Abstract
X-linked lymphoproliferative disease (XLP) is a condition associated with mutations in the signaling lymphocytic activation molecule (SLAM)–associated protein (SAP; SH2D1A). SAP functions as an adaptor, binding to and recruiting signaling molecules to SLAM family receptors expressed on T and natural killer cells. XLP is associated with extreme sensitivity to primary Epstein-Barr virus (EBV) infection, often leading to a lethal infectious mononucleosis. To investigate EBV-specific immunity in XLP patients, we studied 5 individuals who had survived EBV infection and found CD8+ T-cell responses numerically comparable with healthy donors. However, further investigation of in vitro–derived CD8+ T-cell clones established from 2 of these donors showed they efficiently recognized SLAM ligand–negative target cells expressing EBV antigens, but showed impaired recognition of EBV-transformed, SLAM ligand–positive, lymphoblastoid cell lines (LCLs). Importantly, LCL recognition was restored when interactions between the SLAM receptors CD244 and natural killer–, T-, and B-cell antigen (NTBA) and their ligands on LCLs were blocked. We propose that XLP patients' particular sensitivity to EBV, and not to other viruses, reflects at least in part EBV's strict tropism for B lymphocytes and the often inability of the CD8+ T-cell response to contain the primary infection of SLAM ligand–expressing target cells.
Introduction
X-linked lymphoproliferative disease (XLP) is an X-linked primary immunodeficiency of young males most commonly manifest when they become infected with Epstein-Barr virus (EBV), a γ-herpesvirus with growth transforming ability for its principal target, the B lymphocyte. In immunocompetent individuals primary EBV infection is usually asymptomatic but may cause an acute febrile illness, infectious mononucleosis, characterized by extreme but transient expansion of EBV-specific CD8+ T cells and rapid control of B-cell infection in lymphoid tissues.1 In a high proportion of XLP patients, EBV infection results in an exaggerated and often fatal infectious mononucleosis-like disease associated with polyclonal B- and CD8+ T-cell expansions which infiltrate the liver and bone marrow leading to hepatic necrosis and bone marrow aplasia, with some evidence of hemophogocytosis.2 Other phenotypes seen in XLP include the development of malignancies, predominantly of B-cell origin such as Burkitt lymphoma, or hypogammaglobulinaemia, which may predate or be unrelated to EBV exposure.3
The genetic defect in XLP patients has been mapped to mutations in the signaling lymphocytic activation molecule (SLAM; CD150) associated protein (SAP). This small SH2 domain containing protein binds to immunoreceptor tyrosine-based switch motifs present in the cytoplasmic tails of SLAM receptor family membrane proteins, which include CD150, CD244, natural killer (NK)–, T-, and B-cell antigen (NTBA), CD84, CD229, and CD2-like receptor–activating cytotoxic cell (CRACC; reviewed in Ma et al4 ). In the best characterized case of SAP binding to CD150, SAP recruits the protein tyrosine kinase FynT to CD150, allowing phosphorylation of this receptor and other substrates involved in signaling.5 These receptors are expressed in different combinations on T cells, B cells, NK cells, and NKT cells as well as some other cell types and mostly act through homotypic interactions; the exception being CD244 whose ligand is CD48, which is up-regulated on B cells upon EBV infection.6 The functions mediated by this family of receptors are complex and dependent on the cell type being assayed. Ligation of these receptors on NK cells can stimulate cytotoxicity and cytokine secretion,7-9 whereas in the case of T cells, costimulation of these receptors with the T-cell receptor promotes proliferation and modulates cytokine secretion.10,11
The lack of SAP expression by XLP patients causes many defects within the immune system, although how these contribute to sensitivity to EBV infection is not completely understood. These individuals are hypogammaglobulinemic, due to inappropriate B-cell help by T cells.12-14 NKT cells are absent as their development requires appropriate expression of FynT;15-17 however, no obvious role for NKT cells controlling EBV infection has been described. Functionally, NK cells appear normal when triggered through receptors such as CD16; however, they do not show the usual activation of NK cytotoxicity when the SAP binding receptors are engaged.7,8,18,19 XLP NK cells fail to kill SLAM receptor–expressing EBV-infected B-cell targets, leading to the suggestion that this NK defect underlies the patients poor response to EBV.7,8 However, these studies assessed XLP NK cell function against EBV-infected cell lines which lack class I expression or where class I molecules were masked and are not likely to represent in vivo EBV-infected B cells.
Control of EBV infection in immunocompetent donors is critically dependent on T-cell immunity.20 EBV-specific cellular immunity was suggested to be compromised in XLP patients as peripheral blood mononuclear cells (PBMCs) from most patients could not mediate regression of EBV transformed B-cell outgrowth in vitro.21,22 Understanding this defect is difficult, however, as regression likely relies on collaborations between CD4+ and CD8+ T cells and perhaps other cell types in the PBMC population.23 T-cell lines from XLP patients stimulated with EBV transformed B-lymphoblastoid cell lines (LCLs) showed poor function and this was associated with the inability to polarize CD244 and perforin to the immunologic synapse.24,25 However, whether these T-cell lines were EBV-specific was not determined; indeed some were allospecific. Only recently have EBV-specific CD8+ T-cell responses been demonstrated in 2 patients, and the functional ability of these cells is largely unknown.26 In the present study we examined EBV-specific CD8+ T-cell immunity in XLP patients and found that they make responses of a comparable size to healthy donors. The function of these cells was impaired when challenged with their cognate targets, EBV transformed LCLs, but activity could be restored by blocking SLAM receptor interactions.
Methods
Donors
Peripheral blood samples were collected from XLP patients and healthy laboratory donors with a history of EBV infection. Mononuclear cells were separated and cryopreserved using standard procedures. Participants gave written informed consent in accordance with the Declaration of Helsinki. Experiments were approved by the South Birmingham Local Research Ethics Committee or the Central Sydney Area Health Service Human Research Ethics Committee. SAP mutations of the XLP donors have been previously described and do not give rise to detectable protein: XLP1 nucleotide 500 giving a G-to-T change mutating a splice junction (donor P3 in Gilmour et al27 ), XLP8 amino acid 67 glutamic acid to glycine (G. de St Basile, written personal communication, February 2010), XLP11 and 12 amino acid 87 phenylalanine to serine, donor XLP13 amino acid 84 isoleucine to threonine (donors XLP3, 2, and 8, respectively in Hare et al28 ).
ELISpot assays
Enzyme-linked immunosorbent spot (ELISpot) assays were conducted on serial dilutions of thawed PBMCs as described previously.23 Epitope peptides were used at a concentration of 2 μg/mL and are identified by the first 3 or 4 amino acids in the peptide sequence. The epitope peptides used and their source proteins are the human leukocyte antigen (HLA) B*3501-restricted epitopes EPLPQGQLTAY from BZLF1 and YPLHEQHGM from EBNA3A, the B*2705-restricted epitopes, RRIYDLIEL from EBNA3C, HRCQAIRKK from EBNA3B, RRRWRRLTV from LMP2, KRPPIFIRRL from EBNA3A, ARYAYYLQF from BALF2, the B*4402-restricted epitopes VEITPYKPTW from EBNA3B and EENLLDFVRF from EBNA3C, the A*0201-restricted epitopes YVLDHLIVV from BRLF1, GLCTLVAML from BMLF1, TLDYKPLSV from BMRF1, and CLGGLLTMV from LMP2.
T-cell and NK-cell cultures
CD8+ T-cell clones were established from PBMC samples by enriching for antigen-specific cells either by stimulating with epitope peptides and selecting interferon-γ (IFN-γ) secreting cells using an IFN-γ cell enrichment kit (Miltenyi Biotec) or by staining with appropriate phycoerythrin-conjugated HLA class I tetramers and isolating cells with antiphycoerythrin MACS beads (Miltenyi Biotec). Enriched cells were then subjected to limiting dilution cloning and maintained as described previously.29
NK cell clones were derived from PBMCs of donors by depletion of CD3+ cells with OKT3 antibody (eBioscience) and anti-mouse Dynabeads (Invitrogen). Clones were derived from this fraction by limiting dilution cloning29 and maintained in RPMI 1640 medium supplemented with 10% fetal calf serum, 1% human serum, 30% supernatant from the MLA-144 cell line, and 200 U/mL IL-2. Alternatively NK cell lines were generated by selecting CD56-expressing cells from PBMCs using anti-CD56 MACS beads (Miltenyi Biotec) and maintained in the same medium as the NK cell clones.
T-cell and NK-cell recognition experiments
CD8+ T-cell recognition of target cells was measured either by IFN-γ secretion or cytotoxicity assay. For IFN-γ secretion assays, 50 000 target cells were cocultured in triplicate with 5000 T cells in V-bottomed 96-well plates in 100 μL of RPMI 1640 10% fetal calf serum for 18 hours. Fifty microliters of culture supernatant from each well was assayed for IFN-γ by ELISA (Endogen). Target cells were either EBV-transformed B cells generated and maintained as described previously,30 or B-cell blasts generated by culturing PBMCs on CD40-expressing L cells in medium supplemented with IL-4 (100 U/mL), 10% human serum, and cyclosporin A (1 μg/mL), or skin fibroblasts maintained in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum. In cytotoxicity assays, target cells were either infected with recombinant vaccinia viruses expressing EBV antigens, at a multiplicity of infection of 10, cultured for 18 hours, and loaded with sodium chromate 51, or were sensitized with cognate peptide at a concentration of 5 μg/mL while loading with sodium chromate 51. After extensive washing, these cells were incubated with the T cells in standard 5-hour cytotoxicity assays. For NK cell assays, the NK-sensitive cell lines Daudi and K562 were loaded with sodium chromate 51, washed extensively, and incubated with the NK effectors in 5-hour assays. Statistical comparisons made between groups initially used the Kruskal-Wallis test and where significant differences in medians were seen, data were further analyzed by the Mann-Whitney U test to identify significant differences within groups.
Where antibody blocking experiments were conducted, T cells or LCLs were incubated with the relevant antibodies at a final concentration of 10 μg/mL for 30 minutes at room temperature before being mixed in the relevant assay.
Flow cytometric analysis of SLAM receptor expression on CD8+ PBMCs and T-cell clones
PBMCs or T-cell clones were stained with monoclonal antibodies specific for NTBA, CD84, CRACC (R&D Systems), CD150, CD244, CD229, or isotype-matched antibodies (BD Biosciences). Bound antibodies were detected using goat anti-mouse FITC labeled antibodies (Southern Biotech), and the cells costained with Tricolor conjugated anti-CD8 antibodies (Caltag Medsystems). Cells were analyzed on either an Epics II flow cytometer (Beckman Coulter) or an LSR II cytometer (BD Biosciences) and data processed using FlowJo software Version 7.2.2 (TreeStar).
Results
CD8+ T-cell responses to EBV in XLP patients
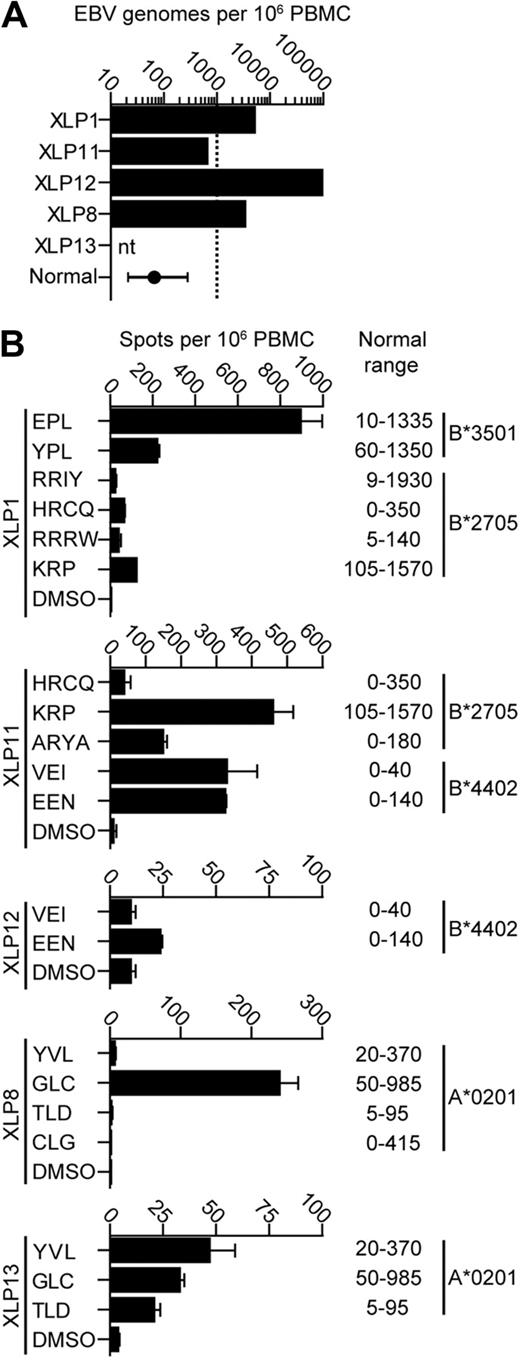
In a previous series of experiments, 4 of 5 XLP donors were characterized for their circulating EBV latent virus loads by quantitative polymerase chain reaction and found to have loads at the upper level or above the normal range seen in healthy carriers31 (Figure 1A). We used PBMCs from the same samples in IFN-γ ELISpot assays to identify EBV-specific CD8+ T-cell responses. Figure 1B shows histograms of ELISpot data from these patients tested against dominant and subdominant epitopes relevant to the donor's HLA type and the range of responses to these epitopes observed in 10 healthy donors. XLP donors made relatively strong responses to immunodominant lytic cycle epitopes such as EPL and GLC, ranging from 200-900 spot-forming units per million PBMCs. Variable but lower responses to the subdominant latent epitopes were seen, with responses ranging from 0-450 spot-forming units per million PBMCs. Overall, the size of responses measured in the donors fell within the normal range for the epitopes tested but, with the exception of XLP11, were lower than the reported median values for such responses.32
EBV virus loads and EBV-specific CD8+T-cell responses in PBMCs from 5 XLP patients. (A) EBV genome load in 106 PBMCs was estimated by quantitative polymerase chain reaction analysis. The normal donor value reported represents the median and the 25-75 percentile range of 600 healthy donors, while the dashed line indicates the upper level of the normal range observed in healthy donors. nt indicates not tested. (B) IFN-γ ELISpot analysis of EBV-specific PBMCs taken from 5 XLP donors. PBMCs were stimulated with known immunodominant EBV epitopes appropriate for their HLA type or the peptide solvent dimethyl sulfoxide (DMSO)as a control. Normal ranges of responses from healthy donors are shown.
