Abstract
The clinical approach to older patients with myeloma has to be modified to take into account comorbidities and the likelihood of higher treatment-related toxicity. Individualization of management and adequate supportive therapy are important to obtain the best response while minimizing adverse effects. Corticosteroids, novel agents, conventional cytotoxic agents, and high-dose chemotherapy with autotransplantation (modalities used in younger patients) are also used in older patients, although the elderly undergo transplantation less frequently. The sequential use of active agents singly and in different combinations has improved response rates and survival of all patients with myeloma, including the elderly.
Introduction
In myeloma, the term “elderly” is sometimes used synonymously with a person ineligible for high-dose chemotherapy and autologous hematopoietic stem cell transplantation (AHSCT). This is not always true. Although younger patients certainly have better outcomes with AHSCT,1 high-dose therapy does have a place in the management of selected older persons.2,3 Age has less impact on event-free survival (EFS) after AHSCT than on overall survival (OS),1 suggesting that older patients may benefit as much from high-dose therapy as younger.
Bearing in mind the pitfalls of choosing therapy based purely on chronologic age, within the so-called elderly group (≥ 65 years; a commonly used if arbitrary and somewhat unkind threshold), our approach to patients up to the age of 70 years, those 71 to 75 years of age, and those more than 75 years (a particularly vulnerable population) does differ. It is reasonable to say that performance status (biologic age) is a bigger determinant of our approach than chronologic age.
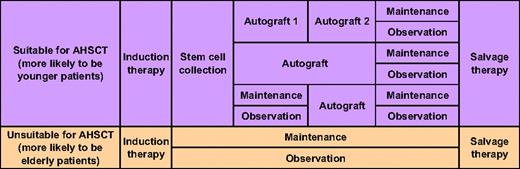
Figure 1 shows a general scheme illustrating the diverse course of disease-specific treatment in myeloma. The transplantation pathway is used more frequently in younger patients and the nontransplantation pathway in older.
Investigations
Table 1 shows the general investigative approach.4,5 With increasing age, some of the more onerous investigations, such as magnetic resonance imaging and positron emission tomography computed tomography, may be unnecessary on a routine basis.
Investigations in elderly patients with myeloma
| Investigation . | Diagnosis . | Prognosis . | Choice of therapy . | Monitoring . | Comments . |
|---|---|---|---|---|---|
| Hemoglobin | + | +/− | − | + | May affect supportive therapy |
| Serum calcium | + | − | − | + | May affect supportive therapy |
| Serum creatinine | + | +/− | + | + | May affect supportive therapy |
| Serum glucose | − | − | +/− | +/− | May affect supportive therapy |
| Serum uric acid | − | − | − | +/− | May affect supportive therapy |
| Bone marrow: plasma cell number, morphology, and plasma cell clonality | + | +/− | − | + | Absence of clonality needed to define stringent CR |
| Bone marrow cytogenetics | − | + | + | +/− | J.M. and S.S. obtain this routinely to determine prognosis for individual patients. M.C. obtains this only in the context of clinical trials or in patients eligible to receive treatment with novel agents. |
| Bone marrow FISH | − | + | + | +/− | J.M. and S.S. obtain this routinely to determine prognosis for individual patients. M.C. obtains this only in the context of clinical trials or in patients eligible to receive treatment with novel agents. |
| LDH | − | + | + | +/− | |
| Skeletal survey | + | − | +/− | +/− | Affects supportive therapy |
| Serum free light chain levels | +/− | − | − | +/− | Essential in patients with nonsecretory or hyposecretory disease; normalization needed to define stringent CR (not validated) |
| Serum albumin | − | + | − | +/− | Essential to apply International Staging System |
| Serum β2-microglobulin | − | + | − | − | Essential to apply International Staging System |
| Serum immunoglobulins | + | − | − | + | |
| Serum protein electrophoresis | + | − | − | + | |
| Serum immunofixation | + | − | − | + | |
| Urine 24-hour protein | + | − | + | + | May affect supportive therapy |
| Urine 24-hour protein electrophoresis | + | − | − | + | |
| Urine 24-hour immunofixation | + | − | − | + | |
| Bone marrow plasma cell labeling index | − | + | − | − | Optional |
| Bone densitometry | − | − | +/− | − | Affects supportive therapy (bisphosphonates may be administered if bone density is low even if there are no osteolytic lesions) |
| Skeletal MRI scan | +/− | − | − | +/− | Optional; may affect supportive therapy (bisphosphonates) and assessment of fracture risk; may show disease activity in patients in serologic CR |
| PET CT | +/− | − | − | +/− | Optional; may show disease activity in patients in serologic CR |
| Echo/MUGA | − | − | +/− | − | Optional; may affect supportive therapy |
| Investigation . | Diagnosis . | Prognosis . | Choice of therapy . | Monitoring . | Comments . |
|---|---|---|---|---|---|
| Hemoglobin | + | +/− | − | + | May affect supportive therapy |
| Serum calcium | + | − | − | + | May affect supportive therapy |
| Serum creatinine | + | +/− | + | + | May affect supportive therapy |
| Serum glucose | − | − | +/− | +/− | May affect supportive therapy |
| Serum uric acid | − | − | − | +/− | May affect supportive therapy |
| Bone marrow: plasma cell number, morphology, and plasma cell clonality | + | +/− | − | + | Absence of clonality needed to define stringent CR |
| Bone marrow cytogenetics | − | + | + | +/− | J.M. and S.S. obtain this routinely to determine prognosis for individual patients. M.C. obtains this only in the context of clinical trials or in patients eligible to receive treatment with novel agents. |
| Bone marrow FISH | − | + | + | +/− | J.M. and S.S. obtain this routinely to determine prognosis for individual patients. M.C. obtains this only in the context of clinical trials or in patients eligible to receive treatment with novel agents. |
| LDH | − | + | + | +/− | |
| Skeletal survey | + | − | +/− | +/− | Affects supportive therapy |
| Serum free light chain levels | +/− | − | − | +/− | Essential in patients with nonsecretory or hyposecretory disease; normalization needed to define stringent CR (not validated) |
| Serum albumin | − | + | − | +/− | Essential to apply International Staging System |
| Serum β2-microglobulin | − | + | − | − | Essential to apply International Staging System |
| Serum immunoglobulins | + | − | − | + | |
| Serum protein electrophoresis | + | − | − | + | |
| Serum immunofixation | + | − | − | + | |
| Urine 24-hour protein | + | − | + | + | May affect supportive therapy |
| Urine 24-hour protein electrophoresis | + | − | − | + | |
| Urine 24-hour immunofixation | + | − | − | + | |
| Bone marrow plasma cell labeling index | − | + | − | − | Optional |
| Bone densitometry | − | − | +/− | − | Affects supportive therapy (bisphosphonates may be administered if bone density is low even if there are no osteolytic lesions) |
| Skeletal MRI scan | +/− | − | − | +/− | Optional; may affect supportive therapy (bisphosphonates) and assessment of fracture risk; may show disease activity in patients in serologic CR |
| PET CT | +/− | − | − | +/− | Optional; may show disease activity in patients in serologic CR |
| Echo/MUGA | − | − | +/− | − | Optional; may affect supportive therapy |
FISH indicates fluorescence in situ hybridization; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; PET, positron emission tomography; CT, computed tomography, +, useful; −, unnecessary; and +/−, usefulness equivocal.
Monitoring other parameters may reduce the risk of toxicity. Brain natriuretic peptide should be monitored in patients with compromised heart function, especially while on therapy that can cause fluid retention. In diabetic patients it is important to monitor hemoglobin A1C to ensure adequate glycemic control. Urine protein should be monitored in all patients because of the high likelihood of proteinuria in the elderly from diabetic or hypertensive renal damage.
When to treat?
When symptomatic myeloma is diagnosed based on the “CRAB” (hypercalcemia, renal failure, anemia, and bone lesions) criteria,6 disease-specific therapy is usually required promptly. Older patients may have age-related osteopenia, which might not indicate the need to start myeloma-specific therapy if there is no other organ dysfunction. Such patients could be started on bisphosphonates and the disease monitored. Mild kidney impairment resulting from diabetes or hypertension may be seen in elderly patients. If anemia seems out of proportion to the disease burden, concurrent causes such as iron, vitamin B12, or folate deficiency, chronic inflammatory diseases, or myelodysplastic syndrome should be sought. Conversely, elderly patients with ischemic heart disease or pulmonary dysfunction may not be able to tolerate modest decreases in hemoglobin or increased blood viscosity, requiring disease-specific therapy despite a modest tumor burden.
The diagnosis of myeloma may be delayed in older persons because early nonspecific symptoms, such as fatigue, bone pain, and susceptibility to infections, may be attributed to other causes.
Goals of therapy
Attainment of complete remission (CR) is an important goal irrespective of age. However, in older patients, the difference between attempting to achieve CR and settling for a lower degree of response may be substantial treatment-related toxicity that could overshadow any benefit derived from achievement of CR. If significant toxicity is seen, obtaining good disease control while maintaining quality of life is reasonable. Prolongation of remission and survival are additional goals. Symptom control is achieved through effective disease-specific and supportive therapy.
There is a small subgroup of much older persons (≥ 80 years; with other serious comorbidities) in whom palliative therapy is a reasonable option. The use of corticosteroids can result in effective palliation as well as some cytoreduction.
Supportive therapy
Supportive therapy includes management of anemia, pain, hypercalcemia, skeletal complications, infections, and nutrition.
Anemia may be better tolerated in older persons if they are not physically very active. On the other hand, anemia is a greater concern in patients with ischemic heart disease, chronic obstructive lung disease, and cerebrovascular disease. Anemia is treated with judicious use of transfusions and erythropoiesis-stimulating agents.
The use of narcotic analgesics should strike a balance between adequate pain relief with resultant improvement in quality of life and adverse effects such as drowsiness, confusion, and constipation. Constipation can be compounded by concomitant administration of thalidomide, and attention to diet and bowel motility is critical before constipation sets in.
Hypercalcemia is treated with hydration, bisphosphonates, occasional calcitonin, and corticosteroid therapy. Once it resolves and regular bisphosphonate therapy is started, calcium and vitamin D are recommended. Typically, pamidronate or zoledronic acid is used. Some prefer pamidronate (90 mg over 2 hours; 30-60 mg with renal dysfunction) because of its more favorable safety profile with regard to renal function and osteonecrosis of the jaw. However, zoledronic acid (4 mg; 2-3 mg with mild to moderate renal impairment) has the advantage of a shorter infusion time (J.M. and S.S. use 30 minutes; M.C. uses 15 minutes). Osteonecrosis is not a major concern with zoledronic acid, provided rigorous preventive strategies are used. Bisphosphonates are usually discontinued after 2 years if myeloma is controlled and skeletal manifestations are better. They are continued beyond 2 years if there is persistent bone disease; sometimes at a reduced frequency (once every 2-4 months).
Kyphoplasty or vertebroplasty should be considered for vertebral collapse resulting in pain unresponsive to medications or to stabilize vertebrae at risk of fracture. Patients with significant kyphoscoliosis should undergo physical therapy and muscle-strengthening exercises to improve posture. Breathing exercises and incentive spirometry are important to reduce the risk of lung infections. Smoking cessation should be encouraged to reduce predisposition to lung problems in the setting of kyphoscoliosis and diminished lung capacity.
Infection prophylaxis is crucial when corticosteroids are used: fluconazole, trimethoprim-sulfamethoxazole, and acyclovir. Older persons are susceptible to varicella-zoster virus reactivation because of age-related decline in varicella-zoster virus–specific cell-mediated immunity and treatment-induced immunosuppression. Acyclovir prophylaxis virtually eliminates the risk of zoster in patients receiving bortezomib.7 It is our practice to administer 200 to 400 mg acyclovir daily to all patients on any type of treatment (J.M., S.S.) or with bortezomib-based regimens and after transplantation (M.C.). The currently available shingles vaccine is not suitable for immunocompromised patients.
Disease-specific therapy
The general scheme of disease-specific therapy is illustrated in Figure 1. Patients eligible for AHSCT should receive induction therapy that excludes melphalan to avoid irreversible stem cell damage. This usually composes 2 or 3 drugs: a novel agent (thalidomide, lenalidomide, or bortezomib) with corticosteroids with or without a cytotoxic agent, or 2 novel agents with corticosteroids. Response rates and EFS are higher with 3-drug combinations compared with 2 drugs in younger patients. Those who are clearly ineligible for AHSCT usually receive induction therapy that is based on one of the novel agents: combined with melphalan-prednisone (MP) or with corticosteroids.
Table 2 shows various induction regimens, several containing novel agents originally developed to treat relapsed/refractory disease.8-10 After the induction phase, thalidomide, bortezomib, and lenalidomide may be used, singly or in combination, as consolidation/maintenance therapy.
Induction therapy regimens
| Therapy type . |
|---|
| Stem cell–sparing |
| Dexamethasone |
| Thalidomide and dexamethasone or prednisone; with or without cyclophosphamide or doxorubicin |
| Bortezomib and dexamethasone or prednisone; with or without thalidomide or doxorubicin |
| Lenalidomide* and low-dose dexamethasone or prednisone |
| Non-stem cell–sparing |
| Melphalan-prednisone (MP) |
| Melphalan-prednisone-thalidomide (MPT) |
| Melphalan-prednisone-bortezomib (MPB) |
| Melphalan-prednisone-lenalidomide (MPL) |
| Therapy type . |
|---|
| Stem cell–sparing |
| Dexamethasone |
| Thalidomide and dexamethasone or prednisone; with or without cyclophosphamide or doxorubicin |
| Bortezomib and dexamethasone or prednisone; with or without thalidomide or doxorubicin |
| Lenalidomide* and low-dose dexamethasone or prednisone |
| Non-stem cell–sparing |
| Melphalan-prednisone (MP) |
| Melphalan-prednisone-thalidomide (MPT) |
| Melphalan-prednisone-bortezomib (MPB) |
| Melphalan-prednisone-lenalidomide (MPL) |
Exposure to more than 3 to 6 cycles of lenalidomide, particularly in elderly patients, can result in difficulty in collecting stem cells. It is therefore better to collect stem cells early in patients receiving lenalidomide. The use of chemotherapy- or plerixafor-containing mobilization regimens increases the likelihood of a successful stem cell collection in lenalidomide-treated patients.
An important element of delivering disease-specific therapy safely is meticulous supportive care. Our practice is illustrated in Table 3. The increased safety and tolerability resulting from close follow-up usually outweigh the inconvenience of intensive monitoring. The frequency of monitoring is reduced in patients who are stable and tolerating treatment well.
Supportive therapy and monitoring in patients on induction or salvage therapy (not applicable to patients on periodic observation with remission, plateau, or stable disease)
| Type . |
|---|
| Infection prophylaxis |
| Fluconazole (if on corticosteroids) |
| Trimethoprim-sulfamethoxazole (if on corticosteroids) |
| Acyclovir (all patients irrespective of whether on therapy or not; J.M., S.S.) |
| Vaccination |
| Seasonal influenza vaccination is appropriate |
| Vaccination against Streptococcus pneumoniae and Haemophilus influenzae may be considered, but immune response may be suboptimal |
| The currently available zoster vaccine is contraindicated in immunocompromised patients and should not be used |
| Ulcer/gastritis prophylaxis (if on corticosteroids; proton pump inhibitor or H2-blocker) |
| Prophylaxis against deep vein thrombosis (if on thalidomide or lenalidomide) |
| Aspirin (81 or 325 mg) if no history of prior thromboembolic phenomena |
| Warfarin if history of prior thromboembolic phenomena or evidence of a high risk of thrombosis |
| Low-molecular weight heparin (a safer alternative to warfarin, particularly in patients with renal failure, although cost may be a barrier) |
| Regular blood counts and chemistry |
| At the time of every infusion of bortezomib on bortezomib-based regimens |
| Every 2 weeks on lenalidomide-containing regimens; the frequency can be reduced after a few weeks if clinical and laboratory parameters stable; if cytopenias are seen, more frequent monitoring may be needed |
| Every 2 to 4 weeks on dexamethasone and thalidomide-containing regimens; frequency can be reduced after a few weeks if clinical and laboratory parameters stable |
| Periodic Hb A1C |
| Regular clinical evaluation |
| Every 1 to 4 weeks to start with based upon the regimen; frequency can be reduced after a few weeks if clinical and laboratory parameters stable |
| Regular blood pressure monitoring if abnormal or known hypertensive (daily self-monitoring if possible) |
| Regular blood sugar monitoring if abnormal levels or known diabetic; if on corticosteroids |
| Type . |
|---|
| Infection prophylaxis |
| Fluconazole (if on corticosteroids) |
| Trimethoprim-sulfamethoxazole (if on corticosteroids) |
| Acyclovir (all patients irrespective of whether on therapy or not; J.M., S.S.) |
| Vaccination |
| Seasonal influenza vaccination is appropriate |
| Vaccination against Streptococcus pneumoniae and Haemophilus influenzae may be considered, but immune response may be suboptimal |
| The currently available zoster vaccine is contraindicated in immunocompromised patients and should not be used |
| Ulcer/gastritis prophylaxis (if on corticosteroids; proton pump inhibitor or H2-blocker) |
| Prophylaxis against deep vein thrombosis (if on thalidomide or lenalidomide) |
| Aspirin (81 or 325 mg) if no history of prior thromboembolic phenomena |
| Warfarin if history of prior thromboembolic phenomena or evidence of a high risk of thrombosis |
| Low-molecular weight heparin (a safer alternative to warfarin, particularly in patients with renal failure, although cost may be a barrier) |
| Regular blood counts and chemistry |
| At the time of every infusion of bortezomib on bortezomib-based regimens |
| Every 2 weeks on lenalidomide-containing regimens; the frequency can be reduced after a few weeks if clinical and laboratory parameters stable; if cytopenias are seen, more frequent monitoring may be needed |
| Every 2 to 4 weeks on dexamethasone and thalidomide-containing regimens; frequency can be reduced after a few weeks if clinical and laboratory parameters stable |
| Periodic Hb A1C |
| Regular clinical evaluation |
| Every 1 to 4 weeks to start with based upon the regimen; frequency can be reduced after a few weeks if clinical and laboratory parameters stable |
| Regular blood pressure monitoring if abnormal or known hypertensive (daily self-monitoring if possible) |
| Regular blood sugar monitoring if abnormal levels or known diabetic; if on corticosteroids |
Duration of therapy
If early AHSCT is planned in a patient 65 to 70 years of age, stem cell–sparing induction therapy (Table 2) is administered for 3 or 4 cycles followed by stem cell collection and transplantation. If CR is achieved with induction therapy, deferring AHSCT until disease progression occurs is a possible alternative to early AHSCT. This is an area of debate because it is unknown whether the depth of CR achieved with novel agent-based induction therapy when not followed by AHSCT is equivalent to the depth of CR attained with novel agents and consolidated with AHSCT. Limited data suggest that the outcome of patients in CR after thalidomide- or lenalidomide-based induction chemotherapy improves further with AHSCT consolidation.11 Prospective randomized studies designed to address this question are ongoing. Our preference in patients 65 to 70 years of age is to proceed to AHSCT even if CR is attained.
In patients who are not candidates for AHSCT, the duration of induction therapy is usually based on the regimen chosen and whether consolidation/maintenance therapy is planned. If no maintenance therapy is planned, 9 to 12 cycles of induction with MP and a novel agent are appropriate.12-15 However, this may be reduced to 6 to 9 cycles if maintenance therapy is planned.16,17 The optimum duration of maintenance fixed versus indefinite (until relapse/toxicity) is unknown.
The treatment duration may have to be individualized based on response and tolerance: a patient whose disease is responding slowly and who is tolerating therapy well may benefit from continued therapy beyond the original plan, whereas a patient whose disease has responded well to therapy but who has significant adverse effects could benefit from interruption or discontinuation of therapy or a reduction in dose intensity.
Sequence of therapy
The various agents and combinations can be used in different sequences. Although there are no data indicating the best sequence, incorporation of novel drugs into initial therapy results in longer disease control. The reported effect of the type of initial therapy on OS has been variable because of heterogeneous salvage therapy. It is important to ensure that, barring any contraindications, patients receive all active agents at some point in the course of the disease. For most patients, more than or equal to 50% of the duration of their disease will fall in the salvage therapy phase (Figure 1). The approach to an elderly patient with relapsed disease needs to be individualized and depends on several factors (Table 4).5,18
Factors determining approach to relapsed disease
| Factor type . |
|---|
| Disease |
| Tumor burden |
| Tempo of progression |
| Biologic nature |
| Patient |
| Age |
| Organ function |
| Concomitant medical problems |
| Bone marrow function |
| Treatment |
| Prior treatment (drugs and combinations) |
| Prior transplantation |
| Response to prior treatment approaches |
| Availability of autologous stem cells |
| Availability of suitable clinical trials |
| Factor type . |
|---|
| Disease |
| Tumor burden |
| Tempo of progression |
| Biologic nature |
| Patient |
| Age |
| Organ function |
| Concomitant medical problems |
| Bone marrow function |
| Treatment |
| Prior treatment (drugs and combinations) |
| Prior transplantation |
| Response to prior treatment approaches |
| Availability of autologous stem cells |
| Availability of suitable clinical trials |
In general, as the disease progresses and patients receive increasing amounts of therapy, performance status declines. This, coupled with advancing age, makes intensive therapy options more difficult to deliver later in the course of the disease. This is particularly important in the small proportion of elderly patients eligible for AHSCT, in whom it may be better to use AHSCT early in salvage therapy.
Corticosteroids
Prednisone and dexamethasone are very active against myeloma with the advantages of oral administration and lack of myelotoxicity. Adverse effects include hypertension, hyperglycemia, gastritis, weight gain, fluid retention, mood swings, opportunistic infections, insomnia, osteopenia, and Cushing syndrome. In our experience, with stringent monitoring and supportive therapy, and dose modification when indicated, it is possible to deliver steroid therapy relatively safely to most patients.
In younger patients, single-agent dexamethasone is typically used in a schedule of 40 mg per day on days 1 to 4, 9 to 12, and 17 to 20 of a 28- or 35-day cycle. In elderly patients, dose modification frequently entails a reduction to 10 to 30 mg. Dexamethasone 40 mg once a week is a common alternative19 for older as well as for younger patients in combination with novel agents. There are no data supporting such reduced doses of dexamethasone as a single agent in newly diagnosed disease or in relapsed disease with a high tumor burden.
An effective alternative that is often tolerated better is prednisone. This can be used intermittently (2 mg/kg daily for 4 days every 4 weeks; M.C.), or continuously (20-60 mg on a daily, alternate day or 3 times a week; J.M., S.S.). In our experience, prednisone 20 to 40 mg daily or 40 to 60 mg 3 times a week can be tolerated well for a period of up to 2 to 3 months (J.M., S.S.). This is associated with more predictable changes in blood pressure and blood sugar, allowing consistent adjustment in supportive therapy.
Low doses of steroids may be considered as a sole option at any stage of therapy in frail patients over the age of 75 to 80 years. We commonly see patients who have “exhausted all treatment options” and have bone marrow failure secondary to disease burden and extensive therapy. A significant proportion of these patients have had limited amounts of corticosteroid therapy. Single-agent corticosteroid therapy is worth considering in such cases (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Melphalan-prednisone and other combinations
MP was a mainstay of therapy for a long time despite low CR rates. Although the addition of other conventional agents to MP improved response rates, the median OS remained unchanged at 3 years.20 The combination of MP with novel agents has improved response tempo and rates and survival.
Thalidomide combinations
The combination of MP with thalidomide (MPT) improves response rates and progression-free survival (PFS), but with increased toxicity.12,13,16 OS has been better when the duration of therapy has been longer.12,13 There is concern that OS differences in some studies partly stem from imbalance in salvage therapy.21
A recent study showed higher response rates but inferior OS with thalidomide-dexamethasone compared with MP because of higher toxicity and early mortality, particularly in patients older than 75 years.22 These data underline the importance of supportive therapy and dose modification in older patients to reduce toxicity.
Cyclophosphamide can be used as a substitute for melphalan. In a recent study, the combination of cyclophosphamide, thalidomide, and dexamethasone (CTD) improved response rates compared with MP. There was a survival benefit in CTD-treated patients with favorable fluorescence in situ hybridization results, although early deaths from infections related to high-dose dexamethasone were worrisome.23
Bortezomib combinations
The addition of twice-weekly bortezomib to MP (MPV) improved response rates, time to progression, treatment-free interval, and OS compared with MP.14 The CR rate with MPV was approximately 30%, the highest in a nontransplantation setting. MPV was superior in all prognostic subgroups, including patients with advanced age, impaired renal function, and high-risk cytogenetics.14,24 Adverse events were more common with MPV, neuropathy being a major concern.
An update of the study24 showed that outcome differences were sustained. However, there were disparities in salvage therapy, reaffirming concerns21 that this may account in part for OS differences. For example, although a similar proportion of relapsing patients in each arm received thalidomide (46% MPV, 47% MP), only 13% of MP patients received lenalidomide compared with 32% of MPV patients (P < .001). A total of 24% of patients in the MPV arm received bortezomib again at relapse whereas only 50% of patients in the MP arm (who had not received bortezomib initially), received the drug at relapse.
Bortezomib once (rather than twice) weekly may be used from the beginning to reduce toxicity. It is worthwhile switching to the once-weekly schedule if the standard schedule is not tolerated well. This approach was based on our observation from the pivotal study of bortezomib9 that patients who tolerated the drug very poorly during the initial phase tolerated it better during the continuation phase where the drug was administered once a week. Weekly bortezomib reduces the risk of side effects, such as peripheral neuropathy, gastrointestinal problems, and thrombocytopenia requiring dose adjustment or interruption in our experience.
The observation of decreased neuropathy with weekly dosing has now been confirmed.17,24,25 In one of the studies,17 bortezomib-melphalan-prednisone (VMP) induction followed by maintenance with bortezomib-thalidomide (VT) or bortezomib-prednisone (VP) was compared with bortezomib-thalidomide-prednisone (VTP) induction followed by VT or VP as maintenance. Treatment discontinuation resulting from adverse events was more probable with VTP compared with VMP. The overall CR rates after induction therapy were similar with VMP and VTP, as were those after VT or VP maintenance. Subgroup analysis, hampered by small sample size, suggested that VMP followed by VT resulted in superior PFS. In another study,25 VMP induction without maintenance was compared with VMP plus thalidomide (VMPT) followed by VT maintenance. VMPT-VT improved response rates and PFS. In both arms, weekly bortezomib was associated with significantly decreased frequency of severe neuropathy.25
Lenalidomide combinations
The combination of MP and lenalidomide (MPL/MPR) is effective in myeloma.26 However, lenalidomide and MP are myelosuppressive, and MPL can result in significant hematologic toxicity, especially in elderly patients.
The preliminary results of a study comparing MP and MPL induction with or without lenalidomide maintenance (MPL-L or MPL) showed that MPL-L was significantly superior to MP with higher response rates and PFS, and superior to MPL in terms of PFS.27 This indicates a beneficial effect of lenalidomide when added to MP induction and of lenalidomide maintenance when given after MPL.
Thalidomide
With the availability of lenalidomide and bortezomib—both more potent than thalidomide—it is easy to overlook the important activity of thalidomide. A key characteristic thalidomide shares with corticosteroids is lack of myelosuppression.8,29 Single-agent thalidomide is a reasonable option in patients with relatively low-grade disease, especially if marrow function is compromised.
The combination of thalidomide and corticosteroids is synergistic in relapsed28,29 and newly diagnosed30,31 disease. Thalidomide-dexamethasone as initial therapy is superior to dexamethasone alone in terms of response rates and PFS, but its impact on OS is unclear.31 Thalidomide is useful as maintenance therapy in the transplantation and nontransplantation settings, but the magnitude of benefit, patient populations that benefit, and optimum duration are not well defined.16,32,33
Bortezomib
Bortezomib with or without corticosteroids has excellent activity in myeloma9 and constitutes reasonable therapy at any stage of the disease. Single-agent bortezomib is superior to dexamethasone for relapsed disease.34 However, it is common practice to combine bortezomib and corticosteroids from the beginning.
Weekly administration of bortezomib is much better tolerated than the standard schedule. This is especially relevant in older patients in whom the better toxicity profile and reduced rate of treatment discontinuation may allow them to stay on therapy for a longer time period, with a higher chance of achieving maximal response.
Selected patients benefit from the addition of pegylated liposomal doxorubicin (PLD) if bortezomib is ineffective.35 We do not typically use the PLD-bortezomib combination without trying bortezomib with corticosteroids first. Although much is made of the “corticosteroid-free” nature of the PLD-bortezomib combination, the practical benefits of eliminating corticosteroids are limited.
Bortezomib and thalidomide have remarkable synergistic activity when used for induction with corticosteroids,17,36 albeit at the cost of increased toxicity compared with VMP.17 These drugs, administered as consolidation/maintenance therapy, increase CR rates17,25,36 and improve PFS.17 We often use bortezomib as maintenance therapy (J.M., S.S.: 1.3 mg/m2 once a month; M.C.: 1.3 mg/m2 twice a month) in selected patients with stable or responding disease irrespective of age (supplemental Figure 2).
Lenalidomide
The pivotal studies of lenalidomide were carried out in patients with relapsed disease where lenalidomide was used at the dose of 25 mg daily for 21 days followed by a 7-day break with high-dose dexamethasone (“pulse dexamethasone”: 40 mg daily on days 1-4, 9-12, and 17-20 every 28 days) (LD).10 In this setting, LD was found to be significantly superior to pulse dexamethasone in terms of response rates, EFS, and OS. The combination was superior in all subgroups of patients, including those previously treated with thalidomide.37 Obviously, in patients with relapsed disease, LD should be used rather than dexamethasone alone.
The paradox between studies of lenalidomide in newly diagnosed and relapsed disease is interesting. A recent study explored low- or high-dose dexamethasone with lenalidomide as first-line therapy in patients of all ages.19 Lenalidomide was administered in the standard fashion. Patients received pulse dexamethasone (480 mg per cycle; LD) or a lower dose (40 mg once a week; 160 mg per cycle; Ld). Response rates were higher with LD as was the toxicity, and there was no difference in OS with 3-year follow-up. This study shows that Ld is safer than LD as first-line therapy with unspecified supportive care. Whether this would hold true if adequate supportive therapy is given is unclear. However, it is reasonable to use lenalidomide with low-dose dexamethasone as first-line therapy with adequate supportive therapy (Table 3) to enhance safety even more.
Lenalidomide and corticosteroids have synergistic activity against myeloma. It is reasonable to expect that they have synergistic activity in causing toxicity, too. Therefore, the extrapolation of the findings of the LD versus Ld study19 to eliminate pulse dexamethasone altogether from the therapeutic armamentarium may be premature.
The role of high-dose chemotherapy
Although age does affect the outcome of AHSCT, biologic characteristics are more powerful determinants of prognosis.1-3,40,41 We consider early (consolidative) AHSCT appropriate in selected patients between 65 and 70 years of age. The dose of melphalan used is 140 to 200 mg/m2. Lower doses (100-140 mg/m2) are used for older patients. The role of amifostine cytoprotection remains to be determined, but we (J.M. and S.S.) use it routinely in elderly patients if their renal function is marginal. We avoid AHSCT in elderly patients with significantly compromised renal function unless it is clearly related to active myeloma that is unresponsive to other therapy.
Over the age of 70 years (generally up to 75-77 years), in the subgroup of patients considered potentially suitable for high-dose chemotherapy, AHSCT is usually reserved as salvage therapy, although stem cells are collected after first-line therapy (J.M. and S.S.). However, collection of stem cells after first-line therapy is not common clinical practice in Europe (M.C.). If stem cells are to be collected after first-line therapy, a stem cell–sparing regimen should used for induction therapy (Table 2).1,19,30,31,36,42,43 In such instances, MP-based regimens can be used later in the course of the disease.
Although only a single high-dose therapy cycle is planned, we collect enough stem cells for more than one cycle (J.M. and S.S.) because a second (salvage) AHSCT procedure is useful in selected patients. The additional cells can also help reconstitute hematopoiesis when bone marrow function is compromised by multiple lines of salvage therapy, including cytotoxic drugs, bortezomib, and lenalidomide. Hematologic recovery attained in this manner improves quality of life (transfusion-independence) and may make patients eligible for additional therapy, including clinical trials.
Treatment decisions
For an elderly patient with a recent diagnosis of symptomatic myeloma, the primary objective is to determine an appropriate treatment approach on the basis of biologic age, performance status, and comorbidities. The basic choice is between active therapy (most patients) and palliation (selected patients). Active therapy choices include stem-cell sparing induction therapy with a view to possible AHSCT at some point, melphalan-prednisone with one of the novel agents, or other multidrug combinations. Interestingly, the Ld combination (lenalidomide and weekly dexamethasone) is commonly used even in patients who are ineligible for HSCT because of its excellent tolerance.19
Our approach is to offer AHSCT to patients 65 to 70 years of age who are fit and motivated. In such patients, we favor the incorporation of novel agents in induction as well as in posttransplantation consolidation/maintenance therapy.
For patients 65 to 75 years who are not eligible for transplantation but can tolerate the increased toxicity profile of novel agents, classic MP is no longer standard treatment but should be combined with novel agents.
In the absence of direct, prospective comparisons, it is not possible to recommend one regimen over another. However, several patient- and disease-related characteristics may suggest one approach over another in specific settings. For example, there is evidence that MPV/VMP and MPL may overcome the adverse prognosis associated with certain high-risk cytogenetic abnormalities.14,24,26,27 Similar data are not available for MPT. Full-dose MPV/VMP and MPT can be administered safely in patients with renal failure, whereas lenalidomide needs dose adjustment. There is no risk of thrombosis with MPV/VMP, whereas this is a significant risk with MPT and MPL. MPV/VMP may be preferable in patients who have a history of thromboembolic phenomena. Bortezomib and thalidomide cause neuropathy, whereas lenalidomide does not. This is relevant in patients with diabetic neuropathy. An all-oral regimen, such as MPT or Ld, is more convenient than a bortezomib-containing combination.
Although MPT and MPV are backed by phase III studies, their choice as first-line therapy in AHSCT-ineligible patients is being challenged by the encouraging results seen with Ld.19 Ld has the advantage of oral administration and may be the treatment of choice in patients with preexisting neuropathy.
In patients over the age of 75 years, we use modified doses of novel agents to minimize toxicity: thalidomide 50 to 100 mg/daily, melphalan 0.15 to 0.20 mg/kg per day for 4 days per cycle, and bortezomib 0.7 to 1.3 mg/m2 once a week. The initial lenalidomide dose should not exceed 10 mg per day and can be adjusted based on blood counts. The dexamethasone dose may need to be reduced from 40 mg once a week to as little as 10 mg.
Finally, if there is a very high likelihood of inability to tolerate the toxicity associated with combination therapy (usually in patients older than 80-85 years), the options are reduced-dose MP, corticosteroids alone at low to modest doses, or palliation. Selected patients benefit from single-agent bortezomib or lenalidomide, agents normally used with corticosteroids.
None of the preferences suggested contradicts alternative options that can be used subsequently. The key aspect of therapy is to use all available drugs and combinations appropriately. Rechallenge with any of the drugs is reasonable, provided it was effective when used previously and relapse did not occur too quickly.
Treatment-induced myelosuppression
Older persons are more susceptible to bortezomib-induced thrombocytopenia and lenalidomide-induced myelosuppression. Conventional cytotoxic chemotherapy also results in more profound and prolonged myelosuppression, and appropriate use of growth factors and prophylactic antimicrobials is essential. If nonmyelosuppressive agents by themselves are inadequate, alternating myelosuppressive and nonmyelosuppressive approaches should be considered in patients who cannot sustain the pace of therapy because of myelosuppression.
We have used cryopreserved stem cells to help marrow recovery after patients have been through multiple cycles of salvage therapy and blood counts are compromised. This is effective if there is no treatment-induced or incidental myelodysplastic syndrome, which should be considered in patients with problematic cytopenias. If myelodysplastic syndrome is confirmed, lenalidomide may be a reasonable choice in these patients because of its action on both diseases.
Special situations
Older patients frequently have age-related comorbidities that can magnify the clinical effects of myeloma and make delivery of effective therapy difficult. Several these comorbidities get aggravated when myeloma is treated. These special situations may result in the need to use one regimen rather than another.
Thromboembolic phenomena
Older patients are more prone to deep vein thrombosis as a result of impaired mobility. Combinations containing thalidomide or lenalidomide increase the risk of deep vein thrombosis, and alternative approaches may be preferable. The risk of thrombosis is limited when these drugs are used alone, an option worth exploring in selected cases.
Renal dysfunction
The use of combinations capable of reducing tumor burden rapidly is important to maximize the chance of restoring renal function. The dose of lenalidomide must be modified with renal dysfunction. Thalidomide and bortezomib can be used at full doses in the presence of renal dysfunction and with hemodialysis. Compared with thalidomide, bortezomib exerts faster and more potent action, which could result in a greater chance of reversal of renal failure. Bortezomib-induced inhibition of nuclear factor-κB and reduction of inflammatory proteins in myeloma kidney are additional factors contributing to reversal of renal failure.44 Corticosteroid-based approaches are particularly useful if renal dysfunction is related to nonspecific proteinuria. Bisphosphonates must be used with caution with renal dysfunction. Old age and renal dysfunction both increase the risk of morbidity and mortality with AHSCT, which may not be appropriate in older persons with significant renal dysfunction that is not the result of active myeloma.
Cardiovascular diseases
Corticosteroids elevate blood pressure, making regimens such as Ld, which reduce the emphasis on corticosteroids, more desirable in hypertensive patients. Thalidomide (and to a lesser extent, lenalidomide) can cause bradycardia. The concomitant use of these drugs with a β-blocker should be avoided to decrease the risk of syncope. Using angiotensin-converting enzyme inhibitors or angiotensin receptor blockers may be desirable in hypertensive patients with proteinuria to reduce protein loss.
Maintaining adequate hemoglobin and starting therapy before serum protein rises to levels that can increase whole blood viscosity significantly are important in patients with cardiovascular disease to minimize the risk of ischemic episodes and heart failure.
Diabetes
Diabetes and end-organ damage are common in elderly persons. Monitoring such patients carefully and modifying the glycemic control regimen when they are on corticosteroids are essential. The problem of peripheral neuropathy may be especially vexing in these persons who are at risk of neuropathic symptoms from diabetes, the plasma cell dyscrasia, and treatment (thalidomide and bortezomib).
Limited drug availability
Despite limitations of the Food and Drug Administration label, novel drugs can usually be used at any stage of the disease in the United States where the availability of AHSCT is also largely unrestricted. There are parts of the world, including several European countries, where certain treatment options are restricted to specific phases of the disease or are not available at all. When drug availability is restricted by disease phase, management is easier. Thus, MPT and MPB are commonly used as induction therapy in Europe, but not lenalidomide combinations. Where there is no access to these drugs at all, the situation is much more difficult. MP or dexamethasone are reasonable choices for induction and salvage therapy under such circumstances. Periodic cyclophosphamide is also a simple, inexpensive, and widely available choice. The simple fact is that AHSCT, and novel agents improve survival substantially and must be considered integral parts of therapy. Issues regarding their limited availability, especially in the “developed world,” are philosophical and economic in nature.
Quality of life
Patients with myeloma frequently have symptoms and treatment toxicity that compromise health-related quality of life (HRQL). Bortezomib therapy results in better HRQL than dexamethasone in patients with relapsed disease,45 partly from better clinical outcomes including survival. High-dose chemotherapy and AHSCT are often thought of as being highly detrimental to quality of life. However, after a temporary drop in HRQL, there is better HRQL with prolonged disease control in AHSCT recipients.46 Similar observations have been made in 2 French studies comparing early and late autotransplantation,47 and transplantation and conventional therapy,48 with better HRQL as measured by the TWiSTT (time without symptoms and treatment toxicity) score when transplantation is used48 and when it is used earlier.47
Prolonged disease-specific therapy, particularly when incorporating novel agents that are more active and often more toxic, can ultimately impair HRQL. Ongoing HRQL assessment is important to balance efficacy and toxicity, especially in elderly, frail patients. Unfortunately, the limited data in the literature are not enough to assist clinical decision-making. Systematic incorporation of HRQL measures into future clinical trials should be strongly encouraged.
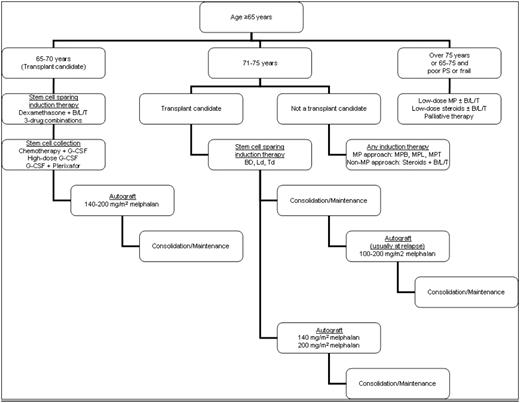
In conclusion, the development of new drugs and enhanced safety of AHSCT have made many treatment options available to elderly myeloma patients. This has improved response rates and survival but has also made delivery of therapy more challenging in such patients with their high rate of comorbid conditions. It is important to take each patient's unique situation into consideration while devising a treatment plan. As subtle differences between the approaches taken by the coauthors, who have similar backgrounds and philosophical approaches to the disease, show, there are many effective ways of treating these patients. A simplified summary is presented in Figure 2.
A simplified approach to the older patient who encompasses the practice of the authors. See text for details as well as some differences in approach used by individual authors.
A simplified approach to the older patient who encompasses the practice of the authors. See text for details as well as some differences in approach used by individual authors.
The online version of this article contains a data supplement.
Authorship
Contribution: J.M., M.C., and S.S. wrote and critically revised the manuscript.
Conflict-of-interest disclosure: J.M. is a consultant and member of the Speakers Bureau, Celgene, and a member of the Speakers Bureau, Takeda (Millennium). M.C. is a consultant with honoraria and membership of the Advisory Board, Janssen-Cilag, Takeda (Millennium), and Novartis honoraria, Celgene. S.S. is a consultant and member of the Speakers Bureau, Takeda (Millennium), is a member of the Speakers Bureau, Celgene, and has received research support from Onyx.
Correspondence: Jayesh Mehta, 676 N St Clair Street, Suite 850, Chicago, IL 60611; e-mail: j-mehta@northwestern.edu.