To the editor:
Primary myelofibrosis (PMF) is a myeloproliferative neoplasm (MPN) with a high propensity to develop acute myeloid leukemia, named at this stage blast phase (BP) of PMF.1 Oncogenic JAK2 signaling is an important event in MPN, but transformation process into BP, although not completely understood, often involves additional genomic lesions.2 Several models have been developed to predict survival in PMF.3-5 Concerning BP occurrence, leukocyte count more than 30 × 109/L,5 blast cell count more than 10%,6 platelet count below 50 × 109/L,6 red blood cell transfusion dependency,7 selected cytogenetic abnormalities,6,8 low JAK2(V617F) allele burden9 have been reported to shorten BP-free survival.
The International Working Group on Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) has recently developed a time-dependent prognostic model, named Dynamic International Prognostic Scoring System (DIPSS) to predict survival anytime in patients with PMF.10 This model includes age older than 65 years, hemoglobin level lower than 10 g/dL, white blood cell count more than 25 × 109/L, peripheral blood blasts equal to or higher than 1%, and constitutional symptoms.
In this study, we investigated whether DIPSS may also predict the occurrence of BP. The study was approved by the Institutional Review Board of each participating center, and the procedures followed were in accordance with the Declaration of Helsinki. On behalf of IWG-MRT, we surveyed the large-scale international database of 525 regularly followed patients with PMF, which allowed the definition of DIPSS.10 Blast phase was defined when a threshold of 20% peripheral blast cells was achieved during follow-up.
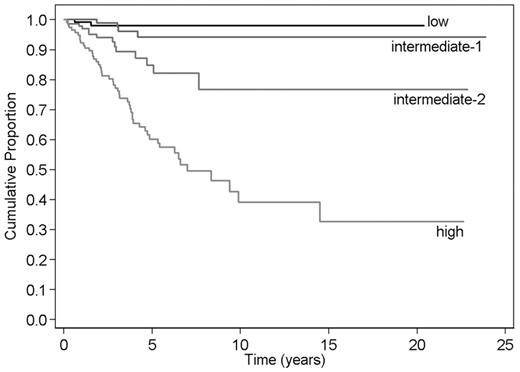
Among 525 patients, 70 (13%) developed BP after a median time of 2.8 years (range, .2-14.5). Median age was 68 years (range, 31-90 years), 58 (83%) patients were male, and 56 (80%) received cytotoxic agents during chronic phase. The incidence of BP was 0.3 (95% confidence interval [CI]: 0.04-1.2) × 100 patient/years in low-risk category, 0.7 (95% CI: 0.2-1.7) × 100 patient/years in intermediate-1, 2.6 (95% CI: 1.4-4.4) × 100 patient/years in intermediate-2, and 8.6 (95% CI: 6.4-11.4) × 100 patient/years in high risk.
We analyzed the categorical DIPSS score as a time-dependent covariate in a Cox survival regression model with BP as outcome (Figure 1). Overall, the Gehan Wilcoxon test showed that DIPSS stratified PMF patients for BP risk (P < .001). The increase in risk when changing risk category was further estimated as a hazard ratio (HR). The estimated HRs were: 2 (95% CI: 0.4-11.3; P = .4) if the risk category shifted from low to intermediate-1, 3.8 (95% CI: 1.2-11.4; P = .019) from intermediate-1 to intermediate-2, and 3.2 (95% CI: 1.8-5.8; P < .001) from intermediate-2 to high. Comparing higher risk categories to low-risk category, HR was 7.8 (95% CI: 1.8-34.2; P = .007) for intermediate-2 and 24.9 (95% CI: 6-102.3; P < .001) for high risk.
Kaplan-Meier estimate of blast phase–free survival in primary myelofibrosis according to the DIPSS. Risk categories were according to the score obtained anytime during follow-up. Values for score calculation are as follows: 1 for age > 65 years, 2 for hemoglobin level < 10 g/dL, 1 for white blood cell count > 25 × 109/L, 1 for peripheral blood blasts ≥ 1%, and 1 for constitutional symptoms. Risk categories are low (score: 0), intermediate-1 (score: 1 or 2), intermediate-2 (score: 3 or 4), and high (score: 5 or 6).
Kaplan-Meier estimate of blast phase–free survival in primary myelofibrosis according to the DIPSS. Risk categories were according to the score obtained anytime during follow-up. Values for score calculation are as follows: 1 for age > 65 years, 2 for hemoglobin level < 10 g/dL, 1 for white blood cell count > 25 × 109/L, 1 for peripheral blood blasts ≥ 1%, and 1 for constitutional symptoms. Risk categories are low (score: 0), intermediate-1 (score: 1 or 2), intermediate-2 (score: 3 or 4), and high (score: 5 or 6).
The study shows that modification of the DIPSS during follow-up of PMF patients may also predict different risks of BP. Patients belonging to the higher risk categories have a notable 7.8-fold and 24.9-fold higher risk of developing BP comparing to those who continue to fit in low-risk category. As BP evolution implies a dismal outcome,1 this observation recommends to approach patients with higher DIPSS categories for intensive or investigative treatments.
Authorship
Contribution: F.P and A.T. designed research; F.P. interpreted results and wrote the paper; F.C., A.M.V., E.M., E.R., and M.C. performed research and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Francesco Passamonti, Department of Hematology Oncology, Division of Hematology, University of Pavia Medical School and Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; e-mail francesco.passamonti@unipv.it.