EBV virus loads and EBV-specific CD8+T-cell responses in PBMCs from 5 XLP patients. (A) EBV genome load in 106 PBMCs was estimated by quantitative polymerase chain reaction analysis. The normal donor value reported represents the median and the 25-75 percentile range of 600 healthy donors, while the dashed line indicates the upper level of the normal range observed in healthy donors. nt indicates not tested. (B) IFN-γ ELISpot analysis of EBV-specific PBMCs taken from 5 XLP donors. PBMCs were stimulated with known immunodominant EBV epitopes appropriate for their HLA type or the peptide solvent dimethyl sulfoxide (DMSO)as a control. Normal ranges of responses from healthy donors are shown.
Impaired recognition of B-cell targets by cell-mediated immune effectors from XLP patients
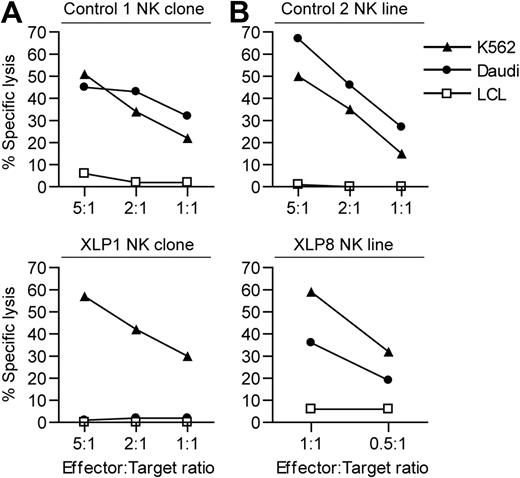
We next examined the in vitro function of the cell-mediated immune response of our 2 most accessible donors, XLP1 and XLP8. To confirm their SAP mutations give a phenotype, their NK cells were tested against 2 NK sensitive targets: Daudi, a Burkitt lymphoma cell line that expresses SLAM family receptors, and K562, which does not (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). XLP NK effectors poorly kill SLAM family–expressing targets due to interactions between these receptors on the effector and target cells.7,8 NK clones and lines were established from XLP1 and XLP8 as well as from healthy donors and were used in cytotoxicity assays. In single experiments, good levels of killing of K562 and Daudi cells were observed by the healthy donor–derived NK cells (Figure 2A-B). XLP-derived NK cells showed good killing of the K562 targets; indeed the XLP8-derived effectors were highly potent. However, in parallel assays XLP1 NK cells were unable to kill Daudi cells, while XLP8 NK cells showed decreased killing relative to that seen against K562, suggesting that these patients' SAP mutations conferred at least some functional defect to the NK cells.
XLP NK cell cytotoxicity against NK-sensitive target cell lines. The NK-sensitive cell lines K562 and Daudi or as a control an LCL were used as targets in chromium release cytotoxicity assays with: (A) NK clones derived from a healthy donor (top panel) or XLP1 (bottom panel) or (B) NK lines established from a healthy donor (top panel) or XLP8 (bottom panel). Results are expressed as the percentage of chromium release from the target cells at the different effector:target ratios.
XLP NK cell cytotoxicity against NK-sensitive target cell lines. The NK-sensitive cell lines K562 and Daudi or as a control an LCL were used as targets in chromium release cytotoxicity assays with: (A) NK clones derived from a healthy donor (top panel) or XLP1 (bottom panel) or (B) NK lines established from a healthy donor (top panel) or XLP8 (bottom panel). Results are expressed as the percentage of chromium release from the target cells at the different effector:target ratios.
To study the function of XLP donors' EBV-specific CD8+ T cells, clones from XLP1 and XLP8 were generated using techniques that efficiently reactivate EBV-specific memory cells from healthy donors. Clones specific to the HLA-B*2705-presented epitope KRP, derived from the EBV latent protein EBNA3A, and the HLA-B*3501-presented epitope EPL, derived from the lytic cycle–expressed BZLF1 protein, were established from XLP1. Clones specific for the HLA-A*0201-presented epitopes YVL and GLC derived from the EBV lytic cycle proteins BRLF1 and BMLF1, respectively, were generated from XLP8.
T-cell function was assessed by measuring IFN-γ production from the clones after challenging them with EBV transformed B-cell lines (LCLs). LCLs constitutively express EBV latent proteins, including EBNA3A, and a small percentage will contain virus undergoing lytic-cycle replication. Two independently derived KRP-specific T-cell clones from XLP1 were compared with equivalent clones derived from healthy donors for their ability to recognize the XLP1 LCL or a negative control HLA mismatched LCL (Figure 3A). Healthy donor–derived T cells produced high levels of IFN-γ on challenge with the XLP1 LCL, indicating this antigen was efficiently processed and presented by XLP LCLs while little IFN-γ was produced in response to the HLA mismatched LCL (Figure 3A left panel, one representative result of 3 clones tested). By contrast, the XLP1-derived T-cell clones produced little IFN-γ when challenged with autologous LCLs in the same experiment (Figure 3A middle and right panels). However, these clones produced high levels of IFN-γ when the LCL was sensitized with KRP-peptide (5 μg/mL), indicating that the T-cell clones could produce IFN-γ and that high peptide display can override the block to LCL recognition. This pattern of results was also observed using other B*2705-expressing target LCLs (data not shown).
