Abstract
The kidney is the main physiologic source of erythropoietin (EPO) in the adult and responds to decreases in tissue oxygenation with increased EPO production. Although studies in mice with liver-specific or global gene inactivation have shown that hypoxia-inducible factor 2 (Hif-2) plays a major role in the regulation of Epo during infancy and in the adult, respectively, the contribution of renal HIF-2 signaling to systemic EPO homeostasis and the role of extrarenal HIF-2 in erythropoiesis, in the absence of kidney EPO, have not been examined directly. Here, we used Cre-loxP recombination to ablate Hif-2α in the kidney, whereas Hif-2–mediated hypoxia responses in the liver and other Epo-producing tissues remained intact. We found that the hypoxic induction of renal Epo is completely Hif-2 dependent and that, in the absence of renal Hif-2, hepatic Hif-2 takes over as the main regulator of serum Epo levels. Furthermore, we provide evidence that hepatocyte-derived Hif-2 is involved in the regulation of iron metabolism genes, supporting a role for HIF-2 in the coordination of EPO synthesis with iron homeostasis.
Introduction
The glycoprotein erythropoietin (EPO) is essential for the regulation of red blood cell mass in response to changes in tissue oxygenation. EPO stimulates erythropoiesis by promoting erythroid precursor cell viability, proliferation, and differentiation, thus enhancing the oxygen-carrying capacity of blood. Its production is tightly regulated by developmental, tissue-specific, and physiologic cues.1,2 Lack of Epo in the embryo, where it is produced by hepatocytes, leads to death from cardiac failure and anemia at embryonic day (E)13.5.3 During late gestation, the site of EPO production switches from the fetal liver to the kidney, where fibroblast-like peritubular interstitial cells become the main physiologic source of EPO synthesis in adults.4-6 Although the liver retains the ability to produce EPO in response to hypoxic stimuli, it does not contribute to the serum EPO pool under normoxic or mild hypoxic conditions.7-9 Therefore, an impairment of renal EPO synthesis, which is typically associated with advanced chronic kidney failure, results in the development of anemia and is treated by administering recombinant EPO.2,10
The primary physiologic stimulus of enhanced EPO gene transcription is tissue hypoxia, which can induce a several hundred-fold increase in circulating serum EPO levels.1 Although in vitro studies, using an 18-nucleotide fragment of the oxygen-sensitive 3′ EPO regulatory element, suggested that hypoxia inducible factor-1 (HIF-1) regulates EPO in Hep3B cells,11-13 recent genetic evidence indicates that Hif-2 has an important role in the maintenance of normal serum EPO levels.14-16 HIF-1 and HIF-2 belong to the PER/arylhydrocarbon-receptor nuclear translocator (ARNT)/single minded family of hypoxia-regulated transcription factors and consist of an oxygen-sensitive α subunit and a constitutively expressed β subunit, also known as ARNT. Both factors facilitate oxygen delivery and cellular adaptation to hypoxia by stimulating erythropoiesis, angiogenesis, and anaerobic glucose metabolism. Under normoxia, HIF-1α and HIF-2α are targeted for rapid proteasomal degradation by the von Hippel-Lindau tumor suppressor, pVHL, which functions as the substrate recognition component of an E3 ubiquitin ligase. pVHL-mediated targeting of HIF-α requires oxygen- and iron-dependent hydroxylation of specific proline residues within the oxygen-dependent degradation domain of HIF-α, carried out by prolyl-4-hydroxylase domain (PHD) proteins (for a recent review on this topic, see Schofield and Ratcliffe17 ).
Even though HIF-1 was initially purified from Hep3B cells, use of small interfering RNAs directed against individual HIF-α homologs showed that the hypoxic induction of endogenous EPO was largely HIF-2 dependent in Hep3B and other cell lines.18,19 The notion that HIF-2 acts as a main regulator of EPO homeostasis in vivo is based on histologic studies, which have shown that the location of Epo-producing renal interstitial fibroblast-like cells coincided with the location of Hif-2α–expressing renal cells,20 and on genetic studies with adult mice that were either germline deficient for Hif-2 or that were made Hif-2 deficient during postnatal life with the use of a ubiquitously expressed Cre-recombinase.14,16 The latter study furthermore showed that widespread postnatal ablation of Hif-1α did not result in anemia, which is in contrast to studies with mice that were heterozygously deficient for Hif-1α.21,22 Global knockout of Phd2, on the other hand, suggested that Hif-1 rather than Hif-2 contributed to increased renal Epo synthesis, because Hif-2α was not detectable in Phd2-deficient kidneys.23 In the liver, however, cell type–specific inactivation showed that Hif-2 is the main regulator of Epo production in hepatocytes.15 Despite these advances in understanding HIF-dependent regulation of erythropoiesis, the role of renal HIF-2 in the control of systemic EPO homeostasis has not been examined directly, in particular it has remained unclear to what degree renal HIF-2 contributes to serum EPO levels after both acute and chronic hypoxic stimulation.
To specifically investigate the role of renal HIF-2 in the regulation of serum EPO homeostasis and to examine the contribution of nonrenal HIF signaling in the absence of hypoxically induced renal EPO, we used Cre-loxP recombination to inactivate Hif-2α in the kidney, whereas Hif-2–mediated hypoxia responses in the liver and other organs remained intact. Our data establish that the hypoxic induction of Epo in the kidney exclusively depends on Hif-2, and that in the absence of renal Hif-2, hepatic Hif-2 takes over as the main regulator of the serum Epo pool. Furthermore, we show that the Epo-producing capability of the liver can be exploited therapeutically, when the kidney, the main physiologic source of Epo in the adult, fails to respond to hypoxia with an appropriate increase in Epo synthesis.
Methods
Generation of mice and genotyping
The generation and genotyping of Hif1a, Hif2a (Epas1), and Vhlh conditional alleles and of P3Pro- and Albumin-Cre transgenes have been described previously.14,24-28 In P3Pro-Cre transgenic mice, which were generated by pronuclear injection, Cre recombinase expression is under the control of the murine Pax3 promoter and is active in neural crest-derived cells and in renal epithelial and interstitial cells.26 The Albumin-Cre transgene used a 2.35-kilobase fragment from the rat albumin enhancer/promoter and targets ≥80%-90% of hepatocytes by 6 weeks of age.24,27,28 Cre-negative littermates were used as controls. All procedures involving mice were performed in accordance with National Institutes of Health guidelines for the use and care of live animals and were approved by the Institutional Animal Care and Use Committees of Vanderbilt University and of the University of Pennsylvania. The following primers were used for the detection of P3Pro- and Albumin-Cre transgenes: P3Pro-Cre: forward, 5′-AATCTTATGGTCACCTGAGTGTTAAATGTCCAATTTAC-3′, reverse, 5′-CATCTTCAGGTTCTGCGGG-3′; Albumin-Cre: forward, 5′-ATGAAATGCGAGGTAAGTATGG-3′, reverse, 5′-CGCCGCATAACCAGTGAAAC-3′.
DNA, RNA, and protein analyses
DNA and RNA were isolated with Trizol reagent (Invitrogen), according to manufacturer's instructions. For real-time polymerase chain reaction (PCR) analysis, 1 μL of cDNA was subjected to PCR amplification either with the use of SYBR Green PCR Master Mix or Taqman Universal PCR Master Mix on an ABI 7300 platform (Applied Biosystems). mRNA expression levels were quantified with the relative standard curve method according to the manufacturer's instructions (Applied Biosystems). 18S ribosomal RNA was used for normalization as previously described.28 Primer sequences for the analysis of Epo and Vegf mRNA levels have been published previously15 ; primer sequences for Phd3 was forward, 5′-TCGCTTCCTCCCGAACTCT-3′, and reverse, 5′-CAGAAACGAGGGTGGCTAACTT-3′. Sequences of primer pairs used for the analysis of iron metabolism gene expression can be found in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For the detection of Hif-1α and Hif-2α by immunoblot, nuclear protein extracts were prepared and analyzed as previously described, and antibodies from Novus Biologicals were used.15 Biliary epithelial and Ito cells were detected with a rat monoclonal anti-CK19 antibody (a gift from Stacey Huppert, Vanderbilt University) and a rabbit antidesmin antibody from Abcam Inc.
Phenotypic analysis of mutant mice
5′-Bromo-4-chloro-3-indolyl-b-d-galactopyranoside (Xgal) staining was carried out according to MacGregor et al29 ; slides were counterstained with Nuclear Fast Red (Sigma). Images were obtained by using a Nikon ECLIPSE E400 microscope (Nikon Plan 10×/0.25 NA and 40×/0.65 NA objectives), using a SPOT, Insight 2 Megapixel CCD digital camera (Model 11.2 Color Mosaic) and SPOT for MAC OS, Version 4.0.4 imaging software (Diagnostic Instruments Inc). Complete blood counts (CBCs) were determined with a Hemavet 950 analyzer (Drew Scientific). Serum Epo levels were determined with a commercially available enzyme-linked immunoabsorbent assay kit according to the manufacturer's instructions (R&D Systems).
Hypoxia experiments
Mice were exposed to 10% O2 for 10 days in an animal hypoxia chamber (Biospherix Ltd) and analyzed immediately after exposure. Anemic hypoxia was generated in anesthetized mice by retro-orbital phlebotomy or by a combination of phlebotomy with phenylhydrazine treatment as previously described.15
Stabilization of HIF-α by prolyl-4-hydroxylase inhibitor or CoCl2 treatment
For chemical stabilization of Hif-α, prolyl-4-hydroxylase inhibitor GSK1002083A (Glaxo-Smith-Kline) was dissolved in a 1% methylcellulose solution and administered by daily oral gavage at a dose of 60 mg/kg for 10 days. For CoCl2 experiments, mice were injected intraperitoneally with 60 mg/kg and analyzed 4 hours after injection.
Statistical analysis
Data reported represent mean values ± SEMs. Statistical analyses were performed with Prism 5.0b software (GraphPad Software) with the use of the unpaired Student t test and the Mann-Whitney U test. P values of < .05 were considered statistically significant.
Results
Generation of conditional knockout mice with Hif-2α inactivation in the kidney
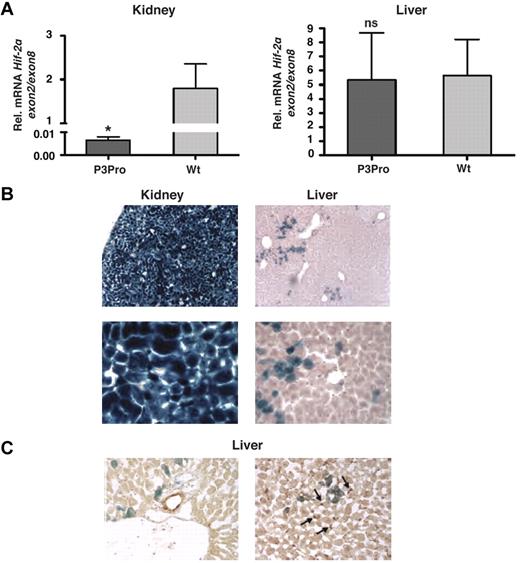
To investigate the role of renal HIF-2 in the regulation of serum EPO levels, P3Pro-Cre transgenic mice were crossed to mice homozygous for the conditional Hif-2α allele.14,26 Recombination of the conditional Hif-2α allele was highly efficient in kidneys as determined by genomic PCR (> 95%; data not shown). This is reflected in an almost complete reduction of Hif-2α exon 2 transcript levels (exon 2 is flanked by loxP sites and targeted for Cre-mediated recombination; Figure 1A). Although recombination was detectable in liver DNA, we did not find a significant difference in the expression levels of Hif-2α exon 2 between mutant and control livers (Figure 1A). As shown in Figure 1B, P3Pro-Cre is widely expressed in the kidney and in a small subpopulation of hepatocytes. It is not expressed in biliary epithelium and Ito cells (Figure 1C). A heterogeneous pattern of limited P3Pro-Cre expression was found in additional tissues, including lung, spleen, and bone marrow (13% of TER119+ and CD71+; data not shown). Bone marrow responsiveness to recombinant EPO was normal in P3Pro mutants (supplemental Figure 1C). Taken together, these studies show that Hif-2α was ablated in most renal cells, whereas loss of Hif-2α in a small percentage of hepatocytes did not significantly affect Hif-2–mediated hypoxia responses in the liver.
Hif-2α deletion in the kidney occurs with high efficiency. (A) Shown is the mean mRNA expression level of Hif-2α exon 2 in kidney and liver extracts by real-time PCR analysis (n = 6). Exon 2 is flanked by loxP site and targeted for Cre-mediated recombination. Exon 2 expression levels were normalized to Hif-2α exon 8, which is not deleted. Bars represent mean values ± SEMs. (B) LacZ staining of kidney and liver tissue from a P3Pro mouse expressing the ROSA26R Cre-reporter transgene. Magnification, ×100 (top) and ×400 (bottom). (C) P3Pro-Cre is not expressed in biliary epithelial or Ito cells. Immunohistochemistry was performed in conjunction with X-gal staining on frozen liver tissue sections. LacZ expression did not colocalize with cytokeratin 19 (CK19), which is specific for biliary epithelial cells (left) nor did it overlap with staining for desmin, which is a histologic marker of Ito cells (right). Shown are LacZ-positive cells (blue) and CK19- or desmin-positive cells (brown) on the left or right, respectively. Wt refers to Cre-negative littermates. Arrows depict desmin-positive cells. Magnification, ×400. *P < .05; ns, not statistically significant.
Hif-2α deletion in the kidney occurs with high efficiency. (A) Shown is the mean mRNA expression level of Hif-2α exon 2 in kidney and liver extracts by real-time PCR analysis (n = 6). Exon 2 is flanked by loxP site and targeted for Cre-mediated recombination. Exon 2 expression levels were normalized to Hif-2α exon 8, which is not deleted. Bars represent mean values ± SEMs. (B) LacZ staining of kidney and liver tissue from a P3Pro mouse expressing the ROSA26R Cre-reporter transgene. Magnification, ×100 (top) and ×400 (bottom). (C) P3Pro-Cre is not expressed in biliary epithelial or Ito cells. Immunohistochemistry was performed in conjunction with X-gal staining on frozen liver tissue sections. LacZ expression did not colocalize with cytokeratin 19 (CK19), which is specific for biliary epithelial cells (left) nor did it overlap with staining for desmin, which is a histologic marker of Ito cells (right). Shown are LacZ-positive cells (blue) and CK19- or desmin-positive cells (brown) on the left or right, respectively. Wt refers to Cre-negative littermates. Arrows depict desmin-positive cells. Magnification, ×400. *P < .05; ns, not statistically significant.
Inactivation of renal Hif-2α results in hypoproliferative anemia
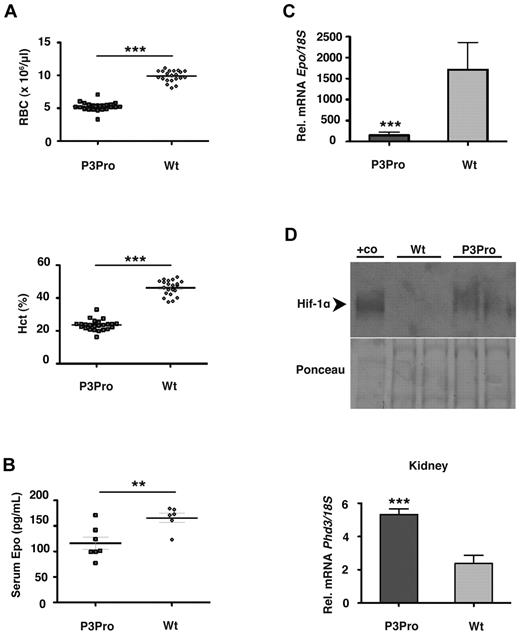
P3Pro mutants were born in Mendelian ratios and developed normally. Although males were fertile, female mutants were not able to generate offspring. The paws of mutant mice were pale, and peripheral blood smears were consistent with normochromic, normocytic anemia. CBC analysis at ages 2-5 months showed that red blood cell (RBC) numbers and hematocrit (Hct) values were substantially reduced in P3Pro mutants (Figure 2A; data not shown). In mutant mice (n = 26) mean RBC counts and Hct levels were 5.27 ± 0.12 × 106/μL and 24% ± 0.6%, respectively, compared with 9.89 ± 0.19 × 106/μL and 46% ± 1.0% in control mice (n = 21). Despite the lower RBC counts, reticulocyte percentages in mutants did not differ from littermate controls and were 2.5% and 2.6%, respectively, which is consistent with hypoproliferative anemia. Deficits in nonerythroid hematopoietic lineages were not present, because numbers of white blood cells (7.9 ± 0.7 × 106/μL) and platelets (935 ± 53 × 106/μL) were within normal limits. This is in contrast to mice with Hif-2α germline inactivation, which developed pancytopenia.16 Serum iron levels and total iron binding capacity were not different between P3Pro mutants and littermate controls, excluding iron deficiency as a contributing cause of anemia (supplemental Figure 1A). Because advanced renal failure is frequently associated with anemia, we assessed kidney function in mutant mice. Blood urea nitrogen and serum creatinine levels were comparable between mutants and controls, and kidneys from mutant mice were histologically normal at 3 months of age, which excluded renal disease as a cause of the anemia (supplemental Figure 1B).
P3Pro-Cre–mediated inactivation of Hif-2α results in severe Epo-deficient anemia. (A) Shown are RBC and Hct values in 2- to 5-month-old P3Pro mutant mice (n = 26) compared with wild-type (Wt) littermate controls of the same age (n = 21). Diamonds represent control mice, P3Pro mutants are depicted by squares. (B) Serum Epo levels in 2-month-old mutants (n = 6) compared with Wt littermates (n = 5). (C) Relative Epo mRNA levels in kidneys from mutant and control animals (n = 9) as determined by real-time PCR analysis. (D) P3Pro mutant kidneys are hypoxic. (Top) Western blot analysis of Hif-1α in kidney extracts from mutant and Wt mice. Nuclear protein extracts from the liver of a Vhlh-deficient mouse were used as positive control (+co). Ponceau S staining is shown to demonstrate equal protein loading. (Bottom) mRNA levels of HIF target gene Phd3 in kidney (n = 6). Wt refers to Cre-negative littermates. Bars represent mean values ± SEMs; **P < .01, ***P < .001.
P3Pro-Cre–mediated inactivation of Hif-2α results in severe Epo-deficient anemia. (A) Shown are RBC and Hct values in 2- to 5-month-old P3Pro mutant mice (n = 26) compared with wild-type (Wt) littermate controls of the same age (n = 21). Diamonds represent control mice, P3Pro mutants are depicted by squares. (B) Serum Epo levels in 2-month-old mutants (n = 6) compared with Wt littermates (n = 5). (C) Relative Epo mRNA levels in kidneys from mutant and control animals (n = 9) as determined by real-time PCR analysis. (D) P3Pro mutant kidneys are hypoxic. (Top) Western blot analysis of Hif-1α in kidney extracts from mutant and Wt mice. Nuclear protein extracts from the liver of a Vhlh-deficient mouse were used as positive control (+co). Ponceau S staining is shown to demonstrate equal protein loading. (Bottom) mRNA levels of HIF target gene Phd3 in kidney (n = 6). Wt refers to Cre-negative littermates. Bars represent mean values ± SEMs; **P < .01, ***P < .001.
Next, we investigated to what degree targeted deletion of Hif-2α in the kidney affected serum Epo levels. We found that, despite the presence of anemia, serum Epo levels were significantly lower in mutant mice than in controls (116 ± 12 pg/mL and 166 ± 9 pg/mL, respectively; P < .01; Figure 2B) and that Epo mRNA in mutant kidneys was decreased by ∼ 12-fold (P < .001; Figure 2C). The anemia in mutant mice was furthermore associated with increased levels of renal Hif-1α and increased expression of HIF target genes Glut1, Pkg, Ldha, and Phd3 (Figure 2D; supplemental Figure 2C; data not shown), indicating the presence of hypoxia. Despite the activation of Hif-1 signaling, mutant kidneys did not respond to anemia with increased Epo production, suggesting that the hypoxic regulation of EPO in the kidney is not HIF-1 dependent.
Hepatic Epo synthesis is not suppressed in P3Pro mutant mice
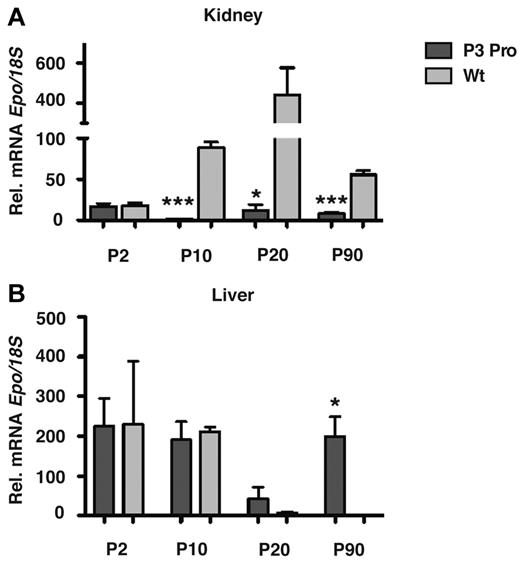
Because the main source of EPO changes from the liver to the kidney perinatally, we investigated whether lack of renal Hif-2 had any effect on the developmental switch in tissue Epo expression. PCR analysis of genomic DNA isolated from mutant kidney extracts at different time points after birth indicated comparable recombination efficiency at postnatal day 2 (P2), 10, 20, and 90 (supplemental Figure 1D). Whereas renal Epo mRNA levels were substantially suppressed at P10, P20, and P90, hepatic Epo in mutant mice did not decline at P10, P20, and P90 compared with littermate control mice, where it became undetectable at P20 and P90 with the use of real-time PCR (Figure 3; n = 6; P < .05). Interestingly, renal Epo mRNA levels at P2 did not differ between mutants and controls, suggesting that the regulation of Epo expression at this stage of postnatal development may be Hif-2 independent.
Hepatic Epo is not suppressed in P3Pro mutants. Relative Epo mRNA expression in kidneys (A) and livers (B) from mutants (dark gray bars) compared with littermate controls (light gray bars) at P2, P10, P20, and P90. Bars represent means ± SEMs (n = 3 for each genotypes and time point). *P < .05, ***P < .001.
Hepatic Epo is not suppressed in P3Pro mutants. Relative Epo mRNA expression in kidneys (A) and livers (B) from mutants (dark gray bars) compared with littermate controls (light gray bars) at P2, P10, P20, and P90. Bars represent means ± SEMs (n = 3 for each genotypes and time point). *P < .05, ***P < .001.
Hypoxic stimulation of erythropoiesis in P3Pro mutants is kidney independent
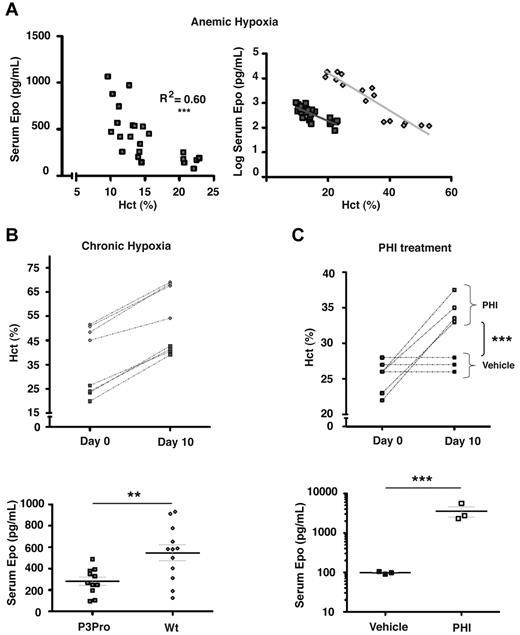
Renal EPO synthesis is stimulated in a graded manner by decreases in tissue pO2.1,2 To further investigate the relative contribution of renal Hif-2 to Epo synthesis in P3Pro mutants, mice were either challenged with acute or chronic hypoxia, or they were treated with HIF-stabilizing agents. Acute anemic hypoxia was induced by phlebotomy in P3Pro mutants, and serum Epo levels were compared with littermate controls with similar Hct levels. A linear decrease in Hct level was associated with an exponential increase in serum Epo levels in both control animals and in P3Pro mutants (Figure 4A; R2 = 0.60; P < .001 for mutants).30 The slopes of increase were similar in P3Pro mutants and in wild-type mice (Figure 4A). To determine to what degree chronic hypoxia was able to trigger erythropoietic responses in P3Pro mutants, mice were subjected to normobaric hypoxia (10% O2) for 10 days. Hct levels rose from a mean of 23% ± 1.3% to 41% ± 0.8% in P3Pro mutants and from 49% ± 1.5% to 65% ± 3.6% in Cre-negative controls (Figure 4B). Although increases in serum Epo levels were found in both groups, mutants generated 2-fold less serum Epo (Figure 4B). The time course of this response was similar between mutants and controls; serum Epo levels peaked at days 3-5 and remained elevated even after 4 weeks of exposure (data not shown). HIF prolyl-4-hydroxylase inhibitors (PHIs) have previously been shown to induce erythropoiesis by enhancing endogenous EPO production.31 To further examine the erythropoietic response after systemic HIF stabilization under normoxic conditions, P3Pro mutants were treated with GSK1002083A, which stabilizes both Hif-1α and Hif-2α in vitro and in vivo, and has been shown to induce erythrocytosis in wild-type mice (supplemental Figure 2A; Figure 4C). Mutants were treated by daily oral gavage for a period of 10 days. After completion of treatment, Hct values increased from 24% ± 1% to 35% ± 1% in mutants, whereas Hct levels in vehicle-treated mice did not change (Figure 4C). The increase in Hct level after PHI treatment was associated with a substantial rise in serum Epo levels 4 hours after drug administration (3564 ± 1041 pg/mL vs 98 ± 4 pg/mL in vehicle-treated mice; Figure 4C). Similarly to hypoxia, mutants generated less serum Epo than control mice (supplemental Figure 2A bottom panel). Whereas both Hif-1α and Hif-2α were stabilized in wild-type kidneys, Hif-2α was not detectable in mutant kidneys, which is consistent with the high recombination efficiency of the conditional Hif-2α allele (supplemental Figure 2B). HIF target genes Ldha and Pgk were significantly induced by PHI treatment in both mutant and wild-type kidneys (supplemental Figure 2C). Our data show that mice, which lack renal Hif-2α have the ability to respond to hypoxic stimuli and to systemic HIF prolyl-4-hydroxylase inhibition with increased erythropoiesis.
Acute and chronic hypoxia triggers erythropoietic responses in P3Pro mutants. (A) In P3Pro mutant mice a linear decline in the Hct value is associated with an exponential increase in serum Epo levels in response to acute anemic hypoxia (R2 = 0.60, P < .0001). Linear regression analysis showed no statistically significant difference between slopes of increase in P3Pro mutants and control mice. Serum Epo levels were measured 24 hours after phlebotomy. (B) Exposure of P3Pro mutant mice to chronic normobaric hypoxia (10% O2 for 10 days) increases Hct values. (Bottom) The serum Epo levels shown in P3Pro mutant (n = 11) and in control mice (n = 12), were measured after 10 days of hypoxia. (C) Prolyl-4-hydroxylase inhibition with GSK1002083A raises Hct values and serum Epo levels in P3Pro mutants. Shown are Hct values before treatment (day 0) and after 10 days of treatment (day 10). Serum Epo levels in P3Pro mutants were measured 4 hours after administration of 2 doses (n = 3). Vehicle indicates treatment with 1% methylcellulose without compound; PHI, treatment with PHD inhibitor GSK 1002083A. **P < .01, ***P < .001.
Acute and chronic hypoxia triggers erythropoietic responses in P3Pro mutants. (A) In P3Pro mutant mice a linear decline in the Hct value is associated with an exponential increase in serum Epo levels in response to acute anemic hypoxia (R2 = 0.60, P < .0001). Linear regression analysis showed no statistically significant difference between slopes of increase in P3Pro mutants and control mice. Serum Epo levels were measured 24 hours after phlebotomy. (B) Exposure of P3Pro mutant mice to chronic normobaric hypoxia (10% O2 for 10 days) increases Hct values. (Bottom) The serum Epo levels shown in P3Pro mutant (n = 11) and in control mice (n = 12), were measured after 10 days of hypoxia. (C) Prolyl-4-hydroxylase inhibition with GSK1002083A raises Hct values and serum Epo levels in P3Pro mutants. Shown are Hct values before treatment (day 0) and after 10 days of treatment (day 10). Serum Epo levels in P3Pro mutants were measured 4 hours after administration of 2 doses (n = 3). Vehicle indicates treatment with 1% methylcellulose without compound; PHI, treatment with PHD inhibitor GSK 1002083A. **P < .01, ***P < .001.
