Abstract
Erythrophagocytosis and inflammation from activated macrophages occur in distinct clinical scenarios. The presence of CD8+ T cells and interferon-γ (IFN-γ) production is required to induce disease in mouse models of hemophagocytic lymphohistiocytosis. We investigated the roles of a different class of proinflammatory cytokines, interleukin-4 (IL-4) and IL-13, in the induction of inflammatory tissue macrophage accumulation and/or hemophagocytosis. We found that large amounts of IL-4, but not IL-13, delivered via an implanted mini-pump or IL-4/anti–IL-4 complexes, lead to substantial YM1+ tissue macrophage accumulation, erythrophagocytosis within the liver, spleen, and bone marrow, decreased hemoglobin and platelet levels, and acute weight loss. This effect is not dependent on the presence of antibody or T cells, as treatment of Rag2−/− mice leads to similar disease, and IFN-γ neutralization during IL-4 treatment had no effect. IL-4 treatment results in suppression of IL-12, elevation of serum IFN-γ, IL-10, and the murine IL-8 homolog KC, but not IL-6, IL-1β, or tumor necrosis factor-α. Finally, mice transgenic for IL-4 production developed tissue macrophage accumulation, disruption of splenic architecture, bone marrow hypocellularity, and extramedullary hematopoiesis. These data describe a novel pathophysiologic pathway for erythrophagocytosis in the context of tissue macrophage accumulation and inflammation involving elevations in IL-4 and alternative macrophage activation.
Introduction
Erythrophagocytosis and accumulation and infiltration of macrophages in tissue occur in a limited set of conditions. Hemophagocytic lymphohistiocytosis (HLH) and macrophage activation syndromes are examples of disorders of abnormal, severe immune activation both characterized by fever, splenomegaly, histiocytic invasion of the liver, spleen, variably bone marrow, and other organs with accompanying engulfment of erythrocytes, and often a cytokine “storm” composed of numerous inflammatory cytokines.1 These conditions are usually fatal if not treated with aggressive chemotherapy and, ultimately, bone marrow transplantation. Mutations in the gene encoding perforin or other genes encoding proteins implicated in perforin release, in the context of an additional trigger, account for many cases of HLH. Acquired or secondary HLH can be induced by viral infection and may occur in visceral Leishmaniasis, autoimmune disease, and malignancy.2 The precise pathogenesis of HLH is not well understood, although it is thought that overproduction of macrophage-activating cytokines by lymphocytes is critical.
Mouse models of HLH involve lymphocytic choriomeningitic virus (LCMV) infection of mice deficient in genes whose absence increase HLH susceptibility in humans, such as Prfl, Unc13d,4 and Rab27a.5 HLH induction in LCMV infection of the Prfl−/− mouse requires both the production of interferon-γ (IFN-γ) and the presence of CD8+ T cells.3
Mediators known to activate macrophages are probably culprits in the induction of disease. These can include IFN-γ and lipopolysaccharide. Inflammatory cytokines, such as IFN-γ, interleukin-2 (IL-12), and tumor necrosis factor-α (TNF-α), are found at high concentrations in HLH patients.6 Alternative macrophage activation, such as that induced by IL-4 or IL-13, can also lead to significant tissue inflammation, as seen in a number of disease models.7 Although Th2 cytokines have not been found in perforin deficiency-associated HLH,6 their role in other forms of HLH is not well defined. Furthermore, the effects of aberrant Th2-induced alternative macrophage activation are not well studied and may be associated with unique pathology resembling, but not identical to, HLH.
We therefore set out to investigate the role of IL-4 and IL-13 in the induction of tissue macrophage accumulation and infiltration, and erythrophagocytosis.
We found that exposure to high concentrations of IL-4 via implanted mini-osmotic pumps or IL-4/anti–IL-4 immune complexes leads to severe histiocytosis and erythrophagocytosis within the liver and spleen, bone marrow erythrophagocytosis, and acute weight loss. The accumulated macrophages were YM1+, indicating alternative activation. This effect is not dependent on the presence of antibody or T cells, as treatment of Rag2−/− mice with IL-4 leads to similar disease. IL-4 treatment results in suppression of IL-12, elevation of serum IFN-γ, IL-10, and KC (the mouse IL-8 analog), but not IL-6, IL-1β, or TNF-α. IFN-γ neutralization during IL-4 treatment actually exacerbates disease.
Interestingly, IL-13 treatment leads to mild expression of YM1 in bile ducts and to serum KC elevation and IL-12 suppression, but no significant pathology. Taken together, these data describe a potentially novel pathophysiologic pathway for erythrophagocytic disorders involving acute elevations in IL-4 and alternative macrophage activation.
Methods
Mice
C57BL6, B6 Rag2−/−, and B6 STAT6−/− mice were obtained from Taconic Farms. IL-4TG.UG B6 mice were housed at the University of Cincinnati animal care facility. All mice experiments were approved by the Animal Care and Use Committee of the Laboratory of Immunology at the National Institute of Allergy and Infectious Diseases and the University of Cincinnati.
Cytokines
Recombinant mouse IL-4 and IL-13 were purchased from PeproTech.
Micro-osmotic pump implantation
Micro-osmotic pumps (Alzet) were filled with cytokine in 100 μL volume as per the manufacturer's instructions and surgically implanted subcutaneously under sterile conditions. Mice were killed 3 days after implantation.
IL-4C injections: IL-4C
Recombinant mouse IL-4 (PeproTech) was mixed with BVD4-1D11 (rat IgG2b anti–mouse IL-4) in an Eppendorf tube at a 1:5 weight (2:1 molar) ratio and incubated on ice for 5 minutes, then diluted to an IL-4 concentration of 50 μg/mL with 1% BALB/c serum in normal saline. Mice were injected intraperitoneally 3 times per week with 0.2 mL (10 μg IL-4/50 μg anti–IL-4).
Antibody treatment
Mice were injected intraperitoneally with 1 mg rat IgG1 anti–IFN-γ (XMG-6) or isotype-matched control monoclonal antibody (GL113). Serum IFN-γ levels were undetectable for up to a week after administration. All hybridomas were originally obtained from Robert Coffman and were grown as ascites in Pristane-primed athymic nude mice and purified by ammonium sulfate fractionation followed by DE-52 (Whatmann) ion-exchange chromatography.
Histology/immunohistochemistry: pathology/immunohistochemistry
Immediately after killing mice in a CO2 chamber, organs were removed, fixed in neutral buffered formalin, and embedded in paraffin. Sections were stained with hematoxylin and eosin. Immunohistochemistry was performed on some tissues with antibodies to Ym-18 by the ABC method (Vector Laboratories). Histopathology was evaluated by a veterinary pathologist.
Isolation of liver macrophages
Immediately after death, mouse livers were perfused with 0.6mM ethylenediaminetetraacetic acid in phosphate-buffered saline (PBS). Livers were then cut into pieces and placed into Hanks balanced salt solution containing 24 μg/mL Liberase (Roche Diagnostics) and 1.6 U/mL DNase I (Roche Diagnostics) for 30 minutes at 37°C. They were then homogenized and strained through a 40-μm filter and plated in RPMI containing 2% fetal calf serum on regular nontissue culture medium–treated Petri dishes overnight. Nonadherent cells were washed, and adherent cells were mobilized for quantitation by F4/80 antibody staining (eBioscience).
Serum ferritin, fibrinogen, triglyceride measurement
Serum from mice was assayed by enzyme-linked immunosorbent assay for ferritin and fibrinogen levels levels (Innovative Research) as per the manufacturer's instructions. Serum triglyceride levels were determined using the GPO-Trinder method for Triglyceride by Roche performed on a Chemwell 2910 Analyzer (Aware Tech).
Reverse-transcribed PCR
Total RNA was isolated from livers using RNeasy kit (QIAGEN) according to the manufacturer's instructions. Equal amounts of total RNA were reverse-transcribed to cDNA using SuperScript II First-Strand Synthesis System for reverse-transcribed polymerase chain reaction (PCR; Invitrogen). Quantitative PCR reactions were performed using a 7500 sequence detection system (Applied Biosystems). The primer/probe sets for detection of Arg1, Tm1 iNOS, and TaqMan Ribosomal RNA Control Reagents for detecting the 18S ribosomal RNA (VIC-MGB probe) were purchased from Applied Biosystems. The mRNA levels of assayed genes were normalized to 18S ribosomal RNA.
Multiplex cytokine analysis
Serum from treated mice was tested for cytokine expression using the Meso Scale 9-plex mouse inflammatory panel as per the manufacturer's instructions.
Microscopy
All micrographs were obtained using an Olympus BX51 microscope, mahnifications 4×/0.16 numeric aperture (NA), 40×/0.95 NA, or 100×/1.4 NA oil. Images were captured with attached Olympus DP70 Digital camera and software Version 2.2.1.227. Images were formatted with Adobe Photoshop CS2.
Results
Continuous infusion of IL-4, but not IL-13, leads to systemic alternative macrophage activation, Kupffer cell hyperplasia, erythrophagocytosis, and tissue macrophage infiltration/accumulation, independent of T cells or antibody
We assessed the effects of large amounts of systemic IL-4 on macrophage-related pathology. Micro-osmotic pumps were filled with IL-4 and surgically implanted in young B6 wild-type mice. Serum IL-4 levels were measured to be between 30 and 50 ng/mL when 1 μg/hour was infused and more than 50 ng/mL when 3 μg/hour was infused. Within 2 to 3 days, the mice developed histopathology characterized by significant macrophage infiltrate of the spleen with increased erythrophagocytosis, red pulp hyperplasia, and disrupted splenic architecture; and Kupffer cell hyperplasia with accumulation of activated macrophages in the lumen of liver sinusoids accompanied by erythrophagocytosis, histiocytic infiltration of lymph nodes, and thymic atrophy (Figure 1A-F, Table 1, supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The bone marrow also showed erythrophagocytosis (Table 1, Figure 1B, supplemental Figure 2). Weight loss (Figure 2A) and even death were noted, although the frequency of lethality varied from experiment to experiment, and death usually occurred after 3 days. Complete blood counts were obtained. IL-4–treated mice had reduced numbers of platelets and hemoglobin levels (Figure 2A) and no evidence of bleeding in the gut or other major organs. In addition to cytopenias and evidence of bone marrow erythrophagocytosis in the absence of malignancy, IL-4–treated mice had significant splenomegaly and elevated triglycerides (Figure 2A; supplemental Figure 3). Although this pathology appears distinct from classic human HLH, it does meet 4 of the clinical criteria for HLH.9 Similarly treated Stat6−/− mice did not have any pathology or changes in platelet count or hematocrit, indicating that the IL-4 effects were dependent on Stat6 signaling (Table 1; Figure 2A).
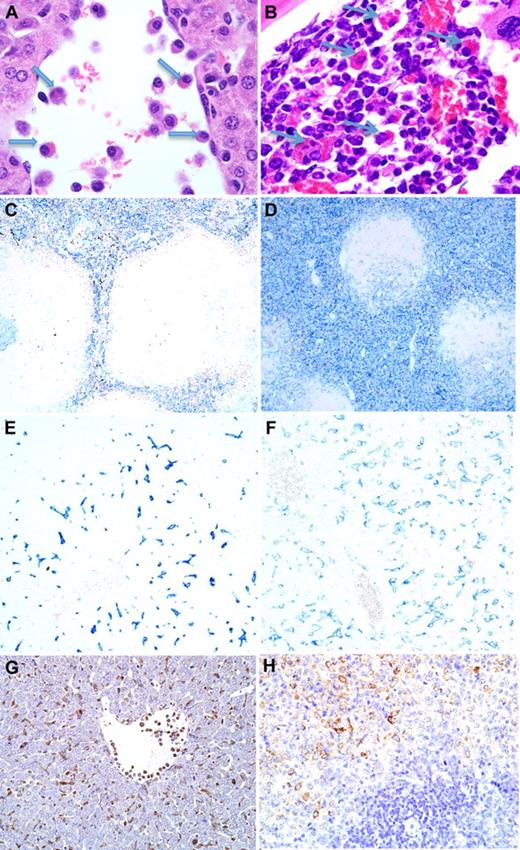
Induction of histiocytosis and erythrophagocytosis by IL-4. (A) Hematoxylin and eosin stain (×100) of tissue from day 3 of IL-4 pump (1 μg/hour), showing activated luminal macrophages and erythrophagocytosis (arrows) within the liver. (B) Hematoxylin and eosin stain (×100) of erythrophagocytosis within the bone marrow. Immunohistochemistry (×40) for F4/80 showing increased cellular density within red pulp of IL-4 mini-pump–treated spleen (D) compared with control (C). Immunohistochemistry (×100) for F4/80 showing more diffuse and larger F4/80+ Kupffer cells within the liver of IL-4 mini-pump–treated mice (F) compared with controls (E). Ym1 immunohistochemistry in (G) liver and (H) spleen (×100) of IL-4 mini-pump–treated mouse.
Induction of histiocytosis and erythrophagocytosis by IL-4. (A) Hematoxylin and eosin stain (×100) of tissue from day 3 of IL-4 pump (1 μg/hour), showing activated luminal macrophages and erythrophagocytosis (arrows) within the liver. (B) Hematoxylin and eosin stain (×100) of erythrophagocytosis within the bone marrow. Immunohistochemistry (×40) for F4/80 showing increased cellular density within red pulp of IL-4 mini-pump–treated spleen (D) compared with control (C). Immunohistochemistry (×100) for F4/80 showing more diffuse and larger F4/80+ Kupffer cells within the liver of IL-4 mini-pump–treated mice (F) compared with controls (E). Ym1 immunohistochemistry in (G) liver and (H) spleen (×100) of IL-4 mini-pump–treated mouse.
Summary of results from mice treated for 3 days with indicated minipump or 10 days with indicated IL-4C treatment
| Recipient . | Dose . | Liver . | Spleen . | Bone marrow . | ||
|---|---|---|---|---|---|---|
| YM1+ Kupffer cell hyperplasia; endothelial cell adhesion . | Erythrophagocytosis . | Erythrophagocytosis . | Histiocytosis, YM1+ macrophages . | Erythrophagocytosis . | ||
| B6 WT | IL-4 3 μg/hour minipump | ++ | ++ | + | ++ | ND |
| IL-4 1 μg/hour minipump | ++ | +++ | + | ++ | ++ | |
| IL-13 1 μg/hour minipump | + | − | − | − | − | |
| IL-4 1 μg/hour minipump + anti-IFNγ | ++ | +++ | + | ++ | ++ | |
| anti-interferon-γ alone | − | − | − | − | ND | |
| IL-4C + anti-interferon-γ | ++ | ++ | − | ++ | ND | |
| IL-4C alone | ++ | ++ | − | + | ND | |
| B6 Rag2−/− | IL-4 3 μg/hour minipump | +++ | ++ | − | ++ | ++ |
| IL-4 1 μg/hour | ++ | ++ | − | ++ | ++ | |
| IL-13 3 μg/hour minipump | + | − | − | − | + | |
| IL-13 1 μg/hour minipump | + | − | − | − | − | |
| B6 Stat6−/− | IL-4 1 μg/hour minipump | − | +/− | − | − | − |
| Recipient . | Dose . | Liver . | Spleen . | Bone marrow . | ||
|---|---|---|---|---|---|---|
| YM1+ Kupffer cell hyperplasia; endothelial cell adhesion . | Erythrophagocytosis . | Erythrophagocytosis . | Histiocytosis, YM1+ macrophages . | Erythrophagocytosis . | ||
| B6 WT | IL-4 3 μg/hour minipump | ++ | ++ | + | ++ | ND |
| IL-4 1 μg/hour minipump | ++ | +++ | + | ++ | ++ | |
| IL-13 1 μg/hour minipump | + | − | − | − | − | |
| IL-4 1 μg/hour minipump + anti-IFNγ | ++ | +++ | + | ++ | ++ | |
| anti-interferon-γ alone | − | − | − | − | ND | |
| IL-4C + anti-interferon-γ | ++ | ++ | − | ++ | ND | |
| IL-4C alone | ++ | ++ | − | + | ND | |
| B6 Rag2−/− | IL-4 3 μg/hour minipump | +++ | ++ | − | ++ | ++ |
| IL-4 1 μg/hour | ++ | ++ | − | ++ | ++ | |
| IL-13 3 μg/hour minipump | + | − | − | − | + | |
| IL-13 1 μg/hour minipump | + | − | − | − | − | |
| B6 Stat6−/− | IL-4 1 μg/hour minipump | − | +/− | − | − | − |
Three to 5 mice/group were treated, and histopathology evaluated in at least 2 separate experiments. Histiocytosis: 0 indicates within normal limits; +, minimal; ++, moderate; +++, severe. Hematopoiesis: 0, within normal limits; +, minimal; ++, mild; +++, moderate; ++++, extensive. Erythrophagocytosis: 0, not observed; +, 1 erythrophagocytic cell per high power field (hpf/40×); ++, 2 erythrophagocytic cells/hpf; +++, 3 erythrophagocytic cells/hpf. ND, not done.
IL-4–treated mice develop weight loss, signs of HLH, and evidence of accumulation of alternatively activated macrophages. (A) Wild-type B6 mice were treated with 1 μg/hour IL-4 mini-pumps or PBS control (5 in each group) for 3 days. The indicated serum and blood cell indices were measured at that time. This experiment was repeated with similar results. (B) Mononuclear cells were isolated from the livers of these mice, enumerated and stained for F4/80 to determine liver macrophage burden (shown are total macrophages/liver), and real-time PCR was performed to evaluate markers of classic and alternative activation (expression levels of untreated mice set at 1; shown are fold increases of expression of the indicated gene over control by IL-4–treated mice).
IL-4–treated mice develop weight loss, signs of HLH, and evidence of accumulation of alternatively activated macrophages. (A) Wild-type B6 mice were treated with 1 μg/hour IL-4 mini-pumps or PBS control (5 in each group) for 3 days. The indicated serum and blood cell indices were measured at that time. This experiment was repeated with similar results. (B) Mononuclear cells were isolated from the livers of these mice, enumerated and stained for F4/80 to determine liver macrophage burden (shown are total macrophages/liver), and real-time PCR was performed to evaluate markers of classic and alternative activation (expression levels of untreated mice set at 1; shown are fold increases of expression of the indicated gene over control by IL-4–treated mice).
To determine whether T cells, B cells, or antibody played a role in the observed histiocytosis and erythrophagocytosis, Rag2−/− mice were treated with IL-4 micro-osmotic pumps. Treatment of Rag2−/− mice resulted in nearly identical pathology to that seen in wild-type animals (Table 1).
Because alternative activation of macrophages can be initiated by IL-4, we stained the liver and spleen infiltrates for YM1, a marker for alternative macrophage activation.10 YM1+ cells were present diffusely in the liver and spleen, and real-time PCR measurement showed a dramatic increase in liver macrophage YM1 as well as arginase, indicating that alternative macrophage activation had occurred (Table 1, Figures 1G-H, 2B). Of interest, inducible nitric oxide synthase levels were not depressed, consistent with the presence of IFN-γ in the serum.
IL-13 uses the type II IL-4 receptor and has also been shown to initiate alternative macrophage activation. IL-13 was therefore infused in a protocol similar to that for IL-4. Even at 300 μg over 4 days, no significant pathology was noted in the IL-13–treated group, with the exception of induction of YM1+ cells in bile ducts (Table 1). This may reflect a specific role for IL-4 in inducing alternative activation, a requirement for much higher concentrations of IL-13 than IL-4 to achieve similar degrees of macrophage activation, and/or more effective neutralization of IL-13 by sIL-13Rα2 than neutralization of IL-4 by sIL-4Rα.11 It is possible that the process of surgical implantation of the micro-osmotic pump could act in concert with IL-4 infusion, through release of inflammatory mediators in response to the procedure. We therefore used IL-4/anti–IL-4 immune complexes (IL-4C) to deliver sustained amounts of IL-4 over a similar time period.12 Injection of IL-4C that contained as little as 5 μg of IL-4 led to histopathology similar to that seen in the IL-4 pump experiments, indicating that the pump does not substantially affect pathology (Table 1; supplemental Figures 4–8).
IL-4 infusion leads to up-regulation of IFN-γ, IL-10, and KC, but not other classic inflammatory cytokines
HLH is associated with a sharp elevation in serum concentrations of IL-1β, TNF-α, and other classic inflammatory cytokines. Multiplex serum cytokine analysis was performed on sera obtained from IL-4–treated Rag2−/− mice on the third day after mini-pump administration. Although IFN-γ, IL-10, and the murine IL-8 homolog KC were up-regulated, there was minimal change in serum concentrations of IL-1β, IL-6, or TNF-α. IL-12 was suppressed. IL-13 treatment led to KC up-regulation and IL-12 suppression as well but had no other significant effect on the measured cytokines. TNF and IL-6 levels, although different, were extremely low and did not approach the levels seen in other mouse models of HLH3 (Figure 3).
Multiplex serum cytokine profile of Rag2−/− mice receiving PBS, IL-4 (1 μg/hour), or IL-13 (1 μg/hour) via micro-osmotic pump for 3 days. Serum cytokine concentrations in B6 Rag2−/− mice treated 3 days earlier with an IL-4– or IL-13–containing mini-pump. Three to 5 mice were used per group, and similar results were obtained in a second similar experiment. *P < .05.
Multiplex serum cytokine profile of Rag2−/− mice receiving PBS, IL-4 (1 μg/hour), or IL-13 (1 μg/hour) via micro-osmotic pump for 3 days. Serum cytokine concentrations in B6 Rag2−/− mice treated 3 days earlier with an IL-4– or IL-13–containing mini-pump. Three to 5 mice were used per group, and similar results were obtained in a second similar experiment. *P < .05.
IFN-γ blockade during IL-4 treatment does not improve tissue macrophage inflammation or erythrophagocytosis
CD8 T cells and IFN-γ production are both necessary for HLH induction in the perforin-deficient LCMV infection model.3 Although the Rag2−/− susceptibility to IL-4–induced disease rules out a requirement of CD8 T cells for pathology, IFN-γ could still play a role because serum levels were up-regulated by IL-4 in a dose-dependent fashion (Figure 3; supplemental Figure 10). However, injection of neutralizing anti–IFN-γ antibody at the time of IL-4C treatment or IL-4 mini-pump placement had little effect, if any, on the degree of macrophage accumulation and erythrophagocytosis, or on hematologic indicators (Table 1; Figure 4).
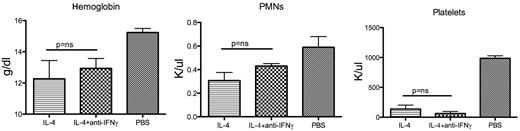
Neutralization of interferon-γ does not ameliorate IL-4–induced disease. Hemoglobin, polymorphonuclear leukocytes, and platelet values for mice treated with micro-osmotic pumps containing PBS or IL-4 (1 μg/hour, 3 mice in each group) for 3 days with or without neutralizing anti–IFN-γ monoclonal antibody. When the neutralizing antibody was present, IFN-γ levels were not detectable in the serum for the duration of the experiment. ns indicates not significant.
Neutralization of interferon-γ does not ameliorate IL-4–induced disease. Hemoglobin, polymorphonuclear leukocytes, and platelet values for mice treated with micro-osmotic pumps containing PBS or IL-4 (1 μg/hour, 3 mice in each group) for 3 days with or without neutralizing anti–IFN-γ monoclonal antibody. When the neutralizing antibody was present, IFN-γ levels were not detectable in the serum for the duration of the experiment. ns indicates not significant.
Mice transgenic for IL-4 production have erythrophagocytosis, extramedullary hematopoiesis, disrupted splenic architecture, and decreased bone marrow cellularity
To assess the effects of long-term IL-4 overproduction, transgenic mice in which expression of IL-4 was driven by an attenuated immunoglobulin promoter13 were killed at different ages and examined for pathology. Splenic macrophage infiltration/accumulation and liver hemophagocytosis were noted (Figure 5A-B; Table 2), as was decreased bone marrow cellularity and increased myeloid/erythroid ratios (Figure 5C; Table 2). Changes in complete blood counts were not noted, nor were there changes in ferritin, fibrinogen, or triglyceride levels; however, these mice did have modestly elevated IFN-γ levels (supplemental Figures 9,11). Marked extramedullary hematopoiesis in the context of normal blood cell indices were noted as the mice aged, suggesting a response to chronic red blood cell loss (Table 2, Figure 5A, supplemental Figure 9). IL-4 levels measured by in vitro cytokine capture assay in these mice were similar to levels seen in mice infected with Schistosoma mansoni (C. Perkins, G. Smulian, L. Gildea, T.O., C. Potter, F. Brombacher, M. Wills-Karp, F.D.F., manuscript in preparation).
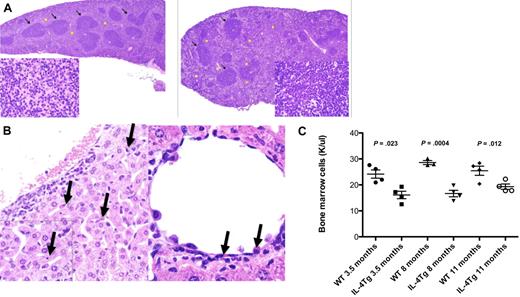
Mice transgenic for IL-4 expression have liver erythrophagocytosis, splenic histiocytosis, and extramedullary hematopoiesis. (A) Hematoxylin and eosin stains of B6 WT spleen (left) and spleen (×4) from IL-4 TG.UG mice (right) showing areas of normal white pulp (arrows). Histiocytosis and extramedullary hematopoiesis are only seen in the TG.UG spleen as shown in areas marked with yellow stars. (Insets) Original magnification ×100 (view of red pulp). Stars in wild-type mice indicate normal red pulp. (B) Hematoxylin and eosin stain (×40) of liver showing erythrophagocytosis (arrows). (Inset) Original magnification ×100 from a representative 11-month-old B6 IL-4 transgenic mouse. (C) Bone marrow cellularity from 1 long bone of IL-4 transgenic and age-matched wild-type B6 mice (4 from each group evaluated).
Mice transgenic for IL-4 expression have liver erythrophagocytosis, splenic histiocytosis, and extramedullary hematopoiesis. (A) Hematoxylin and eosin stains of B6 WT spleen (left) and spleen (×4) from IL-4 TG.UG mice (right) showing areas of normal white pulp (arrows). Histiocytosis and extramedullary hematopoiesis are only seen in the TG.UG spleen as shown in areas marked with yellow stars. (Insets) Original magnification ×100 (view of red pulp). Stars in wild-type mice indicate normal red pulp. (B) Hematoxylin and eosin stain (×40) of liver showing erythrophagocytosis (arrows). (Inset) Original magnification ×100 from a representative 11-month-old B6 IL-4 transgenic mouse. (C) Bone marrow cellularity from 1 long bone of IL-4 transgenic and age-matched wild-type B6 mice (4 from each group evaluated).
Summary of results from IL-4TG.UG mice
| . | Liver . | Spleen . | Bone marrow . | ||||
|---|---|---|---|---|---|---|---|
| Hematopoiesis . | Erythrophagocytosis . | Erythrophagocytosis . | Histiocytosis . | Hematopoiesis . | Erythrophagocytosis . | Myeloid/erythroid ratio . | |
| B6 WT 3 mo | 0 | 0 | 0 | 0 | 0 | 0 | 0.88 |
| IL-4TG.UG 3 mo | ++ | + | 0 | + | ++ | ++ | 2.50 |
| B6 WT 8 mo | 0.75 | ||||||
| IL-4TG.UG 8 mo | ++ | + | 0 | ++ | +++ | ++ | 3.34 |
| B6 WT 11 mo | 0 | 0.88 | |||||
| IL-4 TG.UG 11 mo | ++ | + | 0 | +++ | ++++ | ++ | 2.65 |
| . | Liver . | Spleen . | Bone marrow . | ||||
|---|---|---|---|---|---|---|---|
| Hematopoiesis . | Erythrophagocytosis . | Erythrophagocytosis . | Histiocytosis . | Hematopoiesis . | Erythrophagocytosis . | Myeloid/erythroid ratio . | |
| B6 WT 3 mo | 0 | 0 | 0 | 0 | 0 | 0 | 0.88 |
| IL-4TG.UG 3 mo | ++ | + | 0 | + | ++ | ++ | 2.50 |
| B6 WT 8 mo | 0.75 | ||||||
| IL-4TG.UG 8 mo | ++ | + | 0 | ++ | +++ | ++ | 3.34 |
| B6 WT 11 mo | 0 | 0.88 | |||||
| IL-4 TG.UG 11 mo | ++ | + | 0 | +++ | ++++ | ++ | 2.65 |
Histopathology of indicated mice was performed as in Table 1.
Discussion
Sustained exposure to high levels of IL-4 can induce a striking phenotype of erythrophagocytosis and tissue infiltration and/or accumulation of alternatively activated macrophages. This phenotype resembles, in part, phenotypes seen in HLH. HLH is a severe acute disease of immunodysregulation resulting in life-threatening immunopathology. The genetic and environmental triggers of HLH are heterogeneous, and the underlying mechanism(s) that actually explain the pathology of acute HLH may be heterogeneous as well. Although induction of certain forms of HLH appears to be linked, in a mouse model, to the presence of CD8 T cells and IFN-γ production, the role of other cytokines in other models should also be considered. Other triggers of HLH-like pathology include the macrophage activation syndrome, which is associated with autoimmune disease, in particular. Although IL-4 is not known to play a role in any of those diseases, our observations show that it induces a novel mechanism, distinct from secretory granule defects, which can lead to inflammatory disease associated with erythrophagocytosis and substantial accumulation of tissue macrophages. This mechanism causes a disease that has some of the features of HLH, but not enough of these features to meet current clinical criteria for HLH.
Definitively establishing a role for alternatively activated macrophages in human hemophagocytic syndromes will require clear markers of alternative activation, which are not as robust in humans as in mice.14
Our model differs from that seen in mouse models of HLH in several important aspects. One is the alternative activation pathway, characterized by YM1 positivity within the macrophages, and the lack of detectable increases in IL-1β, IL-6, or TNF-α. Second, the presence of T cells or IFN-γ does not appear to be critical in disease pathogenesis, despite an observed increase in serum IFN-γ as the result of IL-4 exposure. Finally, this phenotype could be observed in wild-type mice with no genetic deficiency in cytotoxic activity.
IL-4 has been shown to have direct cytotoxic effects on hepatocytes, and adenovirus-delivered IL-4 can cause a lethal hepatitis in a similar time frame (3-5 days) to that observed with IL-4 infusion. The observed hepatoxicity and lack of macrophage activation with adenovirus-mediated delivery of IL-4 differ from the findings presented here but could plausibly be explained by unique responses induced by the adenovirus vector itself.15
Transgenic mice producing large amounts of IL-4 develop fatal disease early in life. Mice in whom IL-4 transcription is driven by an attenuated promoter develop pathology consistent with allergic disease in the eye.13 Our examination of older IL-4 transgenic mice showed no reduction in peripheral hemoglobin, but there were reduced cellularity and red blood cell numbers within the bone marrow; liver erythrophagocytosis and extramedullary hematopoiesis were also noted. This suggests that chronic elevations in IL-4 can have hematologic consequences as well.
Potential mechanisms by which alternative activation might predispose to erythrophagocytosis, as opposed to other types of phagocytosis, are not entirely clear. CD163, the scavenger receptor for hemoglobin, is commonly seen in hemophagocytic syndromes of all etiologies,16 and increases in CD163 have been reported to be mediated by IL-4; however, down-regulation of CD163 appears to be the more common effect of IL-4 overexpression.17
The lack of significant pathology induction by IL-13 highlights the differing receptor specificities and sensitivities of IL-13 and IL-4 depending on target tissue. Furthermore, it is consistent with the much higher concentrations of IL-13 required to cause degrees of STAT6 phosphorylation in macrophages and monocytes comparable with that caused by limited amounts of IL-4. Indeed, IL-4 appears to function principally as a regulatory cytokine, whereas IL-13 is an effector and, in this regard, IL-4 overproduction may be more deleterious than IL-13.18,19
IFN-γ production in response to IL-4 has been described to be the result of the action of NK and NKT cells.20 The increase in IFN-γ in response to IL-4 seen in Rag2−/− mice most probably comes from NK cells. It is possible that this increase, together with the IL-4–induced increase in IL-10 production, is a form of counter-regulation to the direct effects of IL-4; however, further study will be necessary to better understand the role of IFN-γ in this system.
In conclusion, we describe a novel mechanism by which the striking phenotype of acute hemophagocytosis and macrophage infiltration and/or accumulation within tissues can be observed. This expands the scope of diseases that may be caused by excess IL-4 production, and provides an alternative potential mechanism for clinically observed disorders of macrophage activation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Alan Remaley, Suh Young Jeong, and Maureen Sampson for technical assistance.
This work was supported in part by the Intramural Research Program of the National Institutes of Health, NIAID, the US Department of Veterans Affairs (merit award), and the National Institutes of Health (grant R01AI070300).
National Institutes of Health
Authorship
Contribution: J.D.M. designed and performed research, and wrote the paper; T.O., G.S., I.J., J.M.W., L.C., and F.T.-V. performed research; S.C.M. contributed vital new reagents; M.B. and F.D.F. designed research and contributed vital new reagents; and W.E.P. designed research and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joshua D. Milner, Laboratory of Immunology, NIAID, 9000 Rockville Pike, NIH Bldg 10, Rm 12S236A, Bethesda, MD 20892; e-mail: jdmilner@niaid.nih.gov.