Recognition of LCLs by EBV-specific CD8+T-cell clones from XLP patients or healthy carriers. ELISA assays were used to estimate IFN-γ secretion from CD8+ T-cell clones incubated overnight with the different target LCLs. (A) IFN-γ secretion by KRP-specific T-cells from a healthy carrier (left panel) or 2 independently derived clones from XLP1 that had been incubated with either XLP1's LCL, an HLA mismatched LCL, or KRP-peptide sensitized XLP1 LCL. (B) IFN-γ secretion by EPL-specific T cells from a healthy carrier (left panel) or 2 independently derived clones from XLP1 that had been incubated with either an HLA B35 matched LCL (BZ+ LCL), an HLA B35 matched BZLF1 knock out EBV lytic antigen–negative LCL (ΔBZ LCL), or HLA B35 matched EPL peptide–sensitized LCL. IFN-γ secretion by (C) GLC-specific T cells and (D) YVL-specific T cells from a healthy carrier (left panels) or 2 independently derived clones from XLP8 that had been incubated with either an HLA A2 matched LCL (BZ+ LCL), an HLA A2–matched BZLF1 knockout EBV lytic antigen–negative LCL (ΔBZ LCL), or an HLA–A2 matched LCL sensitized with the appropriate peptide.
Recognition of LCLs by EBV-specific CD8+T-cell clones from XLP patients or healthy carriers. ELISA assays were used to estimate IFN-γ secretion from CD8+ T-cell clones incubated overnight with the different target LCLs. (A) IFN-γ secretion by KRP-specific T-cells from a healthy carrier (left panel) or 2 independently derived clones from XLP1 that had been incubated with either XLP1's LCL, an HLA mismatched LCL, or KRP-peptide sensitized XLP1 LCL. (B) IFN-γ secretion by EPL-specific T cells from a healthy carrier (left panel) or 2 independently derived clones from XLP1 that had been incubated with either an HLA B35 matched LCL (BZ+ LCL), an HLA B35 matched BZLF1 knock out EBV lytic antigen–negative LCL (ΔBZ LCL), or HLA B35 matched EPL peptide–sensitized LCL. IFN-γ secretion by (C) GLC-specific T cells and (D) YVL-specific T cells from a healthy carrier (left panels) or 2 independently derived clones from XLP8 that had been incubated with either an HLA A2 matched LCL (BZ+ LCL), an HLA A2–matched BZLF1 knockout EBV lytic antigen–negative LCL (ΔBZ LCL), or an HLA–A2 matched LCL sensitized with the appropriate peptide.
The ability of EPL-specific T-cell clones from XLP1 to recognize LCLs was then tested by challenging these with HLA-B*3501-matched LCLs, where approximately 0.5%-5% of LCLs express lytic cycle proteins (BZ+). As a negative control, LCLs were established from the same target cell donor using a recombinant EBV that had the BZLF1 lytic switch gene deleted (ΔBZ), preventing EBV lytic cycle replication in these LCLs and allowing them to act as HLA-matched antigen–negative targets. Consistent with previous findings,29,30 incubation of healthy donor–derived EPL-specific T-cell clones with the BZ+ LCL induced modest IFN-γ production (Figure 3B left panel), reflecting the low percentage of cells expressing lytic-cycle antigens. No IFN-γ was produced when the cells were incubated with the ΔBZ LCL. Incubation of the XLP1 EPL-specific T cells with the BZ+ LCL targets induced much lower amounts of IFN-γ production while no IFN-γ was produced in response to the ΔBZ LCL. In all cases, T cells produced high levels of IFN-γ in response to EPL peptide–sensitized ΔBZ LCL. Similar results were found using a second matched pair of HLA-B*3501 BZ+ and ΔBZ LCL targets (data not shown).
More striking results were observed in recognition experiments using XLP8-derived T-cell clones specific for the lytic epitopes GLC and YVL. These XLP8 specificities produced no IFN-γ when challenged with the BZ+ or ΔBZ LCL (Figure 3C-D, 2 representative clones of 4) while in parallel assays, healthy donor–derived clones produced good levels of IFN-γ in response to the BZ+ LCL. XLP and healthy donor clones produced high levels of IFN-γ in response to peptide-sensitized LCLs. This pattern of results was observed using these T-cell specificities against other HLA matched BZ+ and ΔBZ LCLs (data not shown).
T-cell clone function against antigen-presenting B-cell targets versus non–B-cell targets
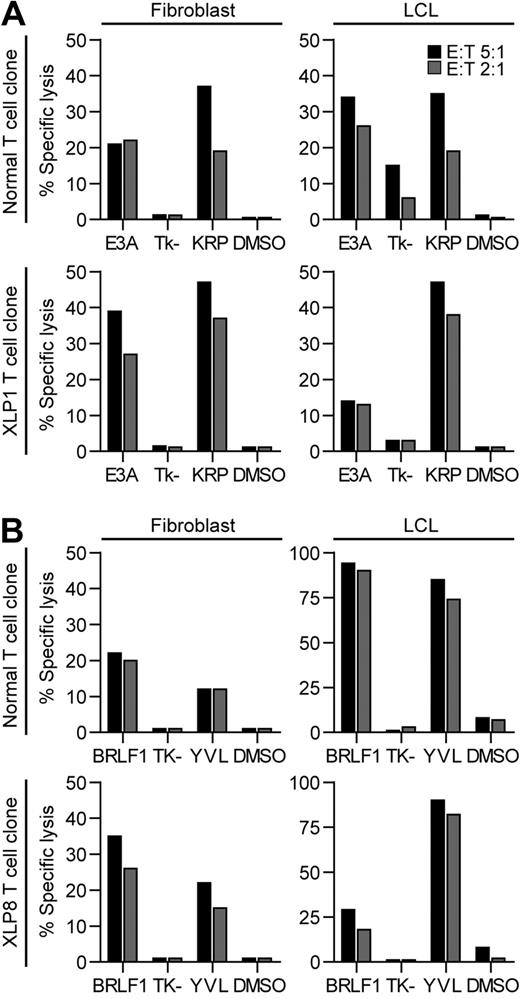
As T cells express SLAM family receptors, we hypothesized that XLP T-cell function is inhibited when these proteins interact with their ligands on target cells, similar to XLP-derived NK cells.7,8 Cytotoxic function of KRP-specific or YVL-specific T cells from XLP1 and XLP8, respectively, were then compared against LCLs that we confirmed to express SLAM family ligands, or fibroblasts that did not (supplemental Figure 2). Cognate antigens EBNA3A or BRLF1, respectively, were expressed in the target cells using vaccinia viruses. Note that although LCLs present epitopes derived from endogenous EBV proteins, they generally show low levels of lysis in cytotoxicity assays,33 but specific killing is much stronger if the target antigen is overexpressed from vaccinia vectors. Figure 4 shows representative results of one assay of 2, using one of 2 independently derived clones for each specificity. Healthy donor and XLP1 KRP-specific T cells killed EBNA3A-expressing fibroblasts but not those expressing a control construct (Tk-), with more efficient killing seen by the XLP1 clone (Figure 4A left panels). In parallel assays, the healthy donor–derived clone killed vaccinia EBNA3A-infected LCLs to a similar level compared with fibroblasts (Figure 4A right panels), yet the XLP1-derived KRP-specific clones killed these targets much less efficiently, despite good killing of the EBNA3A-expressing fibroblasts. Both target cell types were efficiently killed by the clones when sensitized with KRP peptide. Similar cytotoxicity experiments were conducted using YVL-specific T-cell clones. XLP and control clones killed BRLF1-expressing fibroblasts with similar efficiencies (Figure 4B left panels). However, the XLP-derived YVL-specific clone mediated much weaker killing of the BRLF1-expressing LCLs, in contrast to very strong killing by the healthy donor YVL-specific T-cell clone (Figure 4B right panels). Again, both T-cell types efficiently killed LCLs sensitized with cognate YVL peptide.