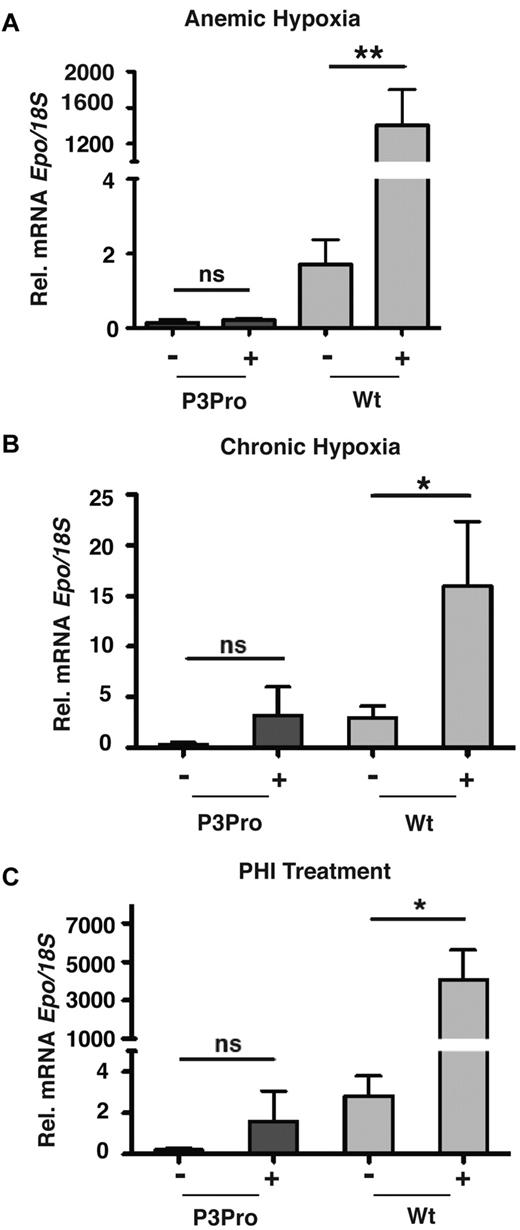
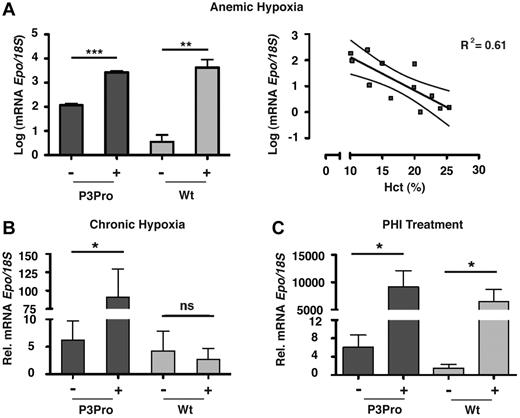
We next investigated whether renal Epo synthesis was stimulated in P3Pro mutants subjected to hypoxia or PHI treatment. We found that, despite up-regulation of HIF target genes (supplemental Figure 2C), Epo mRNA in Hif-2–deficient kidneys was neither induced by hypoxia nor by treatment with GSK1002083A or cobalt chloride (Figure 5; data not shown). Because the liver is the primary site of extrarenal EPO production in hypoxic adults, we next examined whether Epo mRNA in the liver was significantly induced. Expression levels of hepatic Epo in mutants were similar to anemic wild-type mice with comparable Hct levels, suggesting that the liver Epo response to tissue hypoxia was not enhanced in the absence of renal Hif-2 (Figure 6A). Furthermore, we found that, after acute anemic hypoxia, the linear decline in Hct level was associated with a logarithmic increase in hepatic Epo mRNA levels (R2 = 0.61, P < .01), qualitatively resembling the renal Epo response in wild-type animals (Figure 6A). In keeping with this observation, exposure to 10 days of hypoxia or PHI administration led to a ∼ 15- and ∼ 1500-fold increase in hepatic Epo mRNA levels, respectively (Figure 6B-C). In summary, our findings show that in adult mice Hif-2 is required for the hypoxia inducibility of Epo in the kidney and that, in the absence of renal Hif-2, hypoxic stimulation of erythropoiesis is associated with increased hepatic Epo transcription. Furthermore, our data indicate that Hif-1 in adults does not participate in the renal Epo response to hypoxia or to PHD inhibition.
Hypoxia and PHD inhibition do not induce Epo in P3Pro mutant kidneys. Shown are Epo mRNA levels in kidneys from P3Pro mutants and wild-type (Wt) littermate controls. (A) Exposure to acute anemic hypoxia induced by phlebotomy (measurements 24 hours after phlebotomy). (B) Exposure to chronic normobaric hypoxia (10% O2 for 10 days). (C) Oral administration of PHD inhibitor GSK1002083A (PHI) for 2 days. Wt refers to Cre-negative littermates. Bars represent mean Epo mRNA levels ± SEMs (n = 3 or 4 per genotype and time point). *P < .05, **P < .01; ns, not statistically significant.
Hypoxia and PHD inhibition do not induce Epo in P3Pro mutant kidneys. Shown are Epo mRNA levels in kidneys from P3Pro mutants and wild-type (Wt) littermate controls. (A) Exposure to acute anemic hypoxia induced by phlebotomy (measurements 24 hours after phlebotomy). (B) Exposure to chronic normobaric hypoxia (10% O2 for 10 days). (C) Oral administration of PHD inhibitor GSK1002083A (PHI) for 2 days. Wt refers to Cre-negative littermates. Bars represent mean Epo mRNA levels ± SEMs (n = 3 or 4 per genotype and time point). *P < .05, **P < .01; ns, not statistically significant.
Hypoxia and PHD inhibition stimulate hepatic Epo production in P3Pro mutants. Shown are Epo mRNA levels in livers from P3Pro mutants and wild-type (Wt) littermate controls. (A) Exposure to acute anemic hypoxia induced by phlebotomy (log scale; measurements 24 hours after phlebotomy). Shown also is a statistically significant association between the linear decline in Hct value and the exponential increase in hepatic Epo mRNA levels in P3Pro mutants (right; R2 = 0.61, P < .01). (B) Exposure to chronic normobaric hypoxia (10% O2 for 10 days). (C) Oral administration of PHD inhibitor GSK1002083A (PHI) for 2 days. Wt refers to Cre-negative littermates. *P < .05, **P < .01, ***P < .001; ns, not statistically significant.
Hypoxia and PHD inhibition stimulate hepatic Epo production in P3Pro mutants. Shown are Epo mRNA levels in livers from P3Pro mutants and wild-type (Wt) littermate controls. (A) Exposure to acute anemic hypoxia induced by phlebotomy (log scale; measurements 24 hours after phlebotomy). Shown also is a statistically significant association between the linear decline in Hct value and the exponential increase in hepatic Epo mRNA levels in P3Pro mutants (right; R2 = 0.61, P < .01). (B) Exposure to chronic normobaric hypoxia (10% O2 for 10 days). (C) Oral administration of PHD inhibitor GSK1002083A (PHI) for 2 days. Wt refers to Cre-negative littermates. *P < .05, **P < .01, ***P < .001; ns, not statistically significant.
Hepatocyte-derived Hif-2 regulates erythropoiesis in P3Pro mutants
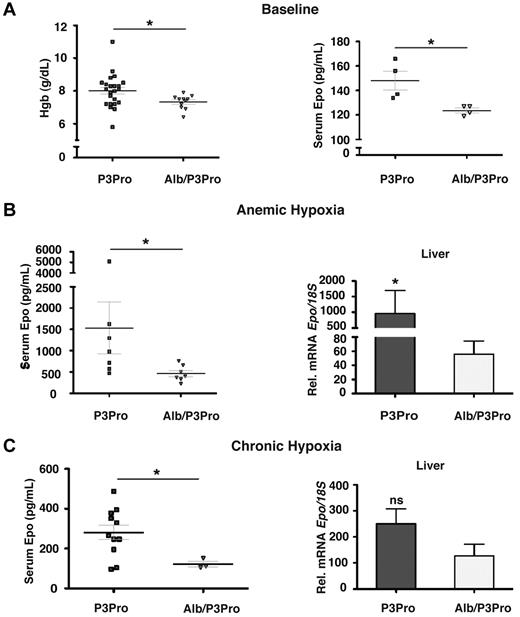
Although hepatocytes have been shown to be the main site of extrarenal EPO production under hypoxia, EPO is also expressed in other organs such as brain, spleen, lung, testis, and uterus.1 To specifically investigate the role of hepatocyte-derived Hif-2 in the hypoxia-stimulated erythropoietic response of P3Pro mutants, we generated Hif-2α conditional knockout mice that lack Hif-2α in the kidney and in hepatocytes by crossing Albumin-Cre transgenic mice with P3Pro mutants, which are from hereon referred to as Alb/P3Pro double mutants. Alb/P3Pro double mutant mice were born at half the expected Mendelian ratios. CBC analysis at ages 2-3 months showed significantly reduced hemoglobin levels of 7.33 ± 0.13 g/dL compared with 8.00 ± 0.2 g/dL for P3Pro littermate controls (P < .05; Figure 7A). The reduction in hemoglobin in double mutants was associated with decreased serum Epo concentrations (124 ± 2 vs 148 ± 8 pg/mL, P < .05; Figure 7A), suggesting that hepatic Hif-2 contributes to the regulation of serum Epo levels in P3Pro mutants under baseline conditions.
Inactivation of hepatocyte-derived Hif-2 in P3Pro mutants blunts erythropoietic responses to hypoxia. (A) Hemoglobin concentrations (n = 23 for P3Pro; n = 11 for Alb/P3Pro) and serum Epo levels in Alb/P3Pro double mutants compared with P3Pro mutants (n = 4) under baseline conditions. (B) Serum Epo levels in P3Pro and Alb/P3Pro mutants after phlebotomy of (n = 7). Epo mRNA levels in livers of P3Pro and Alb/P3Pro mutants at baseline conditions (n = 3) and after phlebotomy (mice were analyzed 18 hours after phlebotomy; n = 7), as determined by real-time PCR. (C) Serum Epo levels in P3Pro (n = 13) and Alb/P3Pro mutants (n = 3) after chronic hypoxia exposure (10% O2 for 10 days) and corresponding Epo mRNA levels in P3Pro and Alb/P3Pro livers (n = 3). *P < .05; ns, not statistically significant.
Inactivation of hepatocyte-derived Hif-2 in P3Pro mutants blunts erythropoietic responses to hypoxia. (A) Hemoglobin concentrations (n = 23 for P3Pro; n = 11 for Alb/P3Pro) and serum Epo levels in Alb/P3Pro double mutants compared with P3Pro mutants (n = 4) under baseline conditions. (B) Serum Epo levels in P3Pro and Alb/P3Pro mutants after phlebotomy of (n = 7). Epo mRNA levels in livers of P3Pro and Alb/P3Pro mutants at baseline conditions (n = 3) and after phlebotomy (mice were analyzed 18 hours after phlebotomy; n = 7), as determined by real-time PCR. (C) Serum Epo levels in P3Pro (n = 13) and Alb/P3Pro mutants (n = 3) after chronic hypoxia exposure (10% O2 for 10 days) and corresponding Epo mRNA levels in P3Pro and Alb/P3Pro livers (n = 3). *P < .05; ns, not statistically significant.
To examine hypoxia-triggered erythropoietic responses in Alb/P3Pro double mutants, mice were challenged with acute anemic hypoxia induced by phlebotomy. Alb/P3Pro mutants exhibited an ∼ 70% reduction in serum Epo levels compared with P3Pro mutants (1535 + 612 pg/mL and 465 + 72 pg/mL, respectively; P < .05), which was associated with a corresponding decrease in liver Epo mRNA levels (Figure 7B). These data suggest that under these conditions ≥ 70% of serum Epo is generated through the activation of hepatocyte-derived Hif-2 signaling. Moreover, our experiments most probably underestimate Hif-2's contribution due to limitations in recombination efficiency, because not all hepatocytes express the Albumin-Cre transgene. Consistent with the findings under acute hypoxia are the results from chronic hypoxia exposure (10% O2 for 10 days), where serum Epo levels in Alb/P3Pro mutants were reduced by ∼ 60% (281 ± 37 pg/mL, compared with 122 ± 15 pg/mL in P3Pro mice; P < .05; Figure 7C). The difference in hepatic Epo mRNA expression under chronic hypoxia, however, did not reach statistical significance (P = .08). This is most probably because of submaximal hypoxic stimulation in this setting, which compared with acute anemia resulted in lower liver Epo output (for P3Pro mutants, 281 ± 37 pg/mL vs 1535 ± 612 pg/mL) and would require a larger sample size to detect statistically significant differences. Nevertheless, taken together, our results suggest that under acute hypoxic conditions hepatocyte-derived Hif-2 regulates ≥ 70% of the serum Epo pool and appears to be the main regulator of serum Epo in the absence of renal Hif-2.
Hif-2–dependent regulation of iron metabolism genes
Hypoxic stimulation of erythropoiesis requires that additional iron is readily made available for heme synthesis. Because the liver plays a central role in the regulation of iron homeostasis, we hypothesized that HIF-2 may coordinate EPO production with iron metabolism in the liver. We therefore examined to what degree hepatocyte-derived Hif-2 participated in the hypoxic regulation of genes involved in iron metabolism. To address this question we made use of 2 different models of Hif activation. In the first model, nonhypoxic Hif stabilization was accomplished by cell type-specific pVHL inactivation in hepatocytes; in the second model, Hif was stabilized by systemic hypoxia in all liver cells. We first determined mRNA levels of iron metabolism genes in littermate control mice, Albumin-Cre/Vhlh mutant mice (Vhlh is the symbol for murine VHL), which express both Hif-1α and Hif-2α in hepatocytes, Albumin-Cre/Vhlh/Hif-1α mutants, which lack Hif-1α and express Hif-2α in hepatocytes, Albumin-Cre/Vhlh/Hif-2α mice, which only express Hif-1α, and Albumin-Cre/Vhlh/Hif-1α/Hif-2α mutants, which lack both Hifs in hepatocytes. We found a Hif-2–dependent increase in the expression levels of ceruloplasmin (Cp), hephaestin (Heph), δ-aminolevulinate synthase 2 (Alas2), divalent metal transporter 1 (Dmt1), mitoferrin (Mfrn), transferrin (Trf), transferrin receptor 1 (Trfc), and lipocalin (Lcn2; Table 1). Hepcidin (Hamp), which regulates intestinal iron uptake and release from body iron stores by facilitating the internalization and degradation of cellular iron export protein ferroportin, was down-regulated ∼ 2-fold in Albumin-Cre/Vhlh livers compared with control, which is most probably a reflection of the increased erythropoietic activity observed in these mice15 (Table 1). No change in serum iron levels and total iron binding capacity was found in Albumin-Cre/Vhlh mutants (supplemental Figure 3). Because inactivation of pVHL results in constitutive activation of the Hif system under normoxia, we next examined to what degree hepatocyte-derived Hif-2 regulated iron metabolism genes under hypoxic conditions in mice with intact pVHL. For this we used a model of severe anemia induced by phenylhydrazine in conjunction with phlebotomy, which results in hypoxic stabilization of both Hif-1α and Hif-2α in hepatocytes and other liver cell types, including endothelial and biliary epithelial cells.15 Iron metabolism gene expression in whole liver homogenates was examined by real-time PCR in anemic mice (Hct levels of ∼ 15%), which expressed both Hif-1 and Hif-2 in the liver, and in anemic mice, which either lacked Hif-1α (Albumin-Cre/Hif-1α), Hif-2α (Albumin-Cre/Hif-2α), or both (Albumin-Cre/Hif-1α/Hif-2α) in hepatocytes specifically. mRNA levels were compared with normocythemic Cre-negative control mice.15 Using this model, we identified Dmt1 as being coregulated by hepatocyte-derived Hif-1 and Hif-2 in a statistically significant manner, whereas the expression levels of other hypoxia-induced genes in the liver were not strongly affected by Hif inactivation in hepatocytes (supplemental Table 2; supplemental Figure 4). Our data indicate that in a pVHL-deficient background iron metabolism gene expression in hepatocytes is predominantly Hif-2 regulated.
Hif-2 regulates iron metabolism gene expression in hepatocytes
| Gene . | VHL . | VHLHif1 . | VHLHif2 . | VHLHif1Hif2 . |
|---|---|---|---|---|
| Epo | 1024.63* | 1046.88* | 23.23* | 2.43 |
| Vegf | 7.97* | 7.33* | 1.29 | 0.91 |
| Cp | 3.58* | 2.47* | 0.67* | 0.98 |
| Heph | 5.76* | 5.20* | 1.16 | 0.91 |
| Aco1 | 0.83 | 1.12 | 0.99 | 0.91 |
| Ireb2 | 0.95 | 0.86 | 0.99 | 1.08 |
| Alas2 | 2.08* | 10.33* | 1.2 | 0.89 |
| Hamp | 0.58* | 0.27* | 0.21* | 1.25 |
| Hfe | 2.28* | 2.15* | 1.55* | 1.36* |
| Hfe2 | 0.61* | 0.84 | 0.97 | 1.56 |
| Dmt1 | 10.52* | 17.22* | 0.88 | 1 |
| Mfrn | 4.46* | 7.42* | 0.8 | 0.99 |
| Fpn | 3.12* | 1.84 | 0.92 | 0.87 |
| Trf | 3.25* | 2.39* | 0.89 | 0.93 |
| Trfc | 8.37* | 4.01* | 1.7 | 0.81 |
| Trfr2 | 0.62* | 0.88 | 1.02 | 0.95 |
| Lcn2 | 74.84* | 49.26* | 0.71 | 1.06 |
| Gene . | VHL . | VHLHif1 . | VHLHif2 . | VHLHif1Hif2 . |
|---|---|---|---|---|
| Epo | 1024.63* | 1046.88* | 23.23* | 2.43 |
| Vegf | 7.97* | 7.33* | 1.29 | 0.91 |
| Cp | 3.58* | 2.47* | 0.67* | 0.98 |
| Heph | 5.76* | 5.20* | 1.16 | 0.91 |
| Aco1 | 0.83 | 1.12 | 0.99 | 0.91 |
| Ireb2 | 0.95 | 0.86 | 0.99 | 1.08 |
| Alas2 | 2.08* | 10.33* | 1.2 | 0.89 |
| Hamp | 0.58* | 0.27* | 0.21* | 1.25 |
| Hfe | 2.28* | 2.15* | 1.55* | 1.36* |
| Hfe2 | 0.61* | 0.84 | 0.97 | 1.56 |
| Dmt1 | 10.52* | 17.22* | 0.88 | 1 |
| Mfrn | 4.46* | 7.42* | 0.8 | 0.99 |
| Fpn | 3.12* | 1.84 | 0.92 | 0.87 |
| Trf | 3.25* | 2.39* | 0.89 | 0.93 |
| Trfc | 8.37* | 4.01* | 1.7 | 0.81 |
| Trfr2 | 0.62* | 0.88 | 1.02 | 0.95 |
| Lcn2 | 74.84* | 49.26* | 0.71 | 1.06 |
Shown are fold changes in mRNA expression levels of genes regulating iron metabolism. Analyzed were Albumin-Cre conditional mutant mice and compared with Cre-negative littermates. First column, pVHL-deficient livers (VHL) from Albumin-Cre/Vhlh mutant mice compared with Cre-negative littermate controls (n = 4-8 each); second column, livers that lack pVHL and Hif-1α and express high levels of Hif-2α (VHLHif1) compared with Cre-negative littermates (n = 4-8 each); third column, livers that lack pVHL and Hif-2α but express high levels of Hif-1α (VHLHif2) compared with Cre-negative littermates (n = 5-8 for mutants and n = 5 for Cre-negative littermates); fourth column, livers that lack pVHL, Hif-1α, and Hif-2α (VHLHif1Hif2) compared with Cre-negative littermates (n = 4-8 each).
Vegf indicates vascular endothelial growth factor; Cp, ceruloplasmin; Heph, hephaestin; Aco1, aconitase; Ireb2, iron regulatory protein 2; Alas2, δ-aminolevulinate synthase 2; Hamp, hepcidin; Hfe, hemochromatosis protein.
P < .05.
Discussion
In this report we have used a genetic approach (1) to specifically investigate the role of renal HIF-2 in the regulation of serum EPO levels and (2) to examine the degree by which nonrenal HIF-2 contributes to hypoxia-stimulated erythropoiesis.
Our data, which showed that renal Epo is exclusively Hif-2 regulated, are compatible with immunohistochemical studies that localized Hif-2α but not Hif-1α to Epo-producing renal cells20 and with genetic studies in patients with familial erythrocytosis in whom only mutations in HIF-2α and not in the HIF-1α gene have been found.32,33 They are furthermore consistent with findings from a tamoxifen-inducible global HIF-1 knockout model in which Hif-1 had no role in the regulation of Epo homeostasis at baseline or in response to acute hemolytic anemia.14 Although responses to chronic hypoxia were not examined in this model, delayed erythrocytosis on exposure to chronic hypoxia (10% O2) and a lack of rise in renal Epo after intermittent hypoxia were observed in mice with heterozygous Hif-1α deficiency.21,22 The discrepancy between our data and observations in Hif-1α haploinsufficient mice may be explained by recent findings that identified epidermal Hif-1 as a regulator of renal Epo production. The skin has been proposed to modulate renal and hepatic EPO synthesis indirectly through HIF-1–dependent, nitric oxide–mediated effects on dermal blood flow,34 which would require intact HIF signaling at the sites of EPO production. Although our studies could not find a role for Hif-1 in the hypoxic induction of renal Epo in adult mice, Hif-1 has been shown to be important in Epo synthesis during early embryonic development.35
P3Pro mutants lacked hypoxia inducibility of renal Epo completely, whereas their hepatic Epo response was normal. Although the kidney is the primary physiologic site of postnatal EPO synthesis, the liver is the main source during embryonic development and produces EPO in adults only under severe hypoxic conditions. Although hepatocytes are the primary EPO-expressing cell type in the liver, Epo has been detected in hepatic stellate cells, which are also known as Ito cells.36,37 The timing of transition from the liver to the kidney as the primary site of EPO production is species dependent and usually occurs late in gestation or around birth.7,38-40 The molecular mechanisms that control this switch are poorly understood and may involve transcriptional repression or reduced expression of transcriptional activators such as globin transcription factor 4 (GATA-4).41 However, we found that hepatic Gata-4 mRNA levels were not different between P3Pro mutants and controls, suggesting that increased Gata-4 levels are not required for persistent hepatic Epo expression found in mutant mice (data not shown). Because the onset of the transition from liver to kidney as the main EPO source was significantly delayed in chronically anemic sheep,38 it is probable that liver pO2 plays a main role in the regulation of this transition.
We found that hepatocyte-derived Hif-2 is the main regulator of serum Epo in the absence of hypoxia-inducible renal Epo, because serum Epo levels were reduced by ∼ 60%-70% in hypoxic P3Pro/Albumin double mutants. It is probable that our study underestimated the contribution of hepatocyte-derived Hif-2 to the serum Epo pool, because only 80%-90% of hepatocytes were targeted by the Albumin-Cre transgene. However, we cannot exclude that Ito cells or other organs have contributed to serum Epo levels in P3Pro/Albumin mutants. Recent studies proposed a significant role for glial Hif-2 in hypoxia-triggered erythropoiesis, suggesting that the brain can drive up to 50% of the erythropoietic response in acutely hypoxic mice.42 Inactivation of glial Hif-2α in P3Pro mutants with the use of GFAP-Cre,43 however, did not produce an additional decrease in Hct level or serum Epo in response to acute anemic hypoxia (data not shown). EPO mRNA has also been detected in lung, testis, and uterus and in other cell types1 ; nevertheless, the contribution of these sites to systemic EPO homeostasis appears to be negligible.7,8 The liver is the main source of extrarenal EPO and has the capacity to generate between 25% and 50% of circulating EPO under moderate to severe hypoxic conditions.7,8 Furthermore, the lack of a significant rise in serum Epo under hypoxic conditions in animals that underwent bilateral nephrectomy combined with subtotal hepatectomy suggests that other organs do not contribute to the serum EPO pool.7,9,44 Nevertheless, the ability of other sites to synthesize EPO can lead to polycythemia in certain pathologic settings, for example, in patients with uterine myomata or cerebellar hemangioblastomas.45,46
The role of HIF-2 in hypoxia-induced erythropoiesis extends beyond the transcriptional control of EPO. Here, we also provide evidence that hepatocyte-derived Hif-2 is involved in the transcriptional regulation of several iron metabolism genes.47 When erythropoiesis is stimulated by hypoxia, iron demand in the bone marrow increases. This necessitates enhanced intestinal iron uptake, an augmentation of serum iron binding capacity and mobilization from body iron stores. We found that in a model of nonhypoxic constitutive Hif activation in hepatocyte-specific pVHL knockout mice, genes involved in iron homeostasis, such as Dmt1, Cp, and Heph, are regulated by Hif-2, suggesting that hepatocyte-derived HIF-2 coordinates EPO synthesis with iron metabolism. This notion is supported by the recent observation that Hif-2 regulates enteral iron uptake through induction of intestinal Dmt1 and DcytB.48,49 However, we were unable to establish an extensive role for hepatocyte-derived Hif-2 in iron metabolism in a model of anemic hypoxia. Although Dmt1 appeared to be coregulated by Hif-1 and Hif-2, the hypoxic induction of other genes, such as Cp, did not depend on hepatocyte-derived Hif in our analysis. This may be due to the use of whole liver homogenates and the contribution of other liver cell types to the RNA pool that was analyzed.
Pharmacologic HIF stabilization induced hepatic Epo mRNA levels and resulted in increased hemoglobin levels and anemia improvement in P3Pro mutants. Clinically, an impairment of the kidney's ability to synthesize adequate amounts of EPO is typically found in patients with chronic kidney disease, resulting in renal anemia.10 Although the VHL/PHD/HIF pathway appears to be operational in EPO-producing renal cells, EPO synthesis in the kidney is inadequate in this setting. The liver, which may also be negatively affected by uremia, only partially compensates for this impairment in renal EPO production,7 unless HIF is stabilized pharmacologically. HIF stabilization through prolyl-4-hydroxylase inhibition with 2-oxoglutarate analogs has the potential to stimulate endogenous EPO synthesis in patients with renal anemia. Oral administration of a PHD inhibitor in nonhuman primates resulted in transient HIF activation and the induction of endogenous EPO synthesis and erythropoiesis.31 Oral administration of the same compound to patients undergoing dialysis, including anephric patients, increased serum EPO levels.50 Although these studies did not address the tissue source of EPO, our study suggests that systemic PHD inhibition can be effectively used to treat renal anemia by inducing hepatic EPO synthesis. Because PHD inhibition results in the stimulation of multiple HIF-regulated biologic processes, such as angiogenesis, careful clinical evaluation of this novel therapeutic approach is warranted before it can be safely used in patients with chronic kidney disease.
In summary, we have shown by genetic means that Hif-2 and not Hif-1 regulates the hypoxic induction of Epo in murine kidneys and that hepatocyte-derived Hif-2 takes over as the main regulator of systemic Epo homeostasis when Epo production in the kidney is impaired.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Heather P. Shutt for technical assistance.
This work was supported by the Krick-Brooks chair in Nephrology (V.H.H.), funds from the Vanderbilt Department of Medicine, and in part by the National Institutes of Health (grants R01-CA100787 and R01-DK081646; V.H.H.).
National Institutes of Health
Authorship
Contribution: P.P.K. and Q.L. contributed equally to the experimental work; P.P.K., Q.L., and V.H.H. conceived the experiments; P.P.K., Q.L., T.L.U., J.R., O.D., and V.H.H. performed experiments; P.P.K., Q.L., T.L.U., and V.H.H. analyzed and interpreted data; B.K., J.A.E., S.L.M., and C.L.E.-M. contributed reagents; and P.P.K. and V.H.H. wrote the manuscript.
Conflict-of-interest disclosure: C.L.-M. is an employee of GlaxoSmithKline and owns stocks. S.L.M. was an employee of GlaxoSmithKline. V.H.H. serves on the Scientific Advisory Board of Akebia Therapeutics, a company that develops prolyl-4-hydroxylase inhibitors for the treatment of anemia. The remaining authors declare no competing financial interests.
Correspondence: Volker H. Haase, Division of Nephrology and Hypertension, Vanderbilt University Medical Center, C-3119A MCN, 1161 21st Ave S, Nashville, TN 37232-2372; e-mail: volker.haase@vanderbilt.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal