In this issue of Blood, Ortona and colleagues identify the complex between vimentin and cardiolipin as an antigenic target in the APS, and demonstrate that affinity-purified antivimentin/cardiolipin antibodies induce IRAK1 phosphorylation and NF-κB activation in endothelial cells.
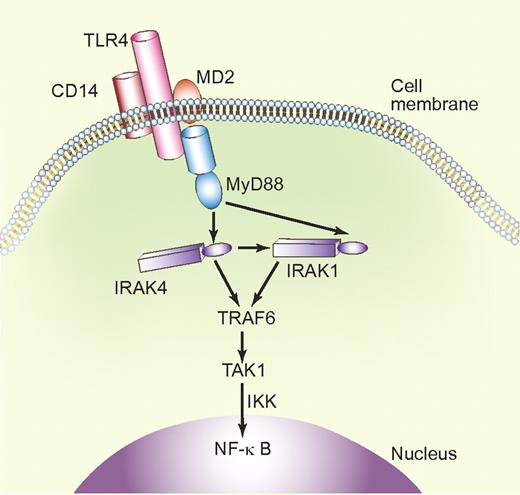
Simplified schematic representation of the TLR4 signaling pathway. Upon interaction with LPS via CD14 and MD2 (myeloid differentiation protein-2), TLR4 recruits the adaptor protein MyD88 and activates IRAK1 and IRAK4 via MyD88-controlled pathways. In combination with TRAF6 (TNFα receptor–associated factor-6), IRAKs activate TAK1 (TGFβ–activated kinase 1). This leads, via phosphorylation of IKKs (IκB kinases), to NF-κB activation and transcription of inflammatory cytokines. Professional illustration by Paulette Dennis.
Simplified schematic representation of the TLR4 signaling pathway. Upon interaction with LPS via CD14 and MD2 (myeloid differentiation protein-2), TLR4 recruits the adaptor protein MyD88 and activates IRAK1 and IRAK4 via MyD88-controlled pathways. In combination with TRAF6 (TNFα receptor–associated factor-6), IRAKs activate TAK1 (TGFβ–activated kinase 1). This leads, via phosphorylation of IKKs (IκB kinases), to NF-κB activation and transcription of inflammatory cytokines. Professional illustration by Paulette Dennis.
The antiphospholipid syndrome (APS) is an autoimmune disease, defined by the presence of so-called antiphospholipid antibodies, in association with clinical manifestations such as thromboembolic events and recurrent abortions and fetal loss. The APS diagnosis strictly relies on the simultaneous identification of clinical manifestations and laboratory findings; the laboratory diagnosis of APS relies on very strict criteria, reached by international consensus.1
Such strict criteria are unavoidable, because antiphospholipid antibodies represent a heterozygous group of antibodies, reactive with several phospholipid-binding plasma proteins. The best-known antigen is β2-glycoprotein I (β2GPI), against which antibodies are found in the majority of APS patients.2 Yet, many more proteins are potential antigen targets in APS, with many implicated in coagulation and anticoagulation regulatory pathways, platelet activation, endothelial cell activation pathways, or in complement activation.1 Another potential mechanism is that APS is characterized by the occurrence of phospholipid-dependent autoimmune antibodies against targets that can trigger different cellular responses, resulting in a variable intensity of clinical manifestations, which, in addition, are determined by antibody specificity and titer. Thromboembolic events and recurrent abortions are the best known manifestations of APS but the clinical symptoms such as neurological and pregnancy complications cannot be explained by thrombotic or ischemic mechanisms.3 Hence, understanding the specific antibody-induced pathways that lead to clinical complications and their pathophysiologic consequences is the focus in current APS research.
To make things even more complex, some patients have clinical signs suggestive of APS, but are persistently negative for phospholipid (eg, cardiolipin)–dependent immune assays, including β2GPI as an antigen and coagulation assays, revealing the lupus anticoagulant activity associated with antiphospholipid antibodies. These “seronegative” APS patients4 suggest the existence of relevant, hitherto unknown antigens in APS.
Ortona and colleagues have analyzed the sera of seronegative APS patients for the presence of a “new” antigen, relevant in APS.5 By autoantibody screening via a proteomic approach and by using as an antigen source the cell-surface membrane proteins of endothelial cells, they identified endothelial cell vimentin as a protein, recognized by patient autoantibodies. Vimentin displayed phospholipid-binding properties and strongly interacted with cardiolipin; it was also found to be present on the endothelial cell surface.5 More importantly, the authors found that more than 90% of APS patients and approximately half of the seronegative APS patients had antivimentin/cardiolipin immunoglobulin G antibodies. These antibodies were present in sera from patients with arterial and venous thrombosis, as well as with pregnancy morbidity. Vimentin is a ubiquitously expressed cytoskeleton intermediate filament protein. Intermediate filaments constitute 1 of the 3 major components of the cytoskeleton, which also includes actin microfilaments and tubulin microtubules. Vimentin and keratin are the major intermediate filament proteins in endothelial cells and endothelial barrier—disrupting agents such as histamine induce vimentin phosphorylation in human umbilical vein endothelial cells and dissociation from adhesive complexes.6
Anti-β2GPI antibodies have been shown to display signaling cascades, similar to those triggered by lipopolysaccharide (LPS) or interleukin-1 (IL-1), and schematically illustrated in the figure; studies on the phosphorylation of IL-1 receptor–associated kinase (IRAK) have shown similar time kinetics upon activation by LPS and anti-β2GPI antibodies.7 These studies have uncovered a role not only for the Toll-like receptors TLR4 in the activation of endothelial cells, but also for monocytes and dendritic cells. Recently, TLR2 was identified as a direct receptor for β2GPI on the endothelial cell surface.8 TLRs induce rapid inflammatory responses and mediate activation of immune effector cells; mediators of the innate immunity are now recognized as additional second hits in the induction of thromboembolic complications in APS, in the presence of antiphospholipid antibodies. In this issue, Ortona and colleagues have shown that affinity-purified antivimentin/cardiolipin antibodies isolated from seronegative patient samples can also induce IRAK1 phosphorylation (primarily coupled to secretion of the cytokine tumor necrosis factor α), a phenomenon which was not observed in incubations with purified antivimentin antibodies.5 Therefore, these experiments (1) illustrate the dependence on phospholipids for the reaction, and (2) couple vimentin via IRAK to endothelial cell activation pathways, similar to those activated via TLRs by antiphospholipid anti-β2GPI antibodies.7 Phosphorylation of IRAK1 triggered NF-κB activation leads to transcriptional activation, similar to NF-κB activation upon LPS stimulation.
It is not yet clear how antivimentin/cardiolipin antibodies trigger vimentin-mediated TLR activation, but autoantibodies to vimentin have recently been shown to potentiate graft vasculopathy in murine cardiac allografts.9 Therefore, the findings reported by Ortona et al in this issue5 have identified vimentin/cardiolipin as a new APS-related antigen. This antigen has limited specificity—because it is also antigenic in patients with systemic lupus erythematosus and rheumatoid arthritis. The illustration by the authors of the existence of antiphospholipid antibody-triggered and vimentin-mediated endothelial cell activation via pathways—characteristic of TLR-mediated signaling, reminiscent of activation pathways triggered by anti-β2GPI antibodies—advances our understanding of APS pathogenic mechanisms. This similarity in cellular activation pathways will help explain how proinflammatory cytokines, blood cells, endothelial cells, procoagulant factors, and complement trigger clinical manifestations in APS.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal