Abstract
The diagnosis of heparin-induced thrombocytopenia (HIT) requires detection of antibodies to the heparin/platelet factor 4 (PF4) complexes via enzyme-linked immunosorbent assay. Addition of excess heparin to the sample decreases the optical density by 50% or more and confirms the presence of these antibodies. One hundred fifteen patients with anti-heparin/PF4 antibodies detected by enzyme-linked immunosorbent assay were classified as clinically HIT-positive or HIT-negative, followed by confirmation with excess heparin. A multivariate logistic regression model was fitted to estimate relationships between patient characteristics, laboratory findings, and clinical HIT status. This model was validated on an independent sample of 97 patients with anti-heparin/PF4 antibodies. No relationship between age, race, or sex and clinical HIT status was found. Maximal optical density and confirmatory positive status independently predicted HIT in multivariate analysis. Predictive accuracy on the training set (c-index 0.78, Brier score 0.17) was maintained when the algorithm was applied to the independent validation population (c-index 0.80, Brier score 0.20). This study quantifies the clinical utility of the confirmatory test to diagnose HIT. On the basis of data from the heparin/PF4 enzyme-linked immunosorbent assay and confirmatory assays, a predictive computer algorithm could distinguish patients likely to have HIT from those who do not.
Introduction
Diagnosis of heparin-induced thrombocytopenia (HIT) requires that patients fulfill certain clinical criteria and demonstrate the presence of antibodies that bind to the complex of heparin and platelet factor 4 (PF4). Clinical criteria for HIT are generally well accepted and include thrombocytopenia with or without thrombosis that develops in temporal association with heparin therapy and in the absence of other causes of platelet count decline.1,2 The diagnosis of HIT can be challenging, however, because critically ill patients can have multiple potential causes of thrombocytopenia. As many as half of all patients with HIT will have a thrombotic complication at presentation, and from retrospective data, it has been demonstrated that half of those without thrombosis at presentation will develop a thrombotic complication subsequently.3 Therefore, prompt recognition of this disorder is necessary so that appropriate treatment can be initiated to prevent the development of thrombotic sequelae.
Laboratory testing for HIT includes both antigen and functional (platelet activation) assays to detect heparin/PF4 antibodies. The 14C-serotonin release assay (SRA), a functional assay that requires the use of radioactive material, is technically demanding and is available at only a few reference laboratories. The most widely available test for HIT is the heparin/PF4 enzyme-linked immunosorbent assay (ELISA). This assay detects antibodies that bind to PF4 complexed to heparin (Diagnostica Stago) or other negatively charged ligands (GTI Diagnostics) coated on microtiter plates. The test is very sensitive to the presence of anti-heparin/PF4 antibodies (> 97%),4 but it is less specific for the clinical syndrome of HIT (50% to 89% specificity) because of the detection of nonpathologic antibodies (antibodies present in the absence of clinical manifestations of HIT).5,6
The manufacturer of 1 commercial immunoassay (GTI Diagnostics) recommends use of a high-dose heparin confirmatory procedure to improve the specificity of the ELISA. In this particular assay, inhibition of a positive ELISA result by 50% or more in the presence of excess heparin (100 U/mL) is considered confirmatory of heparin-dependent antibodies. The significance of a negative confirmatory result is unknown, however, and there are data that suggest that in the cardiac surgery patient population, the confirmatory result does not improve the diagnostic specificity of the heparin/PF4 ELISA.7 In a previous retrospective review of patients with a positive PF4 ELISA at our large university-based tertiary care center, we found that the majority of patients with antibodies and a positive confirmatory test met clinical criteria for HIT.8 This led to a hypothesis that the confirmatory assay provides additional useful information in the laboratory diagnosis of HIT.
To quantify the information contributed by the PF4 ELISA OD value and the confirmatory assay, we developed a predictive statistical model for HIT. The goal of the present study was 2-fold: (1) to determine the diagnostic value of the heparin confirmatory test in the assessment of patients for HIT and (2) to generate a clinically useful predictive tool to facilitate the diagnosis of HIT.
Methods
Patients
This retrospective study was approved by the Institutional Review Board at Duke University Medical Center. With data from the Duke University Medical Center Coagulation Laboratory, all in-patients with a positive anti-heparin/PF4 antibody result determined by a commercial ELISA (GTI Diagnostics) during 2005 (training set) and the first 97 consecutive patients in 2006 (validation set) were included in the present study. A threshold optical density (OD) measurement of 0.40 was defined as positive for the presence of anti-heparin/PF4 antibody.
Data collection
All Duke University Medical Center records were reviewed from the patient's hospitalization and for up to 30 days when records were available, but patients were not contacted. Data collected for the present study included age, race, sex, medical service, dates of platelet count decline, platelet count nadir, platelet count increase and normalization, dates of anti-heparin/PF4 antibody testing, anti-heparin/PF4 antibody OD values, dates and types of heparin administration, dates and types of thromboembolic events, and patient death and cause of death.
All venous thrombotic events documented in patient records were confirmed by review of radiographic reports. Arterial thrombotic events, including stroke and myocardial infarction, were documented via radiographic studies (magnetic resonance imaging, computed tomography, or cardiac catheterization reports) but also included intraoperative assessment of bowel infarction, autopsy findings, and several cases of digital or limb ischemia based on physical examination performed by vascular surgeons.
Chart reviews were conducted systematically with the diagnostic criteria summarized below by 2 of the investigators (N.L.W., T.L.O.). Because all of the patients included in the chart review had positive anti-heparin/PF4 ELISA results, the reviewers could not be blinded to assay results or to the fact that the diagnosis of HIT was clinically possible.
PF4 confirmation
Patients with positive anti-heparin/PF4 antibodies were divided into Confirm+ and Confirm− groups for analysis. The confirmatory step with excess heparin was performed per manufacturer guidelines.9 A positive confirmatory result was defined as a greater than 50% decrease in antibody binding in the presence of heparin (Confirm+). Likewise, a negative confirmatory test (Confirm−) was defined as a decrease of 50% or less in antibody binding in the presence of heparin. For patients with more than 1 PF4 ELISA assay performed, the confirmatory test result of the initial positive PF4 ELISA (OD ≥ 0.4) designated the patient as Confirm+ or Confirm−. The maximal OD value of the positive test results for patients with multiple tests was used for data analysis.
Definition of clinical HIT status
Extensive chart review was undertaken to clinically diagnose all patients with anti-heparin/PF4 antibodies as having HIT or not, as described previously.8 Criteria from the American College of Chest Physicians guidelines were used to make the clinical diagnosis of HIT: (1) Thrombocytopenia, defined as at least a 30% decline in the platelet count, with a platelet count increase after heparin cessation; (2) timing of platelet count fall between 4 and 14 days after heparin exposure or within 24 to 48 hours if heparin exposures was recent (within the last 100 days); and (3) lack of other, predominant causes of thrombocytopenia.2 For patients after cardiac bypass surgery, a platelet count drop of 50% or more from the highest postoperative value that occurred between postoperative days 4 and 14 or that persisted for 6 or more days after surgery was considered consistent with a diagnosis of HIT.2,10,11
For the present study, patients were classified as either having HIT (HIT+) or not (HIT−). Patients who met all 3 criteria for the clinical diagnosis of HIT were classified as HIT+. In addition, patients who met the first 2 criteria for HIT but who also had potential alternative diagnoses to explain the thrombocytopenia5 were also categorized as HIT+. We used this approach for the present study because many of these patients were in the intensive care unit setting and had several potential causes for thrombocytopenia, but HIT could not be clearly eliminated from the differential diagnosis. Patients were also classified as HIT+ in the rare situation of an unexpected thromboembolic event after heparin exposure but before the development of thrombocytopenia. This was based on reports that suggested that anti-heparin/PF4 antibodies can result in a significantly increased risk for thrombosis and heparin-induced thrombocytopenia and thrombosis (HITT) in cardiac patients before the development of a platelet count decline.12,13
Statistical analysis
Statistical analysis and model fitting was performed with SAS 9.1 software (SAS Institute). Univariate analysis was performed on the 2005 patient population after the patients were clinically classified as HIT+ or HIT−. The initial goal was to determine whether there was a relationship between clinical HIT status and age, race, sex, service of admission, PF4 OD value, or confirmatory assay result. Service categories were collapsed into medical (medicine, cardiology, neurology, bone marrow transplantation/oncology) or surgical (orthopedics, general surgery, cardiothoracic surgery) categories. The t test was used for continuous variables (age, PF4 OD value) and χ2 test for dichotomous variables (positive result on confirmatory procedure, sex, race, medical service). A P value of less than .05 was considered statistically significant.
Univariate logistic regression models were fitted to the 2005 data with clinical HIT status (positive or negative) as the dependent variable and log anti-heparin/PF4 OD value, confirmatory-positive status, and service category as the independent variables. Before the construction of a multivariate model, collinearity diagnostics were performed.14 Collinearity exists when there is a pronounced association between 2 independent variables that makes the effect of the independent variables on the dependent variable in regression analysis difficult to quantify. In the present study, collinearity diagnostics were necessary because a positive confirmatory test result conceivably could be associated with high anti-heparin/PF4 OD values. Collinearity is expressed as a variable inflation factor, with large values (ie, > 10) implying that collinearity is present between 2 independent variables.
A multivariate logistic regression model was fitted to the 2005 data. The model was then validated on the independent sample of 97 patients with positive anti-heparin/PF4 antibodies in 2006. Predicted probabilities were generated for each patient by use of an inverse logit transformation. A nomogram summarizing the logistic regression model was developed with the Design library in R 2.9.15
Assessment of model performance
Model performance was assessed with SAS 9.1 (SAS Institute) and R 2.9 (R Project for Statistical Computing, http://www.r-project.org/).15 The most important property of a predictive model is discrimination, the ability of a model to distinguish patients who are likely to have clinical HIT from those who are not.16 Model discrimination was assessed with the concordance probability, or c-index.17 The c-index is equivalent to the area under the receiver operating characteristic curve18 and is related to Somers' Dyx rank correlation [Dyx = (c − 0.5)/0.5]. In other words, the c-index is the probability that of 2 patients drawn randomly from the population, 1 with and the other without clinical HIT, the patient with HIT would have the higher prediction of HIT. A c-index of 0.5 would be equal to chance discrimination (eg, a coin flip); a c-index of 1.0 would be a perfect predictor for HIT.
A second property of predictive models is reliability (precision). In a reliable model, if 100 patients were assigned an 80% probability of HIT, 80 of the patients should actually have clinical HIT. Generally, discrimination is valued more than reliability. A poorly reliable model can be recalibrated, but there is no remedy for a model that discriminates poorly. The Brier score evaluates both discrimination and reliability on a scale from 0 to 1, with lower scores indicating better predictive performance.19 Informative predictive models should not have Brier scores greater than 0.25. Harrell20 further developed methods for fitting a binary logistic model to a new sample to estimate the relationship between the predicted probability and the observed outcome in that sample. This fit provides a simple calibration equation that can be used to quantify unreliability (lack of calibration) and to calibrate the predictions for future use. This logistic calibration leads to indices of unreliability (U), discrimination (D), and overall quality (Q = D−U) that are derived from likelihood ratio tests. Q is a logarithmic scoring rule that is comparable with the Brier score.
Results
Study populations
Demographic characteristics of the 2 patient populations are summarized in Table 1. Patients from the cardiothoracic surgery and general medical services predominated. There were no patients identified from the obstetrics/gynecology or pediatric services. Slightly more than half of the patients in each group met the clinical criteria for HIT (Table 1).
Demographic characteristics of the 2 patient populations
| Characteristic . | Training set (n = 115) . | Validation set (n = 97) . |
|---|---|---|
| Median age, y | 64 | 63 |
| Male sex | 52 (45) | 57 (59) |
| White race | 78 (68) | 66 (68) |
| General surgery service | 17 (15) | 7 (7) |
| Cardiothoracic surgery service | 39 (34) | 33 (34) |
| Medicine service | 35 (31) | 25 (26) |
| Clinical HIT+ | 74 (64) | 56 (58) |
| Characteristic . | Training set (n = 115) . | Validation set (n = 97) . |
|---|---|---|
| Median age, y | 64 | 63 |
| Male sex | 52 (45) | 57 (59) |
| White race | 78 (68) | 66 (68) |
| General surgery service | 17 (15) | 7 (7) |
| Cardiothoracic surgery service | 39 (34) | 33 (34) |
| Medicine service | 35 (31) | 25 (26) |
| Clinical HIT+ | 74 (64) | 56 (58) |
Values are n (%) unless otherwise indicated.
All patients underwent confirmation with excess heparin, with the results for each patient group shown in Table 2, stratified by the presence of clinical HIT. In patients with a clinical diagnosis of HIT, 96% of patients in the training set and 88% in the validation set had positive confirmatory assays. After review of medical records, 36% (41/115) in the training set and 42% (41/97) in the validation set were believed to be HIT−.
Laboratory evaluation of anti-heparin/PF4 antibody–positive patients
| Laboratory result . | Training set . | Validation set . |
|---|---|---|
| All patients, Confirm+ | 98/115 (85) | 77/97 (79) |
| All patients, Confirm− | 17/115 (15) | 20/97 (21) |
| HIT+ patients, Confirm+ | 71/74 (96) | 49/56 (88) |
| HIT+ patients, Confirm− | 3/74 (4) | 7/56 (12) |
| HIT− patients, Confirm+ | 27/41 (66) | 28/41 (68) |
| HIT− patients, Confirm− | 14/41 (34) | 13/41 (32) |
| Laboratory result . | Training set . | Validation set . |
|---|---|---|
| All patients, Confirm+ | 98/115 (85) | 77/97 (79) |
| All patients, Confirm− | 17/115 (15) | 20/97 (21) |
| HIT+ patients, Confirm+ | 71/74 (96) | 49/56 (88) |
| HIT+ patients, Confirm− | 3/74 (4) | 7/56 (12) |
| HIT− patients, Confirm+ | 27/41 (66) | 28/41 (68) |
| HIT− patients, Confirm− | 14/41 (34) | 13/41 (32) |
Values are n/N (%).
Patients were classified as HIT+ and HIT− on the basis of clinical grounds, as described in “Definition of clinical HIT status.”
Univariate analyses
Univariate analysis of the training set demonstrated that the HIT− patients had significantly lower maximal anti-heparin/PF4 OD values than the HIT+ patients (1.36 vs 0.75, P < .001; Table 3). The peak PF4 titer was distributed with considerable skewness to the right. Accordingly, it was log-transformed for the purposes of multivariate modeling. Analysis for collinearity resulted in a variable inflation factor of approximately 1, which indicates an absence of correlation between anti-heparin/PF4 OD values and the high-dose heparin confirmatory test. In the training set, statistically significant univariate associations were noted with an increasing anti-heparin/PF4 OD, presence of a positive excess-heparin confirmatory test, and being a surgical patient (Table 3). No significant relationship was detected between age, race, or sex and HIT status.
Training population, univariate analysis
| Variable . | HIT+ (n = 74) . | HIT− (n = 41) . | P . |
|---|---|---|---|
| Mean age, y | 63 | 61 | .430 |
| White race | 48 (64) | 26 (63) | .400 |
| Male sex | 32 (43) | 20 (49) | .570 |
| Surgical service | 42 (56) | 15 (37) | .038 |
| Maximal heparin/PF4 OD median, (25th, 75th percentiles) | 1.36 (0.61, 1.99) | 0.75 (0.47, 0.93) | < .001 |
| Confirm+ | 71 (96) | 27 (66) | < .001 |
| Variable . | HIT+ (n = 74) . | HIT− (n = 41) . | P . |
|---|---|---|---|
| Mean age, y | 63 | 61 | .430 |
| White race | 48 (64) | 26 (63) | .400 |
| Male sex | 32 (43) | 20 (49) | .570 |
| Surgical service | 42 (56) | 15 (37) | .038 |
| Maximal heparin/PF4 OD median, (25th, 75th percentiles) | 1.36 (0.61, 1.99) | 0.75 (0.47, 0.93) | < .001 |
| Confirm+ | 71 (96) | 27 (66) | < .001 |
Values are n (%) unless otherwise indicated.
Logistic regression analyses
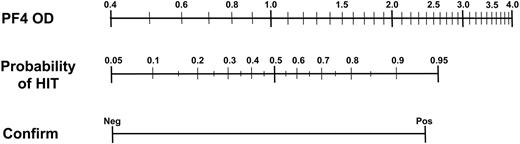
A series of univariate logistic regression models were fitted to the 2005 data with clinical HIT status as the dependent variable (Table 4). The log heparin/PF4 OD and the confirmation assay contributed similar quantities of information in univariate models and more information than clinical service category (medical vs surgical). In a stepwise multivariate logistic model, being a surgical patient did not meet the criterion (P < .05) for entry into the model. In contrast, both the log anti-heparin/PF4 titer and the confirmation assay independently contributed significant information to the multivariate model, with a c-index for the overall model of 0.783 (P < .001; Table 4). This prediction algorithm is graphically summarized as a nomogram in Figure 1.
Univariate and multivariate logistic regression models
| Variable . | Degrees of freedom . | Likelihood ratio, χ2 . | c-index . | P . |
|---|---|---|---|---|
| Log anti-heparin/PF4 | 1 | 15.16 | 0.70 | < .001 |
| Confirm+ | 1 | 18.60 | 0.65 | < .001 |
| Surgical service | 1 | 4.33 | 0.60 | .037 |
| Log anti-heparin/PF4+ and Confirm+ | 2 | 34.42 | 0.78 | < .001 |
| Variable . | Degrees of freedom . | Likelihood ratio, χ2 . | c-index . | P . |
|---|---|---|---|---|
| Log anti-heparin/PF4 | 1 | 15.16 | 0.70 | < .001 |
| Confirm+ | 1 | 18.60 | 0.65 | < .001 |
| Surgical service | 1 | 4.33 | 0.60 | .037 |
| Log anti-heparin/PF4+ and Confirm+ | 2 | 34.42 | 0.78 | < .001 |
c-index indicates the concordance probability, as described in “Methods.”
Nomogram of logistic regression model for predicting HIT. Anti-heparin/PF4 OD and confirmatory assay (Neg indicates negative; and Pos, positive) values are displayed on the outer scales. Connecting a pair of PF4 OD and confirmatory assay results using a straight edge allows one to estimate the probability of HIT from the center scale.
Nomogram of logistic regression model for predicting HIT. Anti-heparin/PF4 OD and confirmatory assay (Neg indicates negative; and Pos, positive) values are displayed on the outer scales. Connecting a pair of PF4 OD and confirmatory assay results using a straight edge allows one to estimate the probability of HIT from the center scale.
Model assessment and validation
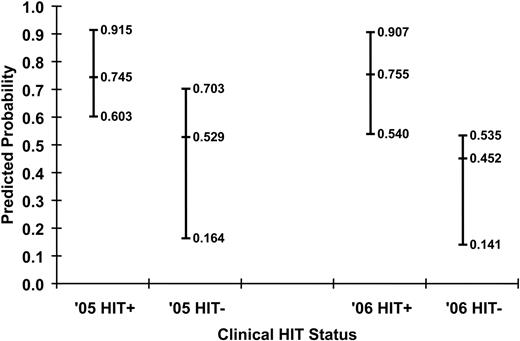
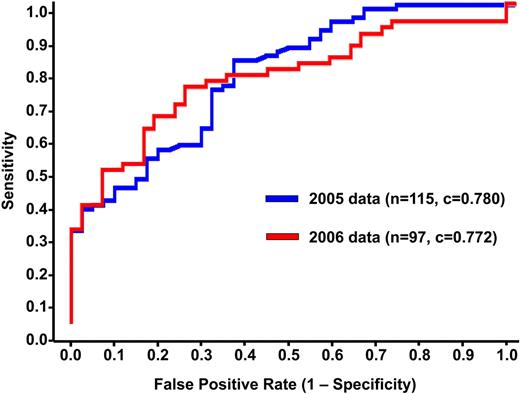
Predicted probabilities of HIT for the training and validation sets are shown in Figure 2. The ability of the model to discriminate between HIT+ and HIT− patients can be appreciated qualitatively by the degree of overlap in predictions for each group. Quantitatively, the discrimination of the algorithm fitted to the training set (c-index 0.783, Somers' Dyx 0.566, Brier 0.170) was maintained when the algorithm was applied to the independent validation set (c-index 0.799, Somers' Dyx 0.597, Brier 0.198). Receiver operator characteristic curves for both populations are shown in Figure 3.
Predicted probabilities of HIT for the training (n = 115) and validation (n = 97) datasets. Median and 25th and 75th percentiles of the predicted probabilities for the HIT+ and HIT− patients using the log–anti-heparin/PF4 OD results and confirmatory status in the training (2005) and validation (2006) populations are shown.
Predicted probabilities of HIT for the training (n = 115) and validation (n = 97) datasets. Median and 25th and 75th percentiles of the predicted probabilities for the HIT+ and HIT− patients using the log–anti-heparin/PF4 OD results and confirmatory status in the training (2005) and validation (2006) populations are shown.
Receiver operating characteristic curves. The discrimination of the algorithm fitted to the 2005 training set (n = 115; blue) was maintained when the algorithm was applied to the independent test population (n = 97; red).
Receiver operating characteristic curves. The discrimination of the algorithm fitted to the 2005 training set (n = 115; blue) was maintained when the algorithm was applied to the independent test population (n = 97; red).
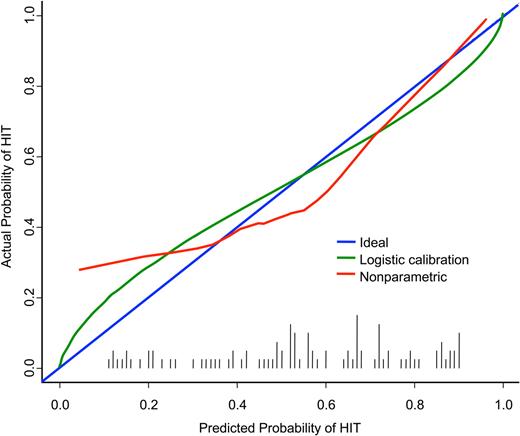
Application of logistic calibration methods17 yielded quality indices (U = 0.008, D = 0.218, Q = 0.210) consistent with the c-index and Brier score measures (Figure 4). The calibrated risk distribution (histogram of logistic-calibrated probabilities) is shown on the x-axis (black vertical bars). The ideal relationship (for a perfect predictor) between the predicted probabilities and the actual probabilities falls on the diagonal (blue line in Figure 4). With use of a smoothed nonparametric calibration curve, the reliability of the statistical model for predicting HIT is close to the ideal relationship (red line in Figure 4). This observation reflects the fact that the prevalence of HIT in the validation set is close to the prevalence in the training set and suggests that the overall need for recalibration is modest. However, the reliability of the model can be improved further by use of a logistic transformation, as shown by the fitted logistic calibration curve (green line in Figure 4). The fact that both curves are near the diagonal indicates that the model reliably predicts clinical HIT status on the basis of the criteria defined in the present study.
Reliability of logistic model in validation set. The calibrated risk distribution (histogram of logistic-calibrated probabilities) is shown (black vertical bars.) The ideal relationship (for a perfect predictor) between the predicted probabilities and the actual probabilities falls on the diagonal (blue). The reliability of the statistical model for predicting HIT is illustrated by a smoothed nonparametric calibration curve (red) and a fitted logistic calibration curve (green).
Reliability of logistic model in validation set. The calibrated risk distribution (histogram of logistic-calibrated probabilities) is shown (black vertical bars.) The ideal relationship (for a perfect predictor) between the predicted probabilities and the actual probabilities falls on the diagonal (blue). The reliability of the statistical model for predicting HIT is illustrated by a smoothed nonparametric calibration curve (red) and a fitted logistic calibration curve (green).
Discussion
HIT has been a recognized complication of heparin treatment for several decades, yet it remains difficult to diagnose because of other clinical contributors to thrombocytopenia. The diagnosis of HIT is a clinicopathologic one,1 and several clinical findings essential to its diagnosis (such as the nadir platelet count) may take days to evolve. Patients with suspected HIT are often at increased risk for bleeding if treated with alternative anticoagulation or at an increased risk for thrombosis if HIT is not recognized or is misdiagnosed. Although specific, the SRA is time-consuming and often requires referral to a reference laboratory. The anti-heparin/PF4 and confirmatory assays are easier to perform and readily available at many hospitals.
The OD value of anti-heparin/PF4 antibodies detected by ELISA correlates with both a clinical diagnosis of HIT and a higher incidence of heparin-induced thrombocytopenia and thrombosis (HITT).8,21,22 A recent study examined whether the absolute OD value could predict the presence of HIT-activating antibodies, defined by a strong positive SRA result. OD values of more than 2.00 resulted in an approximately 90% probability of a strong SRA result, whereas weaker OD values (0.4 to < 1.00) had a less than 5% probability of being associated with a strong positive SRA result.23 Similarly, in univariate analysis, we found that higher absolute OD values correlated with a clinical diagnosis of HIT, and in multivariate logistic regression analyses, they were predictive of clinical HIT. Nevertheless, not all patients who meet clinical criteria for HIT have markedly elevated anti-heparin/PF4 OD values,8,22 and likewise, some high anti-heparin/PF4 OD values can be detected by ELISA in the absence of clinical manifestations of HIT. Consequently, identification of additional laboratory parameters potentially would be useful for the diagnosis of HIT.
Heparin-dependent binding is a defining characteristic of HIT antibodies in both serologic and functional assays. We first reported on the clinical utility of the heparin confirmation test in a retrospective study in which we demonstrated that patients with Confirm+ test results were more likely to meet clinical criteria for HIT than patients who had Confirm− results (72% vs 18%, respectively; P < .001).8 In the present study, we have extended this initial report by demonstrating that in patients who are clinically suspected of having HIT, a confirmatory step with excess heparin added significant diagnostic information to the maximal OD value of the anti-heparin/PF4 ELISA. Patients who were classified by clinical criteria as HIT+ had a high likelihood of having a Confirm+ heparin/PF4 ELISA test result (96% and 88% in the 2 cohorts), whereas those who were classified as HIT− were much less likely to be Confirm+ (66% and 68%, respectively). Stated another way, 32% and 34% of patients in the 2 cohorts who were classified clinically as HIT− demonstrated antibody binding in the presence of excess heparin and therefore were considered as Confirm−.
Furthermore, a predictive statistical model based on both the maximal OD value and the confirmatory result discriminated well in our independent validation sample. Although neither test alone is a perfect predictor for HIT, these tests complement each other in the diagnosis of HIT, as demonstrated by our model. For example, of the 41 patients in the training cohort who were clinically HIT−, 27 of these patients were Confirm+. In univariate analysis, however, it was noted that patients who were HIT+ had a median anti-heparin/PF4 OD value of 1.36 versus 0.75 for those patients who were HIT−. Although a correlation was seen with clinical diagnosis and OD, there was no correlation between OD and results of confirmatory testing. Therefore, in these cases of clinically HIT− patients with Confirm+ results, the absolute anti-heparin/PF4 OD value must be taken into consideration, and the model presented here allows for this.
The results of the present study have the potential to aid in the diagnosis of HIT, yet there are limitations to the data presented. We did not use platelet activation assays to confirm or exclude the diagnosis of HIT in the present study. A sensitive functional assay such as the SRA would have buttressed the laboratory findings in the present study; however, the SRA is not available at our institution and therefore is not used routinely in the laboratory assessment of HIT. The diagnostic approach presented in the present study is nonetheless particularly relevant for clinical practice, because most major medical centers do not offer the SRA or comparable sensitive functional assays.24 Second, the retrospective nature of the study allows only for the inclusion and study of patients who tested positive for the presence of anti-heparin/PF4 antibodies. The knowledge that a clinical suspicion existed for HIT also had the potential to bias the assessment; however, this was mitigated to a large extent by the fact that only patients who are considered to be at risk for the development of HIT (exposure to heparin with thrombocytopenia) undergo anti-heparin/PF4 testing. Third, we did combine patients who met all criteria for HIT with patients who were thrombocytopenic in the correct timeframe in relation to heparin exposure but who also had potential alternative explanations for their thrombocytopenia. Although a subset of these patients may have been assessed subsequently as not having HIT, the purpose of the present study was to assess the value of the high-dose heparin confirmatory test in a “real-world” situation, and many patients with HIT may have more than 1 factor contributing to the overall thrombocytopenia. Lastly, we recognize that clinical requests for anti-heparin/PF4 ELISA assays may have introduced a selection bias on the prevalence of HIT, thus affecting our estimates of model reliability. The maximum Brier score for a predictive model decreases as the prevalence of the predicted event decreases. Consequently, a disadvantage of the Brier score is that its interpretation depends on the frequency of the outcome (ie, clinical HIT+ status). Application of the current predictive model to other patient populations (for example, obstetric patients or pediatric patients) may require additional calibration and validation.
In summary, the confirmatory assay with excess heparin is a valuable adjunct in the laboratory diagnosis of HIT. With a multivariate statistical model, the probability of being clinically HIT+ can be estimated for an individual patient with both the maximal anti-heparin/PF4 OD value and the confirmatory assay result. Accurate predictions of the probabilities of HIT will enable clinicians to initiate appropriate therapy rapidly, thereby reducing complications of HIT.
An Inside Blood analysis of this article appears at the front of this issue.
Presented in abstract form at the 49th annual meeting of the American Society of Hematology, Atlanta, GA, December 10, 2007.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Centers for Disease Control and Prevention (U01-DD000014; T.L.O), a Grant-in-Aid from the American Heart Association (G.M.A.), and the following grants from the National Institutes of Health: T32-HL007057 (N.L.W.), 5K12-HL-087097-04 (A.D.M.), UO1-HL072289 and U54-HL077878 (T.L.O), and RO1-HL081395 (G.M.A.).
National Institutes of Health
Authorship
Contribution: N.L.W. and T.L.O designed and performed the research; D.F.K. analyzed the data and created the figures; N.L.W., T.L.O., G.M.A., and A.D.M. reviewed the data and analyses; and N.L.W., T.L.O., D.F.K., G.M.A, and A.D.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas L. Ortel, Division of Hematology, Department of Medicine, Duke University Medical Center, Box 3422, Rm 0563 Stead Bldg, Durham, NC 27710; e-mail: thomas.ortel@duke.edu.