Recognition of EBV antigen expressing fibroblast or LCL targets in cytotoxicity assays by EBV-specific CD8+T cells derived from healthy controls or XLP patients. (A) HLA-B*2705 fibroblasts (left panels) or LCLs (right panels) were infected with either vaccinia virus expressing EBNA3A or a control vaccinia Tk- construct, or were sensitized with cognate KRP-peptide or DMSO and assayed for recognition by healthy donor–derived KRP-specific clones (top panels) or XLP1 KRP-specific clones (bottom panels). (B) HLA-A*0201 fibroblasts (left panels) or LCLs (right panels) were infected with vaccinia virus expressing either BRLF1 or the control vaccinia Tk- construct, or were sensitized with cognate YVL-peptide or DMSO and assayed for recognition by healthy donor–derived YVL-specific clones (top panels) or XLP8-derived YVL-specific clones (bottom panels). Clones were incubated at the indicated effector:target ratios in 5-hour assays. The standard deviation for the replicates were all within 10% of the value measured.
Recognition of EBV antigen expressing fibroblast or LCL targets in cytotoxicity assays by EBV-specific CD8+T cells derived from healthy controls or XLP patients. (A) HLA-B*2705 fibroblasts (left panels) or LCLs (right panels) were infected with either vaccinia virus expressing EBNA3A or a control vaccinia Tk- construct, or were sensitized with cognate KRP-peptide or DMSO and assayed for recognition by healthy donor–derived KRP-specific clones (top panels) or XLP1 KRP-specific clones (bottom panels). (B) HLA-A*0201 fibroblasts (left panels) or LCLs (right panels) were infected with vaccinia virus expressing either BRLF1 or the control vaccinia Tk- construct, or were sensitized with cognate YVL-peptide or DMSO and assayed for recognition by healthy donor–derived YVL-specific clones (top panels) or XLP8-derived YVL-specific clones (bottom panels). Clones were incubated at the indicated effector:target ratios in 5-hour assays. The standard deviation for the replicates were all within 10% of the value measured.
Sensitivity of B-cell versus fibroblast–target cell recognition by XLP– and healthy donor–derived T cells
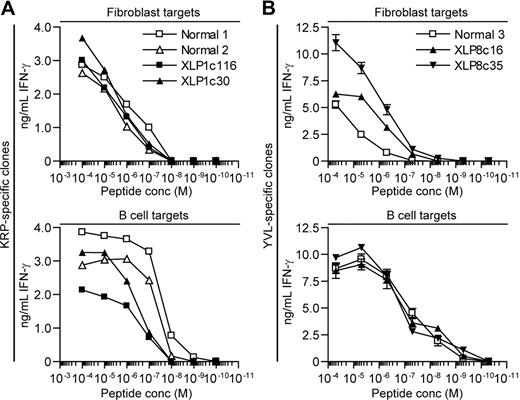
The previous experiments indicated that XLP T-cell clones were poor at recognizing antigen presented by B-cell targets. To further explore this, the relative ability these T cells to recognize B-cell and fibroblast targets sensitized with 10-fold dilutions of cognate peptide was compared. Figure 5A shows representative results from 2 experiments analyzing IFN-γ release from XLP1- and healthy control–derived KRP-specific T-cell clones exposed to peptide-sensitized B-cell blasts, which express SLAM receptors or fibroblasts that do not (supplemental Figure 2). EBV antigen–negative B-cell blasts were used to give a more accurate titration of sensitivity in the absence of endogenously presented antigen by LCLs. Both T-cell clone types incubated with the fibroblast targets showed equivalent sensitivity. However in parallel assays, XLP-derived clones showed approximately 10-fold lower sensitivity compared with healthy donor–derived clones when assayed against B-cell blasts and LCL targets (supplemental Figure 3). Experiments using YVL-specific clones derived from XLP8 and a healthy donor were also conducted (Figure 5B). XLP8-derived clones showed an equivalent sensitivity to the control clones when incubated with peptide-sensitized ΔBZ LCL targets. However the XLP8-derived effectors were 10-fold more sensitive than the corresponding healthy donor–derived cells when assayed against peptide-sensitized fibroblasts, suggesting that the XLP effectors were relatively impaired in their recognition of B-cell targets compared with fibroblasts. Overall, comparing the functional avidity of the T cells by measuring the dose of peptide that gives 50% maximal IFN-γ release, XLP-derived T cells compared with healthy donor–derived T cells show a 10-fold decrease when assayed against B-cell versus fibroblast targets.
Sensitivity of XLP and healthy donor derived CD8+T-cell clones assayed against peptide sensitized fibroblasts and B-cell targets. (A) HLA-B*2705 fibroblasts (top panel) or B-cell blasts (bottom panel) were sensitized with 10-fold dilutions of KRP-peptide, incubated overnight with KRP-specific T-cell clones derived from XLP1 or healthy donors and recognition assessed by measuring IFN-γ secreted by the T cells. (B) HLA-A*0201 fibroblasts (top panel) or LCLs (bottom panel) were sensitized with 10-fold dilutions of YVL-peptide, incubated overnight with YVL-specific T-cell clones derived from XLP8 or healthy donors, and recognition assessed by measuring IFN-γ secreted by the T cells.
Sensitivity of XLP and healthy donor derived CD8+T-cell clones assayed against peptide sensitized fibroblasts and B-cell targets. (A) HLA-B*2705 fibroblasts (top panel) or B-cell blasts (bottom panel) were sensitized with 10-fold dilutions of KRP-peptide, incubated overnight with KRP-specific T-cell clones derived from XLP1 or healthy donors and recognition assessed by measuring IFN-γ secreted by the T cells. (B) HLA-A*0201 fibroblasts (top panel) or LCLs (bottom panel) were sensitized with 10-fold dilutions of YVL-peptide, incubated overnight with YVL-specific T-cell clones derived from XLP8 or healthy donors, and recognition assessed by measuring IFN-γ secreted by the T cells.
Blocking SLAM family interactions restores T-cell function against B-cell targets
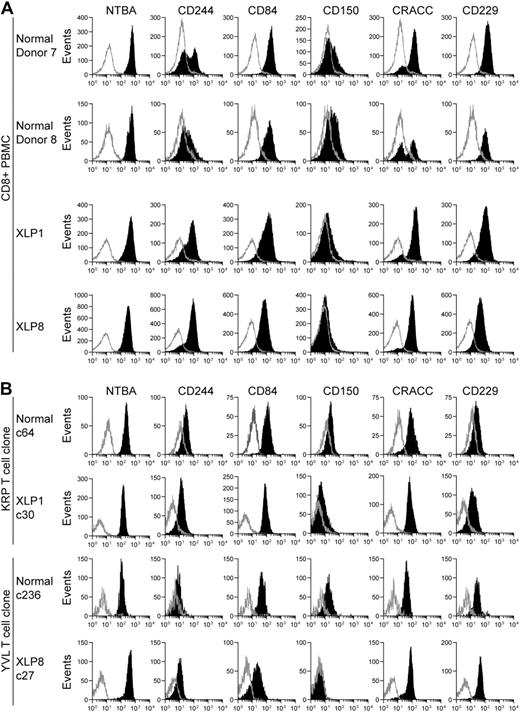
The previous observations suggested that XLP T-cell function was inhibited when assayed against B-cell targets. As SLAM receptor interactions can inhibit XLP-derived NK cell function,7,8 the surface expression of these receptors on peripheral CD8+ T cells from 2 healthy donors and XLP1 and XLP8 was examined by flow cytometry (Figure 6A). Consistent with previous observations,8,9,34,35 CD8+ T cells expressed high levels of NTBA and also expressed CD84, CD229, and CRACC, although one healthy donor showed a biphasic distribution of CRACC on their cells. The majority of XLP-derived cells expressed CD244 while healthy donors had a low or biphasic distribution of this molecule. Low levels of CD150 were detected on cells from healthy donors and little expression detected on XLP-derived cells.
SLAM family receptor expression on XLP or healthy donor CD8+PBMC or T-cell clones. Flow cytometry was used to measure surface level expression of SLAM family receptors on CD8+ PBMCs (A) or T-cell clones (B) from 2 healthy donors or XLP1 and XLP8. Cells were stained with antibodies specific for CD8 and costained with either antibodies specific to the indicated SLAM receptors or isotype control antibodies. Histograms present the analysis gated on CD8+ positive cells, with the intensity of SLAM family receptor staining shown in black or isotype staining in gray.
SLAM family receptor expression on XLP or healthy donor CD8+PBMC or T-cell clones. Flow cytometry was used to measure surface level expression of SLAM family receptors on CD8+ PBMCs (A) or T-cell clones (B) from 2 healthy donors or XLP1 and XLP8. Cells were stained with antibodies specific for CD8 and costained with either antibodies specific to the indicated SLAM receptors or isotype control antibodies. Histograms present the analysis gated on CD8+ positive cells, with the intensity of SLAM family receptor staining shown in black or isotype staining in gray.
Surface levels of the SLAM family receptors expressed on representative CD8+ T-cell clones used throughout this analysis were similarly assessed. Figure 6B shows histograms of receptor staining intensity on XLP1-derived KRP-specific and XLP8-derived YVL-specific clones, and equivalent healthy donor–derived specificities. All clones expressed high levels of NTBA and intermediate levels of CD84 and CRACC. Control clones expressed CD150 weakly with XLP-derived clones expressing low levels or none of this marker, while CD229 and CD244 was expressed slightly more on the XLP-derived clones relative to the control clones.
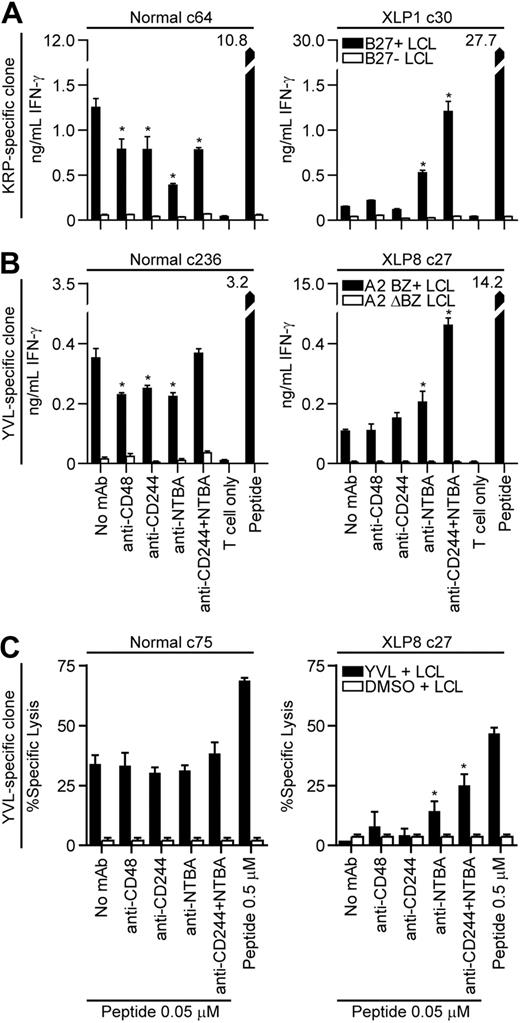
To determine whether SLAM family receptor interactions can inhibit XLP CD8+ T-cell function, clones characterized above were used in recognition assays against LCL targets where the CD244-CD48 and NTBA interactions were inhibited using monoclonal antibodies with known blocking ability.7,8 Clones from XLP or control donors were incubated with CD244- or NTBA-specific antibodies either singly or in combination, before being mixed with targets that had been incubated with antibodies specific for CD48 or NTBA, and IFN-γ secretion assessed. Figure 7A-B show representative results of 2 repeated blocking assays conducted on 2 different specificity clones. Healthy donor–derived HLA-B*2705-restricted KRP-specific CD8+ T cells assayed against HLA-B*2705 matched or mismatched LCLs showed varying levels of inhibition of IFN-γ secretion when incubated with the antibodies but never any increase. Target cell recognition by the XLP-derived KRP-specific clone was unaffected when CD48 or CD244 interactions were blocked; however, inhibiting NTBA interactions significantly increased LCL recognition, while simultaneous blocking of both CD244 and NTBA synergistically increased IFN-γ secretion. Similar results were observed when YVL-specific clones were assessed in these assays (Figure 7B). No effect of blocking CD244 or CD48 interactions in XLP-derived cells was observed. However, significant increases in IFN-γ secretion upon stimulation with the LCL were seen when NTBA interactions were blocked and again, a marked synergistic effect was seen when both CD244 and NTBA interactions were blocked.
Recognition of LCL targets in the presence of SLAM receptor blocking antibodies by CD8+T-cell clones derived from healthy donors or XLP patients. (A) CD8+ T cells specific for the KRP-epitope derived from a healthy donor (left panel) or XLP1 (right panel) and HLA-B*2705 matched or mismatched LCLs or (B) CD8+ T cells specific for the YVL-epitope derived from a healthy donor (left panel) or XLP8 (right panel) and BZ+ HLA-A*0201 matched or ΔBZ LCLs were incubated with the indicated antibodies at a concentration of 10 μg/mL for 30 minutes. The CD8+ T cells and targets were then mixed in the presence of the antibody and T-cell recognition assessed by measuring IFN-γ secretion after overnight incubation. LCLs sensitized with the cognate peptide served as positive controls. (C) CD8+ T cells specific for the YVL-epitope derived from a healthy donor (left panel) or XLP8 (right panel) were incubated with target LCLs sensitized with either a high concentration of peptide (0.5μM) or a suboptimal concentration of peptide (0.05μM) or DMSO as a control. LCLs sensitized with the suboptimal concentration of peptide or DMSO and the T cells were incubated with the indicated antibodies as above and these used in 5-hour chromium 51 release cytotoxicity assays. Asterisks indicate results which are significantly different from the no antibody treatment control using the Mann-Whitney U test (P < .05).
Recognition of LCL targets in the presence of SLAM receptor blocking antibodies by CD8+T-cell clones derived from healthy donors or XLP patients. (A) CD8+ T cells specific for the KRP-epitope derived from a healthy donor (left panel) or XLP1 (right panel) and HLA-B*2705 matched or mismatched LCLs or (B) CD8+ T cells specific for the YVL-epitope derived from a healthy donor (left panel) or XLP8 (right panel) and BZ+ HLA-A*0201 matched or ΔBZ LCLs were incubated with the indicated antibodies at a concentration of 10 μg/mL for 30 minutes. The CD8+ T cells and targets were then mixed in the presence of the antibody and T-cell recognition assessed by measuring IFN-γ secretion after overnight incubation. LCLs sensitized with the cognate peptide served as positive controls. (C) CD8+ T cells specific for the YVL-epitope derived from a healthy donor (left panel) or XLP8 (right panel) were incubated with target LCLs sensitized with either a high concentration of peptide (0.5μM) or a suboptimal concentration of peptide (0.05μM) or DMSO as a control. LCLs sensitized with the suboptimal concentration of peptide or DMSO and the T cells were incubated with the indicated antibodies as above and these used in 5-hour chromium 51 release cytotoxicity assays. Asterisks indicate results which are significantly different from the no antibody treatment control using the Mann-Whitney U test (P < .05).
Cytotoxic function of the XLP-derived clones was examined against LCL targets when CD244-CD48 and NTBA interactions were blocked. YVL-specific clones from XLP8 and a control clone were incubated with LCLs sensitized with either a high dose of cognate peptide (0.5μM), or a suboptimal dose of peptide (0.05μM) where the XLP T cells show little function relative to the control clone. LCLs sensitized with the suboptimal peptide dose and T cells were incubated with the antibody combinations described above and used in cytotoxicity assays. Figure 7C shows representative results of one of 3 experiments. The antibodies had little effect on the cytotoxic function of the healthy donor–derived clone. However, inhibiting NTBA interactions between the XLP-derived clone and targets significantly increased killing in 2 of 3 experiments with a trend to increased killing in the third. Inhibiting both NTBA and CD244 interactions significantly increased killing in all experiments.
Discussion
Previous studies investigating functional EBV-specific memory responses in XLP patients focused mostly on assays measuring PBMC-mediated control of EBV transformation of B cells, known as regression assay analysis.21,22 In these complex assays, multiple lymphocyte effector populations contribute to the control of transformation, giving a global measure of immunity but making it difficult to interpret the function of individual effector subsets.23 Nevertheless, the studies demonstrated impairment of in vitro recall responses in XLP patients. Given the high viral loads measured in these donors, one may postulate this reflects a high antigen load, a situation that in other chronic viral infections has been related to deletion and inactivation of virus-specific responses.36 However, the current analysis shows that XLP patients retain EBV-specific memory CD8+ T-cell responses and these are of a comparable size and repertoire breadth to those seen in healthy EBV-infected donors.
Although EBV-specific T-cell responses were detected ex vivo in XLP patients by ELISpot, EBV-specific T-cell clones generated from them responded poorly to antigen-expressing B-cell targets compared with equivalent healthy donor–derived clones. We attribute this contrast in ex vivo and in vitro function of XLP T cells to the levels of peptide used to stimulate the T cells in the respective assays. Thus, antigen-expressing B cells display low levels of endogenously processed peptide, which inefficiently stimulates XLP-derived T cells (Figure 3). However, when high concentrations of synthetic epitope peptide are used to sensitize B-cell targets, levels similar to those used in ELISpot assays, T-cell function is restored (Figures 3 and 5). In contrast to the poor response to antigen-expressing B cells, XLP-derived T-cell clones tested against antigen-expressing fibroblasts were equally or more sensitive than equivalent healthy donor–derived T-cell clones. This difference in sensitivity was at least 10-fold, which perhaps surprisingly seems modest given the poor control of EBV but may suggest a fine balance between inhibition and stimulation in this context. Nevertheless, the XLP-derived T-cell clones described here and in other studies consistently showed poor recognition of cognate or allogenic EBV-transformed B-cell targets expressing endogenous antigens.24,25 Our results extend these findings by showing that XLP EBV-specific CD8+ T-cell clones are capable of effector function, but are dependent on the cellular context in which the antigen is presented.
As XLP NK cell function is inhibited against B-cell targets through CD244 and NTBA on the NK cell interacting with their ligands on B cells, we reasoned that such interactions may inhibit T-cell function.7,8 Blocking NTBA interactions restored partial T-cell recognition of LCLs while blocking both NTBA and CD244-CD48 interactions synergistically increased recognition of antigen-expressing LCLs. We believe these increases are due to inhibiting receptor interactions rather than stimulating T cells through Fc-mediated presentation of the antibodies by the LCLs; increased function was not seen with healthy donor–derived clones assayed in parallel that expressed similar receptor levels compared with the XLP-derived clones. Furthermore, we were unable to induce such Fc-directed killing using LCLs to present the T-cell stimulatory antibody OKT3 (data not shown). These results suggest that in XLP T cells, both NTBA and CD244 can deliver negative signals to the cell. These findings are consistent with those using XLP-derived NK cells by Parolini et al and Bottino et al,7,8 but show some contrast with other studies18,19 where XLP-derived NK cells were unable to be activated by signaling induced through CD244, unlike healthy donor–derived NK cells. Why these differences have occurred is unclear but may be related to the use of redirected cytotoxicity assays in these latter experiments.
How T-cell function in vivo is modulated by SLAM receptor signaling in XLP patients remains to be determined. Murine knockout models suggest that another SAP adapter family molecule, EAT-2, can modify immune cell function. Thus, EAT-2 knockout mice show increased NK cell responsiveness and overexpression reduces function of these cells to certain stimuli, implying that EAT-2 acts as an inhibitor in this system.37 As human CD8+ T cells express this protein,38 it is tempting to speculate that it has a similar function in these effectors and in the absence of SAP may dictate the outcome of SLAM family receptor signaling. However, studies from multiple SAP adaptor family knockout mice suggest that EAT-2s function is more complex and may cooperate with SAP.39 Alternatively, in vitro experiments have shown some evidence for inhibitory molecules such as SHP-1, SHP-2, SHIP, or Csk binding to SLAM family receptors.40,41 In vivo experiments do not support a role for modulating signaling through the direct binding of these molecules to these receptors.7 However, as peripheral CD8+ T cells in these patients express high levels of NTBA and CD244 (Figure 6A), we would expect these to show poor effector function against EBV-infected B cells; a finding consistent with the elevated viral loads detected in these patients. Potential therapeutic interventions might include reducing the expression of these molecules using virally vectored siRNA, although the most durable intervention is likely to come from restoring appropriate SAP expression in patients hematopoeitic stem cells.
Whether other members of the SLAM family affect the outcome of T-cell–B-cell interactions in XLP remains to be fully resolved. The availability of XLP-derived antigen–specific CD8+ T-cell clones will allow investigation of other receptor interactions, particularly ones for which blocking reagents are unavailable, through engineering expression of SLAM receptors either singly or in combination on negative cells or reducing expression of these molecules with siRNA. However, the low levels of CD150 expressed on the T-cell effectors in this and another study24 suggests its influence is likely to be minimal. CRACC interactions may not be relevant, as SAP does not bind to this protein and stimulation of CRACC on XLP-derived NK cells induces cytotoxicity.9 CD84 ligation on XLP T cells costimulated with anti-CD3 mAb undergo enhanced proliferation, much like healthy donor–derived T cells. However, other effector functions and the cells' response to cognate antigen stimulation remain to be examined.34 The nature of the antigenic stimulus is likely important in such experiments, as a mitogenic T-cell stimulus such as anti-CD3 may overcome the SAP deficiency similar to loading supraphysiologic levels of cognate peptide on B-cell targets in our experiments.
The data from these experiments and other studies suggest that XLP, rather than reflecting an inability to cope with EBV per se, is a disease of ineffective T-cell and NK cell function when engaging B cells.7,8,12,14,24,25 The exquisite B-cell tropic nature of EBV and the mechanism it uses to establish infection provides an explanation as to why it and not other viruses encountered by these patients specifically triggers severe disease. Thus, upon infection of B cells EBV expresses its latent genes, inducing proliferation of these cells that harbor the viral genome in an episomal state, allowing the establishment and amplification of a reservoir of infected cells (reviewed in Rickinson and Kieff42 ). In healthy carriers, latently infected B cells and cells reactivating virus from latency into lytic cycle are eventually controlled by the T-cell response. However, as XLP patients CD8 T cells would be unlikely to kill antigen-expressing B cells, these cells and thus this source of antigen would not be cleared. Given that in the sap−/− murine model priming of T-cell responses by dendritic cells, which express much lower levels and a restricted range of SLAM family receptors compared with B cells,14,35,43,44 is apparently normal, cross presentation by dendritic cells of an increasingly high antigen load would continue to generate activated T cells unable to clear the antigen source. Such a scenario would explain the abundance of highly activated T cells and EBV-transformed B cells seen in XLP patients with fulminant infectious mononucleosis.45-47 Note also that the inability of XLP T cells and NK cells to recognize B-cell targets in general (rather than EBV-transformed B cells in particular) may also explain why B-cell malignancies such as Burkitt lymphoma, including the EBV-negative cases of this tumor, are more frequent in this population,48,49 since such tumors express SLAM family ligands. Consistent with the ideas proposed by Qi14 and Schwartzberg,50 the impairment of EBV-specific CD8+ T-cell function observed here probably reflects a broader functional deficit that affects all CD8+ T cells in XLP patients, mirroring the impairment already shown for XLP NK cells.7,8 This global deficit in T-cell–B-cell and NK-cell–B-cell cross-talk likely underlies these patients' extreme susceptibility to B-cell pathologies.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients and healthy donors who donated blood samples for this study. We thank Dr Geneviève de St Basile for providing sequence and protein expression analysis of XLP8 SAP mutation.
This work was supported by a New Investigator Award from the Medical Research Council United Kingdom (G0501074) to A.D.H. and a program grant from the Medical Research Council United Kingdom (G9901294). U.P. and S.G.T. are supported by the XLP Research Trust United Kingdom and The Cancer Council New South Wales, Australia.
Authorship
Contribution: A.D.H., U.P., and A.M.L. performed experiments; P.D.A., S.G.T., H.B.G., A.C.L., P.S.R., and A.M. provided essential reagents; A.D.H. designed the experiments; A.D.H. and P.D.A. analyzed the data; and A.D.H. and A.B.R. interpreted the results and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew D. Hislop, School of Cancer Sciences and Medical Research Council Centre for Immune Regulation, University of Birmingham, Edgbaston, Birmingham, B15 2TT, United Kingdom; e-mail: a.d.hislop@bham.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal