Abstract
Polyploidization of megakaryocytes (MKs), the platelet precursors, occurs by endomitosis, a mitotic process that fails at late stages of cytokinesis. Expression and function of Aurora B kinase during endomitosis remain controversial. Here, we report that Aurora B is normally expressed during the human MK endomitotic process. Aurora B localized normally in the midzone or midbody during anaphase and telophase in low ploidy megakaryocytes and in up to 16N rare endomitotic MKs was observed. Aurora B was also functional during cytokinesis as attested by phosphorylation of both its activation site and MgcRacGAP, its main substrate. However, despite its activation, Aurora B did not prevent furrow regression. Inhibition of Aurora B by AZD1152-HQPA decreased cell cycle entry both in 2N to 4N and polyploid MKs and induced apoptosis mainly in 2N to 4N cells. In both MK classes, AZD1152-HQPA induced p53 activation and retinoblastoma hypophosphorylation. Resistance of polyploid MKs to apoptosis correlated to a high BclxL level. Aurora B inhibition did not impair MK polyploidization but profoundly modified the endomitotic process by inducing a mis-segregation of chromosomes and a mitotic failure in anaphase. This indicates that Aurora B is dispensable for MK polyploidization but is necessary to achieve a normal endomitotic process.
Introduction
Megakaryocytes (MKs) are the hematopoietic cells that produce platelets. During differentiation, MKs undergo a unique mode of cell cycle called endomitosis that corresponds to an incomplete multipolar mitosis characterized by failure of both nuclear (karyokinesis) and cytoplasmic division (cytokinesis). It was considered that MK endomitosis was similar to a mitotic failure in anaphase without cleavage furrow formation.1,2 However, 3 recent reports have shown that the switch from mitosis to endomitosis corresponds to a late failure of cytokinesis,3-5 probably because of an abnormal contractile ring.4 The structure of the central spindle in MKs, another major regulator of cytokinesis, has also been a matter of debate.4,6-10 However, recent reports in human MKs underscored its normal organization.4,6
Aurora B kinase is a member of a growing family of chromosomal passenger proteins that change their subcellular localization during mitosis. Aurora B is associated with chromosomes during prophase, with inner centromeres during metaphase and early anaphase, and with the midzone and midbody during late anaphase and telophase.11-13 Aurora B activity depends on its association with other chromosome passenger proteins (INCENP, Borealin, and survivin) as well as its autophosphorylation at threonine 232 (Thr232) located in the activating loop.14 Aurora B is essential for mitosis and cytokinesis.15,16 Aurora B is involved in chromosome congression at metaphase and chromosome separation at anaphase. One of the main Aurora B kinase substrate is MgcRacGAP. Phosphorylation of the GAP domain at serine 387 (Ser387) by Aurora B converts MgcRacGAP to an active RhoA GAP that controls formation of the cleavage furrow.17 Recently, it has been shown that Aurora B kinase also plays a main role in late phases of cytokinesis by regulating mitotic kinesin-like protein 1 phosphorylation,18 which controls the abscission checkpoint to prevent tetraploid cell occurrence.19 Inhibition of Aurora B with the use of various strategies was associated with chromosome mis-segregation, abnormal cytokinesis, and polyploidy.20 Especially, the Aurora B inhibitor AZD1152-HQPA effectively induced growth arrest, polyploidy, and apoptosis in several types of cancer cell lines. Aurora B may also inhibit endoreduplication by regulating retinoblastoma (Rb) phosphorylation after an abnormal mitosis.21
Aurora B, as well as other chromosome passengers such as survivin, has a controversial role in MK endomitosis. It was initially reported that Aurora B was not expressed or only at a low level in endomitotic murine MKs, and it was postulated that this deregulated expression was responsible for MK polyploidy.22,23 Subsequently, it was shown that Aurora B was expressed and functional during the early endomitosis phases of human MKs.7 Presently, these differences seen in Aurora B expression could be related either to the antibodies used in the different studies or to distinct endomitotic processes between the 2 species or to the lower ploidy of human MKs in culture. However, the exact role of Aurora B in a mitotic cycle lacking cytokinesis such as an endomitosis remains elusive. For this reason, we reinvestigated the expression of Aurora B and its role in the process of MK endomitosis. We demonstrate that Aurora B was clearly expressed in human MKs all along the endomitosis and whatever the ploidy. In addition, Aurora B was functional at both the metaphase/anaphase transition and during the late phases of endomitosis as its different substrates were phosphorylated. Surprisingly, inhibition of Aurora B by AZD1152-HQPA did not alter MK polyploidization, but induced growth arrest and apoptosis in 2N to 4N MK cells in mitosis. However, this apparently normal ploidization was associated with profound changes in the endomitotic process itself with an aborted mitosis in anaphase. Thus, Aurora B is dispensable for MK polyploidization, but its inhibition profoundly modifies the endomitotic process.
Methods
In vitro culture of MKs derived from human CD34+ cells in liquid serum-free medium
CD34+ cells were obtained, in agreement with and with approval from our Institute Ethic Committee (Assistance Publique des Hôpitaux de Paris), from leukapheresis samples after mobilization. CD34+ cells were isolated by a positive selection with the use of an immunomagnetic cell sorting system (AutoMacs; Miltenyi Biotec) and were cultured in serum-free medium in the presence of recombinant human thrombopoietin (TPO; 10 ng/mL; Kirin Brewery) to induce MK differentiation. Ingredients used to prepare the serum-free medium were as previously described.24 AZD1152-HQPA (provided by AstraZeneca Pharmaceuticals) was dissolved at 10mM in dimethyl sulfoxide and diluted in culture medium at a final concentration of 100nM. The same volume of dimethyl sulfoxide was added in culture medium as a control. This compound has a high specificity for Aurora B (Aurora A, Ki: 1.369nM; Aurora B, Ki: 0.3nM).
Cell transduction
CD34+ cells isolated from leukapheresis were cultured 4 days in serum-free medium in the presence of TPO (10 ng/mL) and were then transfected with 2 μg of the pEF1a-H2BGFP plasmid4 by the Amaxa electroporation system. For BclxL overexpression, we used a retroviral vector MIGR encoding human BclxL.25 Retrovirus particle production was achieved as previously described.26 CD34+ cells were grown in the presence of TPO and stem cell factor and subsequently infected at days 1 and 2 of culture.
Real-time quantitative reverse transcription polymerase chain reaction
Primers for quantitative reverse transcription polymerase chain reaction were designed with the use of the Primer Express Software (Perkin-Elmer Applied Biosystems) and were synthesized by Eurogentec as follows: BclxL (5′-TGTTCCCATAGAGTTCCACAAAAGT and 5′-AATGACCACCTAGAGCCTTGGAT) and hypoxanthine phosphoribosyl transferase (5′-GGCAGTATAATCCAAAGATGGTCAA and 5′-TCAAATCCAACAAAGTCTGGCTTATAT). Polymerase chain reactions were carried out in the ASI Prism GeneAmp 5700 Sequence Detection System (Perkin-Elmer Applied Biosystems) with the use of the Power SYBR-Green PCR Master Mix (ABI) containing the specific primers (1.2mM). The expression level of BclxL was expressed relatively to hypoxanthine phosphoribosyl transferase.
Live cell imaging by confocal video-microscopy
After 24 hours of transfection, the CD41+/GFP+ cells were sorted and cultured in serum-free medium with or without AZD1152-HQPA for 12 hours. Then the cells were stained by CellTracker Orange by following the protocol of the product data sheet (Invitrogen) and plated in glass-bottom culture dish (MatTek). The cells were imaged under a LSM 510 laser scanning microscope (Carl Zeiss) using a 40×/1.2W Apochromat plan objective at 37°C and 5% CO2 in air. Serial images were obtained at 3- or 5-minute intervals. Image analysis was performed with LSM Image Examiner 4.2 software (Carl Zeiss).
Immunofluorescence
Immunofluorescence was performed on MKs at day 6 of culture after incubation during 48 hours with 100nM AZD1152-HQPA or without, as described previously.26 The following antibodies were used: mouse anti-Aurora B (1:100; BD Biosciences), rabbit anti-Survivin (1:100; Abcam), rabbit anti-Aurora B pThr232 (1:100; Tebu-Bio), rabbit anti-MgcRacGAP pSer387 (1:100; from T. Kitamura), mouse anti-lamin A+C (1:100; SC Biotechnology), mouse anti-α and anti-β tubulin monoclonal antibodies (MoAbs; 1:200; Sigma-Aldrich). The secondary antibodies were used as described previously.4 Images were captured with a Zeiss laser scanning microscope (LSM 510; Carl Zeiss) with a 63×/1.4 NA oil objective. Image analysis was performed with LSM Image Examiner 4.2 software (Carl Zeiss).
Cell viability and cell counting
Cell viability during the treatment with AZD1152-HQPA was measured by trypan blue exclusion, and cell proliferation was quantified by manual cell counting every 24 hours during 4 days.
Measurement of ploidy
Hoechst 33342 (10 μg/mL; Sigma-Aldrich) was added in the MK culture for 2 hours at 37°C. Cells were then stained with directly coupled MoAbs: anti-CD41 allophycocyanin (APC) and anti-CD42 phycoerythrin (1:10; BD Biosciences) for 30 minutes at 4°C. The ploidy was measured in the CD41+/CD42+ cell population by a LSRII flow cytometer equipped with 3 lasers (360nM, 480nM, and 560nM excitation). Approximately 50 000 cells were analyzed with the CellQuest software package (BD Biosciences). The ploidy of MK culture was measured as described.4
Western blot analysis
After 48 hours of treatment with or without AZD1152-HQPA, MKs were incubated during 2 hours at 37°C with Hoechst 33342 (10 μg/mL; Sigma-Aldrich). After anti-CD41 APC staining, cells were sorted as previously reported into CD41+/2N to 4N and CD41+/8N or greater cell fractions.27 Western blot analysis was performed as described previously.26 The following antibodies were used: mouse anti–Aurora B (1:250; BD Biosciences), mouse anti-p53 (1:100; Calbiochem), mouse anti-bax (1:1000; SC Biotechnology), mouse anti-p21 (1:1000; BD Biosciences), mouse anti-Rb total (1:1000;Cell Signaling), mouse anti–cyclin D3 (1:1000; SC Biotechnology), rabbit anti-Rb780 (1:1000; Cell Signaling), rabbit anti-Rb608 (1:1000; Cell Signaling), rabbit anti–caspase-3 cleaved (1:1000; Cell Signaling), and rat anti-Hsc70 (1:5000; Nventa Biopharmaceuticals). Primary antibodies were shown with appropriate secondary antibodies conjugated with horseradish peroxidase and an enhanced chemiluminescence system (Thermo Fisher Scientific). We used Hsc70 to normalize the protein quantity. Western blots were quantified by ImageJ (National Institutes of Health).
Apoptosis assay
After treatment for 48 hours with AZD1152-HQPA, apoptosis was measured by Annexin V–fluorescein isothiocyanate (FITC) detection kit according to the manufacturer's instruction (BD PharMingen). Hoechst 33342 (10 μg/mL; Sigma-Aldrich) was added in the MKs culture for 2 hours at 37°C before annexin V staining.
Histone H3 phosphorylation status
The phosphorylation status of histone H3 was determined by flow cytometry. MK culture at day 5 was treated with 50nM, 100nM, 150nM, and 200nM AZD1152-HQPA for 48 hours, then fixed in ice-cold 70% ethanol, and kept at −20°C overnight. Cells were rehydrated in phosphate-buffered saline (PBS) and incubated with 0.25% Triton X-100 (Sigma-Aldrich) on ice for 10 minutes. After washing with PBS, cells were incubated with rabbit anti–phospho-serine 10 (Ser10) histone H3 antibody (1:1000; Upstate Biotechnology) at 4°C. After an overnight incubation, cells were washed with PBS and incubated with a Alexa 488–conjugated goat anti–rabbit immunoglobulin G (1:100; Molecular Probes) for 1 hour. Cells were washed and resuspended in PBS containing 10 μg/mL RNAase (Sigma-Aldrich) and 10 μg/mL propidium iodide (PI; Sigma-Aldrich). After an overnight incubation in PI solution, cell samples were analyzed on a FACSort (BD Biosciences) equipped with an argon laser (15 mW, 480 nM excitation). Fluorescence data were analyzed with the CellQuest software package (BD Biosciences).
5′-Bromo-2′-deoxyuridine assay
After treatment for 48 hours with or without AZD1152-HQPA, cells were incubated with 10μM 5′-bromo-2′-deoxyuridine (BrdU) for 1 hour at 37°C. Then the cells were stained with the anti-CD41 APC (BD PharMingen) for 30 minutes at 4°C. BrdU staining was performed by the BrdU flow kit according to the manufacturer's instruction (BD PharMingen). BrdU incorporation was analyzed in CD41+ cells stained with PI on a LSRII. Approximately 50 000 cells were analyzed with the CellQuest software package (BD Biosciences).
Results
Aurora B kinase is present and colocalizes correctly with survivin in endomitotic MKs
The expression of Aurora B in MKs is controversial. Because endomitosis is related to a failure in cytokinesis and because Aurora B plays a key role in this process, it was important to know if Aurora B was present during endomitosis and if it was appropriately localized in late phases of endomitosis.
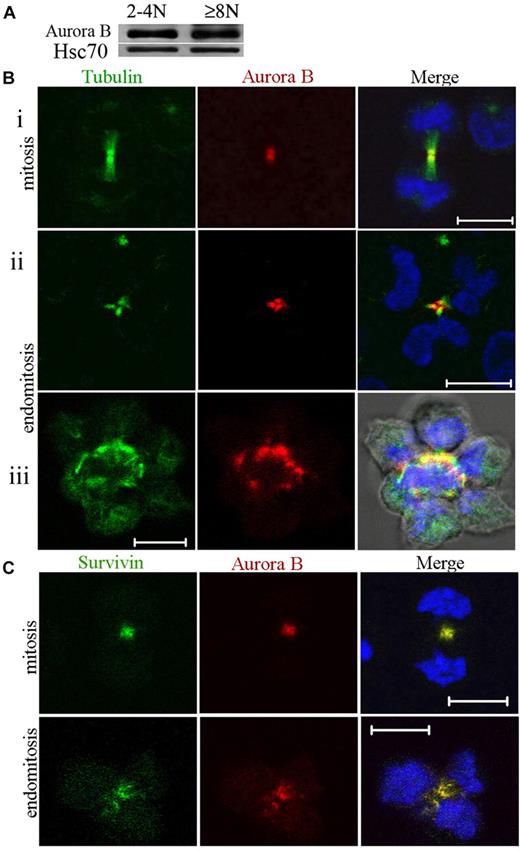
Expression of Aurora B was investigated during polyploidization. MKs at day 7 of culture were sorted after Hoechst 33342 and anti-CD41 APC staining into 2 cell fractions (CD41+/2N-4N and CD41+/≥ 8N). Equal amounts of total cell protein were size-fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and the proteins were analyzed by Western blotting for the presence of Aurora B. As shown in Figure 1A, Aurora B was expressed in 2N to 4N cells and in 8N or greater MKs at a very similar level in comparison to Hsc70.
Aurora B kinase is expressed in polyploid MKs and localizes normally to the central spindle during endomitosis. (A) Study of Aurora B expression in CD41+ 2N to 4N and 8N or greater cells. The cells were sorted after incubation with Hoechst 33342 and CD41 staining. Western blot analysis showed that Aurora B protein was present in 2N to 4N and 8N or greater MK without marked differences between the 2 cell fractions. (Bi-iii) MKs were stained for tubulin (α and β, green), Aurora B (red), and DNA (blue). During telophase, the central spindle was highly concentrated, forming intercellular bridge (midbody). Aurora B was present in the midbody of the central spindle for dipolar mitosis (Bi) or endomitosis (8N, 4 poles) (Bii-iii). (Biii) For the high ploidy MK (16N, 8 poles), the merge image showed the staining for tubulin, Aurora B, DNA, and phase-contrast light. (C) During telophase Aurora B colocalized correctly with survivin to the central spindle of dipolar or multipolar endomitotic MKs as described in classic mitosis. Scale bars represent 10 μm.
Aurora B kinase is expressed in polyploid MKs and localizes normally to the central spindle during endomitosis. (A) Study of Aurora B expression in CD41+ 2N to 4N and 8N or greater cells. The cells were sorted after incubation with Hoechst 33342 and CD41 staining. Western blot analysis showed that Aurora B protein was present in 2N to 4N and 8N or greater MK without marked differences between the 2 cell fractions. (Bi-iii) MKs were stained for tubulin (α and β, green), Aurora B (red), and DNA (blue). During telophase, the central spindle was highly concentrated, forming intercellular bridge (midbody). Aurora B was present in the midbody of the central spindle for dipolar mitosis (Bi) or endomitosis (8N, 4 poles) (Bii-iii). (Biii) For the high ploidy MK (16N, 8 poles), the merge image showed the staining for tubulin, Aurora B, DNA, and phase-contrast light. (C) During telophase Aurora B colocalized correctly with survivin to the central spindle of dipolar or multipolar endomitotic MKs as described in classic mitosis. Scale bars represent 10 μm.
To confirm this expression and to investigate its subcellular localization during endomitosis, we prepared primary MKs for immunostaining. In agreement with the results of Geddis and Kaushansky,7 we found that Aurora B was present and localized to the centromeres and then to the midzone in endomitotic MKs in anaphase. In addition, at later stages of multipolar endomitosis characterized by decondensing chromosomes, Aurora B was present in the midbody or midzone, including in the rare high polyploid MKs that we could observe (Figure 1B). Therefore, Aurora B localizes to the midzone and the midbody in multipolar endomitotic cells, as it does in bipolar mitosis.
We examined also if Aurora B colocalized with survivin, another chromosome passenger protein. In multipolar endomitotic MKs, colocalization of Aurora B and survivin was seen all along the endomitotic cycle from prophase to telophase. This was also true in late telophase whereby both molecules were localized in the midbody (Figure 1C). These results indicated no difference in Aurora B or survivin localization between an endomitotic and a mitotic cell cycle in human MKs.
Aurora B kinase is active in the late phases of endomitosis
Aurora B activity depends on phosphorylation of a Thr232 in its activation loop, and this phosphorylation site is indispensable for cytokinesis.14 Nevertheless, Aurora B localization to the midzone is not dependent on its kinase activity.28 We thus investigated whether Aurora B was phosphorylated on Thr232 with the use of a specific antibody (Figure 2A). Phospho-T232 Aurora B was detected in the midbody of all mitotic cells (n = 15) as previously reported.14 For multipolar endomitotic cells (n = 7), phospho-T232 Aurora B was also essentially detected in the midbody during late telophase. This result suggests that Aurora B remains active throughout the entire telophase in multipolar endomitotic cells as in mitosis.
Aurora B kinase is active in telophase of endomitosis. (A) MKs were stained for tubulin (α and β, green), phospho-T232-Aurora B (red), and DNA (blue). Phosphorylated Aurora B was present in the midbody of dipolar mitotic cells. For multipolar endomitotic cells, phosphorylated Aurora B was essentially detected in late telophase midbody (arrowhead). (B) MKs were stained for tubulin (α and β, green), phospho-Ser387-MgcRacGAP (red), and DNA (blue). During telophase, phospho-Ser387-MgcRacGAP was strongly enriched in the midbody for dipolar mitosis. A similar labeling pattern was observed for multipolar endomitosis (arrowhead). Scale bars represent 10 μm.
Aurora B kinase is active in telophase of endomitosis. (A) MKs were stained for tubulin (α and β, green), phospho-T232-Aurora B (red), and DNA (blue). Phosphorylated Aurora B was present in the midbody of dipolar mitotic cells. For multipolar endomitotic cells, phosphorylated Aurora B was essentially detected in late telophase midbody (arrowhead). (B) MKs were stained for tubulin (α and β, green), phospho-Ser387-MgcRacGAP (red), and DNA (blue). During telophase, phospho-Ser387-MgcRacGAP was strongly enriched in the midbody for dipolar mitosis. A similar labeling pattern was observed for multipolar endomitosis (arrowhead). Scale bars represent 10 μm.
In a second set of experiments aiming to confirm that Aurora B was active in telophase of endomitotic MKs, we studied if Aurora B could phosphorylate Ser387 of one of its main substrate, MgcRacGAP.17 In cells containing multipolar spindles, MgcRacGAP was detected on the cortex of the cleavage furrow (data not shown) as previously shown.6 Then, with the use of an antibody against the phospho-Ser387 epitope, we studied the spatiotemporal phosphorylation of MgcRacGAP in mitosis and in endomitosis. No difference was found as shown in Figure 2B. Accumulation of phospho-Ser387-MgcRacGAP to the midzone of the bipolar mitotic spindle could be detected at the beginning of telophase. Later on, phospho-Ser387-MgcRacGAP was strongly enriched in the midbody. A similar pattern of labeling was seen in multipolar endomitosis (Figure 2B). Together, these data show that Aurora B had a very close activity in late stages of mitosis and endomitosis.
Inhibition of Aurora B induces a chromosome segregation defect in endomitotic MKs
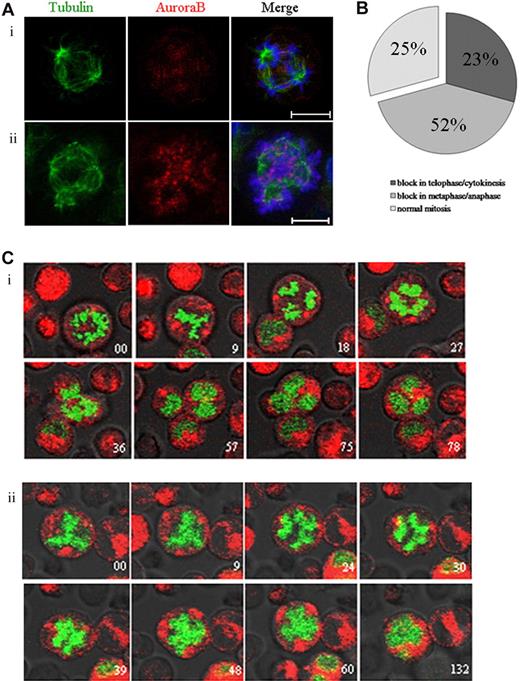
Aurora B kinase regulates the spindle checkpoint and cytokinesis in many cell types.29-31 To study the role of Aurora B in endomitosis, we used the AZD1152-HQPA compound that has been described as a highly potent and selective inhibitor of Aurora B.32 By immunofluorescence, we examined the endomitotic process and the localization of Aurora B in MKs cultured without or in the presence of AZD1152-HQPA (Figure 3A). Aurora B localized on the microtubules between separating chromosomes in anaphase of multipolar endomitosis in untreated cells (Figure 3Ai). After AZD1152-HQPA treatment, chromosomes were poorly condensed at anaphase in multipolar endomitosis, and, subsequently, chromosome segregation was incomplete. Aurora B remained localized in centromeres of segregating chromosomes, whereas in untreated cells Aurora B accumulated in the midzone.
Inhibition of Aurora B by AZD1152-HQPA induces a chromosome segregation defect in mitotic and endomitotic MKs. (A) MKs were stained for tubulin (α and β, green), Aurora B (red), and DNA (blue). (Ai) In MK from untreated cultures, Aurora B accumulated in the midzone of multipolar endomitosis. (Aii) After AZD1152-HQPA treatment Aurora B localized in centromeres of segregating chromosomes. (B) The figure summarizes the percentage of MK mitosis abnormalities after incubation with AZD1152-HQPA: 52% cells were blocked in metaphase/anaphase, 23% in telophase/cytokinesis, 25% in normal mitosis; for multipolar endomitosis we did not find the telophase after incubation with AZD1152-HQPA. (C) Time-lapse images of H2B-GFP–expressing MKs were obtained by confocal video microscopy. Time relative to the first image is indicated in minutes on each image. (Ci) Endomitosis of control cell (8N, 4 poles) from metaphase to telophase. (Cii) Endomitosis of cell treated with AZD1152-HQPA (8N, 4 poles): metaphase, anaphase with slightly segregated chromosomes, and absence of telophase. Scale bars represent 10 μm.
Inhibition of Aurora B by AZD1152-HQPA induces a chromosome segregation defect in mitotic and endomitotic MKs. (A) MKs were stained for tubulin (α and β, green), Aurora B (red), and DNA (blue). (Ai) In MK from untreated cultures, Aurora B accumulated in the midzone of multipolar endomitosis. (Aii) After AZD1152-HQPA treatment Aurora B localized in centromeres of segregating chromosomes. (B) The figure summarizes the percentage of MK mitosis abnormalities after incubation with AZD1152-HQPA: 52% cells were blocked in metaphase/anaphase, 23% in telophase/cytokinesis, 25% in normal mitosis; for multipolar endomitosis we did not find the telophase after incubation with AZD1152-HQPA. (C) Time-lapse images of H2B-GFP–expressing MKs were obtained by confocal video microscopy. Time relative to the first image is indicated in minutes on each image. (Ci) Endomitosis of control cell (8N, 4 poles) from metaphase to telophase. (Cii) Endomitosis of cell treated with AZD1152-HQPA (8N, 4 poles): metaphase, anaphase with slightly segregated chromosomes, and absence of telophase. Scale bars represent 10 μm.
To better study the effects of the Aurora B inhibitor on endomitosis, we used real-time video microscopy in H2B-GFP–expressing MKs treated with AZD1152-HQPA for 12 hours. For MK bipolar mitosis (25 MKs were analyzed in 3 independent experiments), chromosomes in 52% of the cells segregated slightly at the onset of anaphase, then the 2 chromatin masses stuck together without telophase. For 23% of other mitosis, we observed the presence of a true telophase with the formation of a normal cleavage furrow, but the cells did not progress further and stayed blocked in this state. The remaining cells (25%) divided normally (Figure 3B). As illustrated in Figure 3C, most endomitosis was abnormal in cultures treated with the Aurora B inhibitor. Multipolar endomitotic MKs (9 MKs were analyzed in 3 independent experiments) remained round, the chromatin mass appeared to be stretched toward opposite poles, chromosomes were slightly segregated into distinct masses corresponding to the beginning of anaphase (Figure 3Cii). Anaphase did not progress further and finished with the fusion of the different chromatin masses; then the chromosomal masses decondensed to form an irregularly polylobulated nucleus. In consequence, we did not observe any true telophase. This multipolar endomitosis markedly contrasted with normal endomitosis occurring in untreated MKs (5 endomitosis with 4 poles analyzed; n = 3). The condensed chromosomes remained separated until late anaphase, and then cell elongation generated 4 cells that remained linked by a cytoplasm connection. In late telophase, chromosomes decondensed; the cell shape subsequently shrunk, leading to the fusion of the 4 chromatin masses and formation of a single polylobulated nucleus. These observations show that a chromosome segregation defect was induced by the AZD1152-HQPA treatment in multipolar endomitosis. However, the cells could finish their endomitosis by aborting the mitotic process at anaphase with a fusion of the chromatin masses and the formation of a polylobulated nucleus.
Aurora B is indispensable for MK precursor mitosis but is dispensable for polyploidization
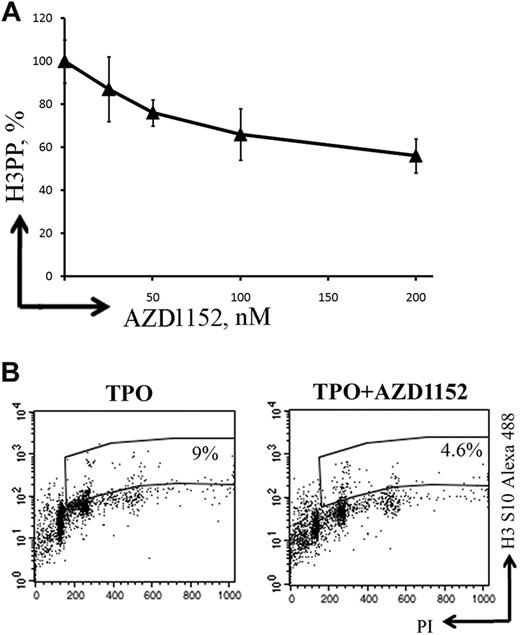
To study whether Aurora B was implicated in the regulation of MK polyploidization, MKs were grown in the presence or absence of AZD1152-HQPA. To determine the concentration of AZD1152-HQPA to be used for the study, we analyzed Ser10 phosphorylation of histone H3 by flow cytometry (Figure 4A). One disadvantage of using this approach is that it was shown that Ser10 phosphorylation of histone H3 is controlled by both Aurora A and Aurora B kinases.33-35 Expression of phosphorylated histone H3 Ser10 was approximately 40% diminished in the presence of 100nM AZD1152-HQPA compared with control cultures without inhibitor (Figure 4B). This AZD1152-HQPA concentration was used for all experiments.
AZD1152-HQPA inhibits histone H3 Ser10 phosphorylation in MKs. (A) A dose-response curve showing inhibition of histone H3 phosphorylation (H3PP) in MKs at day 7 of culture after 48 hours of exposure to AZD1152-HQPA. (B) Flow cytometry was used to determine percentage of cells with phosphorylated histone H3. The plots show propidium iodide staining, used to show the DNA content of cells against the level of phosphorylated histone H3 for each cell measured. At 48 hours, the untreated population had 9% phosphorylated histone H3 (TPO) and AZD1152-HQPA treated cells (100nM) 4.6% (TPO + AZD1152-HQPA) shown in the selected region on these plots.
AZD1152-HQPA inhibits histone H3 Ser10 phosphorylation in MKs. (A) A dose-response curve showing inhibition of histone H3 phosphorylation (H3PP) in MKs at day 7 of culture after 48 hours of exposure to AZD1152-HQPA. (B) Flow cytometry was used to determine percentage of cells with phosphorylated histone H3. The plots show propidium iodide staining, used to show the DNA content of cells against the level of phosphorylated histone H3 for each cell measured. At 48 hours, the untreated population had 9% phosphorylated histone H3 (TPO) and AZD1152-HQPA treated cells (100nM) 4.6% (TPO + AZD1152-HQPA) shown in the selected region on these plots.
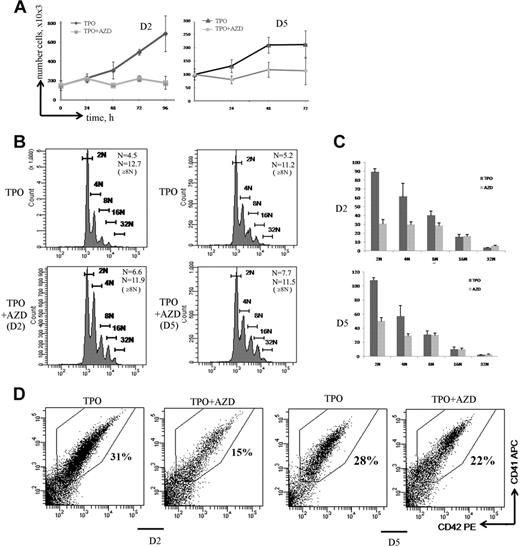
During MK differentiation, MK precursors switch from a classic mitosis to an endomitosis. To investigate the role of Aurora B in mitosis and endomitosis, respectively, we added the Aurora B inhibitor in MK culture at day 2 when most of the cells are proliferating and at day 5, which approximately corresponds to the beginning of the endomitotic process. Whether the inhibitor was added at day 2 or day 5 of culture, incubation with AZD1152-HQPA during 72 hours completely inhibited cell proliferation (Figure 5A).
Aurora B is indispensable for MK precursor mitosis but is dispensable for polyploidization. (A) Effect of AZD1152-HQPA on cell proliferation. MK cultures were treated with AZD1152-HQPA at day 2 (D2) and at day 5 (D5). The cell number with or without inhibitor was counted at time points indicated (n = 3). (B) Effects of Aurora B inhibitor on MK polyploidization. MK cells were treated with AZD1152-HQPA at day 2 (D2) and at day 5 (D5), and after 72 hours the ploidy level was analyzed in the CD41+/CD42+ cell population. The mean ploidy was calculated from the number of cells of each ploidy class in 3 independent experiments. (C) The number of MKs obtained in each ploidy class after 72 hours of treatment with AZD1152-HQPA at day 2 and at day 5. (D) Effect of AZD1152-HQPA on MK differentiation. AZD1152-HQPA was added to the culture at day 2 or at day 5 for 72 hours. The percentage of mature MKs expressing CD41 and CD42 antigens was analyzed by flow cytometry. Dot plots are from a representative experiment (n = 3).
Aurora B is indispensable for MK precursor mitosis but is dispensable for polyploidization. (A) Effect of AZD1152-HQPA on cell proliferation. MK cultures were treated with AZD1152-HQPA at day 2 (D2) and at day 5 (D5). The cell number with or without inhibitor was counted at time points indicated (n = 3). (B) Effects of Aurora B inhibitor on MK polyploidization. MK cells were treated with AZD1152-HQPA at day 2 (D2) and at day 5 (D5), and after 72 hours the ploidy level was analyzed in the CD41+/CD42+ cell population. The mean ploidy was calculated from the number of cells of each ploidy class in 3 independent experiments. (C) The number of MKs obtained in each ploidy class after 72 hours of treatment with AZD1152-HQPA at day 2 and at day 5. (D) Effect of AZD1152-HQPA on MK differentiation. AZD1152-HQPA was added to the culture at day 2 or at day 5 for 72 hours. The percentage of mature MKs expressing CD41 and CD42 antigens was analyzed by flow cytometry. Dot plots are from a representative experiment (n = 3).
Interestingly, AZD1152-HQPA increased the mean ploidy level of MKs (CD41+/CD42+ cells). The mean ploidy was increased from 4.5N to 6.6N (n = 3; P < .05) when AZD1152-HQPA was added at day 2, and from 5.2N to 7.7N (n = 4; P < .04) when added at day 5 (Figure 5B). However, AZD1152-HQPA did not further increase the ploidy level in polyploid MKs. Indeed, when AZD1152-HQPA was added at day 5, the mean ploidy level of 8N or greater MKs was 11.2 for control and 11.5 for AZD1152-HQPA–treated MKs (n = 4; P = .52). Similarly, when AZD1152-HQPA was added at day 2, the mean ploidy level of 8N or greater MKs was 12.7 for control MKs and 11.9 for AZD1152-HQPA–treated MKs (n = 3; P = .33). This result led us to quantify the number of MKs generated in each ploidy class. As shown in Figure 5C, when AZD1152-HQPA was added at day 2 of culture, the number of 2N MKs was diminished by approximately 67%, 4N MKs by 50%, and 8N MKs by 25%; in contrast, when AZD1152-HQPA was added at day 5 of culture, the number of 2N MK cells was decreased by approximately 55% and 4N MKs by 49%; in contrast, the number of 8N or greater MKs was not significantly changed (n = 4; P = .82).
In addition, when the Aurora B inhibitor was added at day 2 (Figure 5D), but not when added at day 5, the percentage of maturing MKs expressing CD41/CD42 was significantly decreased from 35% in control to 15% in treated culture (Figure 5D; n = 3; P < .022). Thus, inhibition of Aurora B markedly affected MK differentiation of 2N to 4N cells but strikingly did not impair MK polyploidization. Thus, it appears that the increase in mean ploidy induced by AZD1152-HQPA is more a selection of polyploid cells versus 2N to 4N cells than a true induction of polyploidization. For this reason, we studied in detail the cell cycle and apoptosis of these 2N to 4N and 8N or greater MK populations.
Inhibition of Aurora B kinase decreases G1/S phase transition
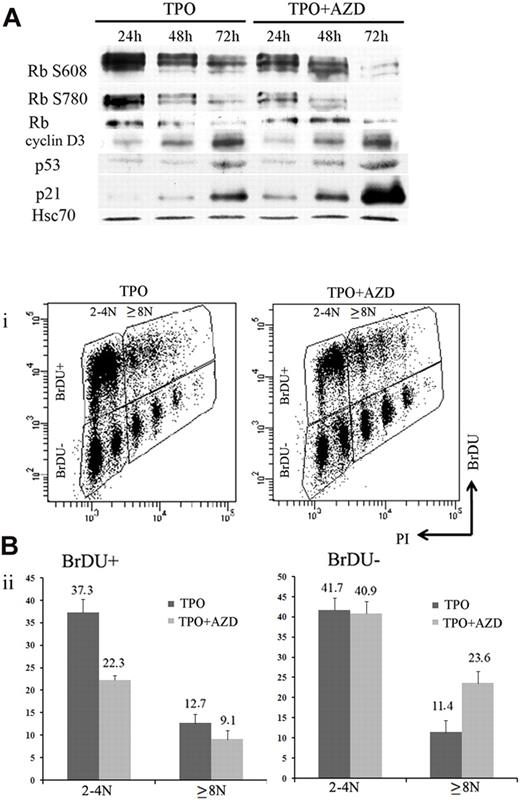
During MK differentiation, levels of cyclin D3, p21, and p19 proteins increase with ploidy.27,36,37 MK polyploidization also requires phosphorylation of the Rb protein, and the endomitotic arrest is associated with a hypophosphorylated form of Rb.36 Previous studies that used inhibitors of Aurora B kinase reported that induction of polyploidy or pseudo-G1 arrest was dependent on the p53/p21 and Rb pathways.21,38 To analyze how Aurora B inhibition modifies cell cycle protein expression, 100nM AZD1152-HQPA were added to MK cultures on day 5, and cells were collected every 24 hours to perform Western blotting. Compared with control cultures, cyclin D3, p21, and p53 protein levels were induced after AZD1152-HQPA treatment at 48 and 72 hours. In addition, Rb was hypophosphorylated, and especially phosphorylation at Ser780 was markedly inhibited within 24 hours (Figure 6A). This result is in agreement with recent data showing that Ser780 Rb is a direct phosphorylation site of Aurora B.21
Inhibition of Aurora B kinase decreases G1/S phase transition in MK cells. (A) Western blot analysis with or without AZD1152-HQPA added at day 5. Western blot showed a time-dependent induction of p53 and p21 and the appearance of the hypophosphorylated form of Rb onAZD1152-HQPA treatment. (Bi) Flow cytometric analysis of BrdU incorporation in CD41+ cells of day 7 MK culture incubated with or without inhibitor during 48 hours. The plots show propidium iodide staining, used to show the DNA content of cells against the level of BrdU for each cell measured. (Bii) The BrdU-positive or -negative populations in 2N to 4N and 8N or greater MKs with or without inhibitor were compared. BrdU incorporation diminished in 2N to 4N population and changed slightly in 8N or greater onAZD1152 treatment. BrdU-negative accumulation in 8N or greater after AZD1152 treatment was more important than 2N to 4N. Dot plots are from a representative experiment (n = 3).
Inhibition of Aurora B kinase decreases G1/S phase transition in MK cells. (A) Western blot analysis with or without AZD1152-HQPA added at day 5. Western blot showed a time-dependent induction of p53 and p21 and the appearance of the hypophosphorylated form of Rb onAZD1152-HQPA treatment. (Bi) Flow cytometric analysis of BrdU incorporation in CD41+ cells of day 7 MK culture incubated with or without inhibitor during 48 hours. The plots show propidium iodide staining, used to show the DNA content of cells against the level of BrdU for each cell measured. (Bii) The BrdU-positive or -negative populations in 2N to 4N and 8N or greater MKs with or without inhibitor were compared. BrdU incorporation diminished in 2N to 4N population and changed slightly in 8N or greater onAZD1152 treatment. BrdU-negative accumulation in 8N or greater after AZD1152 treatment was more important than 2N to 4N. Dot plots are from a representative experiment (n = 3).
To investigate if Aurora B inhibition induces an arrest in the cell cycle, we compared BrdU incorporation in CD41-expressing cells 48 hours after AZD1152-HQPA was added to MK cultures on day 5 and in untreated cultures. A marked decrease (from 50% to 31.4%) of BrdU incorporation was observed after Aurora B inhibition. When the analysis was performed in the 2N to 4N and the 8N or greater MK populations (Figure 6B), the percentage of BrdU-positive cells was decreased from 37.3% to 22% in the 2N to 4N cell population (n = 3; P < .03) and from 12.7% to 9.1% (n = 3; P < .04) in the 8N or greater population. When the percentage of BrdU-negative 8N or greater MKs was quantified, an increase from 11.4% to 23.6% (n = 3; P < .025) was also observed; this result showed that the cell cycle entry was also affected by Aurora B inhibition in polyploid cells. These data show that inhibition of Aurora B leads to a decrease in MK cells progressing in the cell cycle at all ploidy levels, but with a more marked effect on the 2N to 4N cell population. This result further reinforces the hypothesis that Aurora B inhibition does not promote MK polyploidization.
Inhibition of Aurora B kinase induces apoptosis in 2N to 4N cells
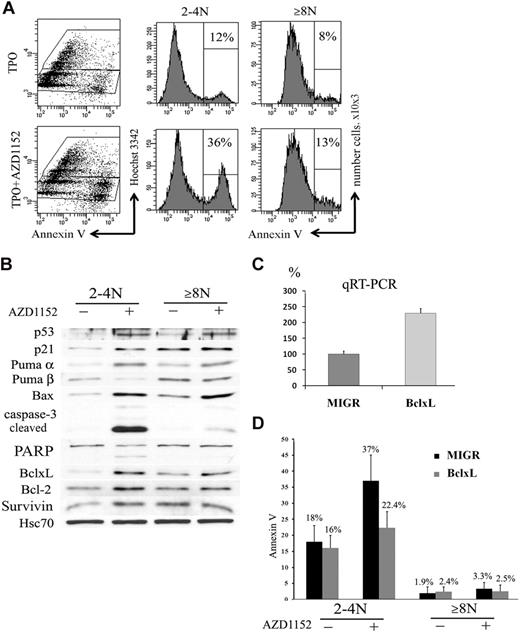
In many cell types Aurora B inhibition not only blocks proliferation but also induces apoptosis.39 We thus studied if the decrease of the CD41+ cell population observed after Aurora B inhibition could be related to apoptosis and if the 2N to 4N and 8N or greater MK cell populations had a similar response. To perform these experiments, CD41+ cells were sorted at day 6 and cultured in the presence or the absence of 100nM AZD1152-HQPA for 48 hours. An increase in the percentage of Annexin V+ cells was observed in both 2N to 4N MKs (36% vs 12% in controls; n = 3; P < .033) and 8N or greater MKs (13% vs 8% in controls; n = 4; P < .05) (Figure 7A).
Inhibition of Aurora B kinase induces apoptosis in 2N to 4N cells. (A) Flow cytometry analysis of Annexin V binding. CD41+ sorted cells were cultured with or without AZD1152-HQPA for 48 hours. The plots show Hoechst 33342 staining, used to show the DNA content of cells against the level of Annexin V for each cell measured. After AZD1152-HQPA treatment the Annexin V+ cell population was 36% versus 12% in 2N to 4N fraction and 13% versus 8% in 8N or greater. Dot plots are from a representative experiment (n = 3). (B) AZD1152-HQPA induces caspase-3 activation in 2N to 4N population of MKs. Cells were cultured with or without AZD1152-HQPA for 48 hours. Then, CD41- and Hoechst 33342–stained cells with or without inhibitor were sorted into CD4+ 2N to 4N and 8N or greater. Western blot analysis showed that AZD1152-HQPA induced expression of p53 and p21 in both MK fractions. In contrast, the cleavage of caspase-3 and poly (adenosine diphosphate–ribose) polymerase were markedly induced in 2N to 4N MK-sorted cell population, whereas these cleavage forms were barely detectable in 8N or greater cells. (C) Quantitative reverse transcription polymerase chain reaction for BclxL with the use of RNA isolated from CD41+/2N to 4N and CD41+/8N or greater sorted cells. (D) The percentages of Annexin V+ cells in 2N to 4N and 8N or greater MKs overexpressing BclxL or in control (MIGR) with or without AZD1152-HQPA were compared. In the presence of AZD1152-HQPA, Annexin V+ cells were diminished in the 2N to 4N cell population overexpressing BclxL.
Inhibition of Aurora B kinase induces apoptosis in 2N to 4N cells. (A) Flow cytometry analysis of Annexin V binding. CD41+ sorted cells were cultured with or without AZD1152-HQPA for 48 hours. The plots show Hoechst 33342 staining, used to show the DNA content of cells against the level of Annexin V for each cell measured. After AZD1152-HQPA treatment the Annexin V+ cell population was 36% versus 12% in 2N to 4N fraction and 13% versus 8% in 8N or greater. Dot plots are from a representative experiment (n = 3). (B) AZD1152-HQPA induces caspase-3 activation in 2N to 4N population of MKs. Cells were cultured with or without AZD1152-HQPA for 48 hours. Then, CD41- and Hoechst 33342–stained cells with or without inhibitor were sorted into CD4+ 2N to 4N and 8N or greater. Western blot analysis showed that AZD1152-HQPA induced expression of p53 and p21 in both MK fractions. In contrast, the cleavage of caspase-3 and poly (adenosine diphosphate–ribose) polymerase were markedly induced in 2N to 4N MK-sorted cell population, whereas these cleavage forms were barely detectable in 8N or greater cells. (C) Quantitative reverse transcription polymerase chain reaction for BclxL with the use of RNA isolated from CD41+/2N to 4N and CD41+/8N or greater sorted cells. (D) The percentages of Annexin V+ cells in 2N to 4N and 8N or greater MKs overexpressing BclxL or in control (MIGR) with or without AZD1152-HQPA were compared. In the presence of AZD1152-HQPA, Annexin V+ cells were diminished in the 2N to 4N cell population overexpressing BclxL.
For Western blot analysis, cells at day 5 of culture were cultured with AZD1152-HQPA for 48 hours. Then cells were sorted into CD41+/2N to 4N and CD41+/8N or greater cell fractions. As shown in Figure 7B, AZD1152-HQPA treatment markedly induced the cleavage of caspase 3 and poly (adenosine diphosphate–ribose) polymerase in 2N to 4N MK-sorted cell population, whereas these cleavage forms were barely detectable in 8N or greater cells. Surprisingly, p53 was induced in both MK cell fractions. This was associated, in the 2N to 4N MK fraction, with a marked induction of Bax, Puma α, and p21, all being targets of p53, but also with an unexpected induction of antiapoptotic proteins such as BclxL, Bcl-2, and survivin. In contrast, the 8N or greater MK fraction had a lower induction of p53 proapoptotic targets such as Bax and Puma α, but a high constitutive expression of antiapoptotic proteins such as BclxL. Thus, these results suggest that the apoptotic resistance to Aurora B inhibition of the 8N or greater MKs is not due to an absence of p53 activation but to a high constitutive expression of antiapoptotic proteins. The marked increase in BclxL in the 2N to 4N cells after AZD1152-HQPA might be related to a selection of surviving cells after p53 activation.
To confirm that BclxL overexpression could effectively inhibit the apoptosis induced by AZD1152-HQPA, we used a retroviral vector MIGR encoding human BclxL. CD34+ cells were grown in the presence of TPO and stem cell factor and infected at days 1 and 2 of culture. At day 5 of culture (72 hours after transduction), cells were harvested and GFP+ cells were sorted. The expression of BclxL in GFP+ cells was investigated by quantitative reverse transcription polymerase chain reaction. The BclxL retrovirus increased approximately 2.3-fold the BclxL transcripts in comparison to the control (Figure 7C). After sorting, GFP+ cells were incubated with AZD1152-HQPA for 48 hours. A decrease in the percentage of Annexin V+ cells was observed in 2N to 4N MKs overexpressing BclxL (22.4% vs 37% in MIGR; n = 3; P < .033) and was not significantly changed in 8N or greater MKs (2.5% vs 3.3% in MIGR; n = 3; P = .5) (Figure 7D). Together, these results further suggest that the increase in MK mean ploidy caused by AZD1152-HQPA was related to a cell selection due to an apoptosis resistance of polyploid cells.
Discussion
Aurora B is a kinase that plays a main role in accurate chromosome segregation and implementation of cytokinesis.33 Aurora B inhibitors are now used as anticancer agents because inhibition of the kinase activity leads to a mitotic catastrophe, which induces massive genomic instability and cell death.39 Aurora B inhibition or genetic disruption also leads to tetraploidization and polyploidization.19,21 The role of Aurora B in MK polyploidization is poorly understood. It was suggested that MK polyploidization was related to an absence of Aurora B,22 but expression of Aurora B as well as other chromosomal passenger proteins during MK endomitosis remains controversial.7,23 We here report that, like survivin, Aurora B is expressed and functional during human MK endomitosis whatever the ploidy. This result confirms the data reported by Geddis and Kaushansky,7 who used a similar approach and human MKs. In addition, we could show that Aurora B is also expressed in late stages of endomitosis, including the midbody. Thus, Aurora B does not seem to be degraded in early anaphase as it was previously suggested with mouse MKs.22 However, in contrast to the mouse system, rare greater than 8N human MKs are observed in culture.
In mitosis, Aurora B is localized in the centromeres at metaphase/anaphase and mediates phosphorylation of histone H3 on Ser10, explaining the occurrence of chromosome segregation.33,34 In MK endomitosis, asymmetrical chromosome segregation to the poles is seen, but this is mostly due to the organization of a multipolar spindle.8 Nevertheless, our data provide evidence that Aurora B is active in late stages of the endomitotic process because we could detect phosphorylation of Thr232 in its activating loop of its main substrate MgcRacGAP. This suggests that Aurora B plays a role in the regulation of furrow formation and ingression in endomitotic MKs.
However, when Aurora B was functionally inactivated by AZD1152-HQPA, MK polyploidization occurred, and, even more, an increase in mean ploidy was observed. This could be interpreted as evidence for a negative role of Aurora B in MK polyploidization. Indeed, in many cell types, Aurora B inhibition results in polyploidy by induction of abnormal mitosis with the absence of cytokinesis followed by endoreplication.19,21 However, our detailed analysis of the different MK ploidy classes showed that the number of 2N and 4N MKs was greatly diminished because of increased apoptosis and cell cycle arrest. This effect was even more striking when AZD1152-HQPA was added at the beginning of the culture (day 2) when MK precursors are proliferating. In contrast, although the overall mean ploidy was increased, the number of polyploid MKs was not augmented, and the ploidy distribution in greater than 4N cells was quite similar in treated and untreated cultures. Therefore, inhibition of Aurora B does not induce a true increase in polyploidy in MKs but rather a selection of surviving polyploid cells at the expense of the 2N to 4N cells. Interestingly, in both 2N to 4N and greater than 4N cell populations, Aurora B inhibition led to the induction of p53 and to the appearance of a hypophosphorylated form of Rb that was partly due to an inhibition of Ser780 phosphorylation. This Ser780 is considered to be a direct site of phosphorylation by Aurora B, and it has been suggested that the direct effect of Aurora B on Rb plays a role in polyploidy induction by allowing endoreplication after an abnormal mitosis.21 In fact, a slight decrease in cell cycle entry was observed in polyploid MK cells after Aurora B inhibition. It is thus possible that, in polyploid MKs, abnormal chromosome segregation is not “sensored” as an abnormal mitosis; thus, regulation of new entry in cell cycle requires a phosphorylated form of Rb as in normal endomitosis.40,41 Interestingly, p53 was activated in both 2N to 4N MKs and 8N or greater MKs. Although expression of apoptotic molecules, such as Bax, was induced in both MKs populations, apoptosis was not significantly augmented in the 8N or greater MK population. This seems to be correlated to the high constitutive expression of antiapoptotic molecules in the polyploid cell population because BclxL overexpression decreased the apoptosis induced by the Aurora B inhibitor in the 2N to 4N MKs.
The present results are reminiscent to those recently described for survivin,9 another protein of the chromatin passenger complex. When survivin is specifically deleted in the MK lineage in vivo, MK precursors die by apoptosis, and MK differentiation is supported by cells, which have not excised the survivin gene. In vitro, survivin knockout leads to an increased degree of polyploidization related to a decrease in 2N and 4N MK cells. That both survivin and Aurora B inactivation leads to the same phenotype is quite expected because function of both proteins is interdependent with Aurora B regulating survivin localization by phosphorylation, and survivin is being required for appropriate Aurora B localization.42,43 However, our data show that, whereas Aurora B inactivation is dispensable for MK polyploidization, it is absolutely required for accomplishment of a complete endomitotic process. Indeed, in most Aurora B inhibitor–treated endomitotic MKs, mitotic failure occurs at the metaphase/anaphase transition because of incomplete chromosome segregation and not because of a failure in late cytokinesis. This defect in the metaphase/anaphase transition may be related to the abnormal localization of Aurora B due to inhibition of its kinase activity. Thus, we cannot exclude that a true knockdown of Aurora B will differently affect the endomitotic process. However, approaches aiming to inactivate Aurora B in vivo will be extremely difficult to perform as underscored by the survivin knockout mice because Aurora B is required for cell survival of MK precursors.
Altogether our study provides evidence that Aurora B is active all along the endomitotic process but does not prevent furrow regression and tetraploidization. However, Aurora B activation is required for the occurrence of a complete endomitotic process characterized by failure in late cytokinesis, but it is dispensable for MK polyploidization, as previously shown for survivin.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank F. Wendling for critically reading the manuscript.
This work was supported by grants from the Association de la Recherche contre le Cancer (W.V.), the Inserm (Y.C. and L.L.), the Agence Nationale pour la Recherche (Y.C. and L.L.) and the Ligue Nationale Contre le Cancer (équipe labellisée 2010).
Authorship
Contribution: L.L. designed, performed experiments, analyzed data, and wrote the paper; Y.C. designed, performed experiments, and critically read the paper; A.J. performed experiments and analyzed data; Y.L. and F.L. performed sorting experiments; F.A., L.G., and J.L. performed experiments; T. Kawashima and T. Kitamura performed the antibodies, analyzed data, and critically read the paper; N.D. designed and performed experiments; W.V. designed the work, supervised the experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William Vainchenker, Inserm, U1009, Hématopoièse et Cellules Souches Normales et Pathologiques, Institut Gustave Roussy, 39 rue Camille Desmoulins, 94805 Villejuif cedex, France; e-mail: verpre@igr.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal