaemiehilfe Steiermark.
Authorship
Contributions: H.W.A., N.D., and P.S. conceived the study; H.W.A., P.S., and C.B.S. performed the experiments; H.W.A. wrote the manuscript; and P.S. and N.D. revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Holger W. Auner, Gene Regulation and Chromatin Group, MRC Clinical Sciences Centre, Imperial College Faculty of Medicine, Hammersmith Hospital Campus, Du Cane Rd, London W12 0NN, United Kingdom; e-mail: holger.auner@csc.mrc.ac.uk; or Pierangela Sabbattini, Gene Regulation and Chromatin Group, MRC Clinical Sciences Centre, Imperial College Faculty of Medicine, Hammersmith Hospital Campus, Du Cane Rd, London W12 0NN, United Kingdom; e-mail: pierangela.sabbattini@csc.mrc.ac.uk.

![Figure 2. Stabilization of a safety-catch in caspase-3 in plasma cells. (A) Immunoblotting on whole cell extracts prepared from undifferentiated (d0) and plasmacytic (d3) I.29μ+ cells that had been treated for 9 hours with 10μM PAC-1 or DMSO (control). Images are from the same blot. (B) Fold increase in apoptotic loss of ΔψM in undifferentiated (d0) and plasmacytic (d3) I.29μ+ cells after treatment with PAC-1 for 9 hours (mean and SEM of 2 independent experiments). P values refer to the comparison between PAC-1 treated and control cells, and statistically significant differences between means are indicated by an asterisk. (C) Apoptosis (positive staining with annexinV-FITC and/or propidium iodide) after 14 hours of treatment with PAC-1 in primary resting B and plasma cells. To account for different baseline viabilities, relative cell death (percent dead cells/untreated control) was expressed as the difference in cell death in the absence (A) and presence (P) of a drug, normalized by the cell death achievable in the control cells [(P - A)/(100 - A) × 100]. Mean and SEM of 2 independent experiments. (D) Immunoblotting of whole cell extracts from splenic B cells 3 days after in vitro activation with LPS and freshly isolated in vitro–differentiated plasma cells. (E) Immunoblotting of whole cell extracts from the indicated human (KMS-11, RPMI8226, U266, OPM-2) and mouse (MPC-11) myeloma cell lines that had been treated with PAC-1 for 14 hours.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/18/10.1182_blood-2009-10-250423/4/m_zh89991059340002.jpeg?Expires=1764993051&Signature=Ur5NZtroVeeEv8cY7v7dzd6iNhDD9VM5ejRNNjQh8tlgmJcldlBmRnrffPRxf9okKdBoAZp1Ilw7rUhrosIAzm6iV1Xrt76HxvE0-W~lEeCmObAiuPU~3o99LeohPAx-qIynKOcIarOGhwSBsVOVRIL~9ih2F47zcCcpAQKQuFukREH94n-ZCqiDj6UtWU8fb5fOg8BYxqLVbhmbQrZ29MQWaY~i6f8w~BHOPK3GZLYoeJUS79LxeCQXNQAW37-OFdFYJC-ZLD574nk9q61~SGcjWfCI2AZ8YfzzH-uCn3SGHWAsV1KGtK0mLCtbzUYGBWxs3aDPE6cCwFiqO8aDRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal