Abstract
Platelets undergo several modifications during storage that reduce their posttransfusion survival and functionality. One important feature of these changes, which are known as platelet storage lesion, is the shedding of the surface glycoproteins GPIb-α and GPV. We recently demonstrated that tumor necrosis factor-α converting enzyme (TACE/ADAM17) mediates mitochondrial injury-induced shedding of adhesion receptors and that TACE activity correlates with reduced posttransfusion survival of these cells. We now confirm that TACE mediates receptor shedding and clearance of platelets stored for 16 hours at 37°C or 22°C. We further demonstrate that both storage and mitochondrial injury lead to the phosphorylation of p38 mitogen-activated kinase (MAPK) in platelets and that TACE-mediated receptor shedding from mouse and human platelets requires p38 MAP kinase signaling. Protein kinase C, extracellular regulated-signal kinase MAPK, and caspases were not involved in TACE activation. Both inhibition of p38 MAPK and inactivation of TACE during platelet storage led to a markedly improved posttransfusion recovery and hemostatic function of platelets in mice. p38 MAPK inhibitors had only minor effects on the aggregation of fresh platelets under static or flow conditions in vitro. In summary, our data suggest that inhibition of p38 MAPK or TACE during storage may significantly improve the quality of stored platelets.
Introduction
Patients with a low platelet count or hyporeactive platelets are at increased risk of spontaneous bleeding or hemorrhage after injury or surgery. To maintain a normal hemostatic state, they may require a transfusion of platelets. After collection and processing, human platelets are stored in plasma for only 5 to 7 days at 22°C, mainly because a longer storage period would dangerously increase the risk of bacterial contamination. However, improved methods of pathogen inactivation could make it possible to extend platelet shelf life. During storage, platelets unfortunately undergo numerous modifications that alter their functional integrity and structure. These changes are summarized as platelet storage lesion (PSL) and are strongly associated with a decrease in platelet posttransfusion survival and function.1 The main characteristics of PSL are: (1) shape change, (2) reduced activation in response to agonists, such as adenosine diphosphate (ADP), thromboxane A2 (TxA2), or epinephrine, (3) secretion of platelet granules, and (4) exposure of phosphatidylserine on the outer leaflet of the plasma membrane accompanied by blebbing of microparticles.2 Furthermore, the surface expression of the glycoproteins GPIb-α and GPV, subunits of the von Willebrand factor (VWF) receptor complex, is altered during long-term storage,3,4 mainly by metalloproteinase-mediated proteolysis of their ectodomain. The major sheddase for GPIb-α and GPV is tumor necrosis factor-α converting enzyme (TACE; ADAM17),5,6 which is a type I metalloproteinase involved in the shedding of several transmembrane proteins (cytokines, growth factors, receptors, or adhesion molecules) and implicated in developmental and inflammatory processes.7 As a result of TACE activation on platelets, 130-kDa (glycocalicin) and 80-kDa soluble fragments of GPIb-α and GPV, respectively, are released. GPIb-α shedding was proposed as a platelet clearance mechanism in a study of human platelets transfused in rabbits where the surface levels of GPIb-α correlated with the platelets' overall clearance.8 Our own studies demonstrated that TACE mediates cleavage of GPIb-α from injured mouse platelets and that TACE activity leads to a reduced posttransfusion recovery of these cells.5,9
The p38 mitogen-activated kinase (MAPK) belongs to a family of serine-threonine kinases, which are activated by dual phosphorylation of threonine and tyrosine residues separated by a single amino acid. Human platelets possess 4 isoforms of p38 MAPK (α, β, γ, and δ), but the most abundant forms are p38 MAPK-α and -β. p38 MAPK-α (named p38 MAPK) was shown to be activated in response to several agonists, including thrombin,10,11 TxA2,12 collagen,13 ADP,14 and VWF,15 but its role in platelet function remains controversial. Importantly, inhibition of p38 MAPK showed only minor effects on platelet aggregation induced by threshold concentrations of agonists,12,16 and this effect, at least in part, may be the result of cross-reactivity of p38 inhibitors with cyclo-oxygenases and thus impairment of TxA2 generation.17 Recently, p38 MAPK inhibition has been proposed and investigated as a new strategy to treat inflammatory disorders, such as atherosclerosis,18 rheumatoid arthritis, and septic shock.19 All of these pathologies involve the production and/or the release of TNF-α, the prototypical substrate of TACE.
In the present study, we confirm that TACE mediates the shedding of GPIb-α and GPV from stored platelets, and we demonstrate that TACE is activated via a p38 MAPK-dependent pathway. We also propose that p38 MAPK inhibition during storage improves the posttransfusion recovery without affecting the hemostatic function of platelets in vitro and in vivo.
Methods
Animals
C57BL/6J wild-type mice were purchased from The Jackson Laboratory. TACE+/ΔZn mutant mice (C57BL/6J/129Sv background)20 were kindly provided by Jaques Peschon (Amgen, Seattle, WA). TACEΔZn/ΔZn and TACE+/+ chimeric mice were generated by injecting fetal liver cells from TACEΔZn/ΔZn and TACE+/+ sibling embryos at day 16.5 of development into irradiated C57BL/6J recipient mice (12.5 Gy; 107 cells per mouse). IL4Rα/GPIb-α21 transgenic mice were kindly provided by Jerry Ware (University of Arkansas for Medical Sciences, Little Rock, AR). All mice were bred and housed in our facilities. The Animal Care and Use Committee of the Immune Disease Institute approved experimental procedures.
Reagents and antibodies
The following were purchased: enoxaparin (Aventis Pharmaceuticals Products); heparin-coated microcapillaries (VWR Scientific); collagen reagent Horm (Nycomed); prostaglandin I2, caspase-1 and -3 inhibitor N-benzyloxycarbonyl-Val-Ala-Asp(O-Me) fluoromethyl ketone (Z-VAD), mouse thrombin, FeCl3, carbonyl cyanide m-chlorophenylhydrazone (CCCP), and sodium pyruvate (all from Sigma-Aldrich); p38 MAPK inhibitors SB203580 and SB202190, protein kinase C (PKC) inhibitor Ro31-8220, the extracellular regulated-signal kinase (ERK) inhibitor PD98059 (all from EMD Chemicals); ADP (Bio/Data Corporation); murine PAR4 receptor-activating peptide H GYPGKF-NH2 (PAR4-AP; Advanced ChemTech); annexin V-phycoerythrin and carboxyfluorescein succinimidyl ester (CFSE; Invitrogen); monoclonal antibody directed against human GPIb-α, mouse P-selectin, and tetraspanin (CD9; all from BD Biosciences); and monoclonal antibodies against murine GPIb-α, GPV, GPVI, and GPIX (emfret Analytics).
Platelet preparation
Mouse platelets.
Mice were anesthetized with isoflurane (Abbott Laboratories). Blood was obtained by retro-orbital bleeding and was collected into tubes containing 100 μg/mL enoxaparin in phosphate-buffered saline. Enoxaparin was used as an anticoagulant for mouse blood instead of calcium-chelating agents, such as acid citrate dextrose or citrate because mouse integrins require extracellular Ca2+ concentrations of more than 100μM for their activation.22 Platelet-rich plasma (PRP) was prepared by centrifugation at 100g for 7 minutes. PRP and buffy coat were transferred into new tubes and spun a second time at 100g for 5 minutes. PRP was incubated with prostaglandin I2 (0.1 μg/mL) at 37°C for 5 minutes. When indicated, platelets were pelleted at 1000g for 3.5 minutes and washed in modified Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer (137mM NaCl, 0.3mM Na2HPO4, 2mM KCL, 12mM NaHCO3, 5mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 5mM glucose, pH 7.4).
Human platelets.
Human blood (9 volumes) from healthy volunteers was collected in acid citrate dextrose (1 volume). All blood donors gave written informed consent, and the research was approved by the Immune Disease Institute's Institutional Review Board in accordance with the Declaration of Helsinki. Whole blood was centrifuged at 100g for 10 minutes, plasma and buffy coat were transferred to new tubes, and PRP was obtained by centrifugation at 100g for 5 minutes.
In vitro platelet storage
PRP was treated as indicated in the respective figures and then stored at 37°C (or 22°C when indicated) under agitation for 16 hours for mouse PRP and 72 hours for human PRP in Eppendorf tubes.
Flow cytometry
PRP or washed platelets were stained for 10 minutes with a saturating amount of fluorophore-conjugated antibodies. After incubation, platelets were immediately analyzed on a FACSCalibur flow cytometer (BD Biosciences). Platelets were gated by their forward scatter/side scatter FSC/SSC characteristics.
Immunoblot analysis
PRP or washed platelets were centrifuged at 1000g for 5 minutes. Cells were lysed with radioimmunoprecipitation Assay buffer containing protease inhibitor cocktail Complete EDTA-free (Roche Diagnostics). Total protein amount from the cell lysate and plasma was assayed according to the specification of the BCA Protein Assay kit (Pierce Protein Research Products; Thermo Fisher Scientific). A total of 30 μg of proteins from platelet lysates was used to immunoprecipitate the phosphorylated form of p38 MAPK using a specific antibody immobilized on agarose hydrazide beads (Cell Signaling Technology). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. p38 MAPK was detected with a monoclonal antibody against mouse total p38 MAPK (Cell Signaling Technology) and a goat anti–rabbit-horseradish peroxidase coupled secondary antibody (Bio-Rad). Proteins were visualized by enhanced chemiluminescence.
p38 MAPK activity
A p38 MAPK activity kit was obtained from Cell Signaling Technology. Assays of p38 MAPK activity were performed exactly as directed by the manufacturer using whole platelet lysates.
Aggregometry
PRP was diluted with modified Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer containing 1mM CaCl2 in a prewarmed (37°C) aggregometer cuvette to achieve a platelet concentration of 2 × 108/mL and stirred. Agonists were added, and light transmission was recorded for 20 minutes on a 4-channel optical aggregation system (Chrono-log).
Flow chamber
Blood preparation.
Mouse blood was drawn from the retro-orbital plexus into heparinized tubes (30 U/mL low molecular weight heparin, enoxaparin). Platelets were labeled for 5 minutes with an Alexa488-labeled antibody against murine GPIX (emfret Analytics). Human blood (9 volumes) was drawn into 3.8% sodium citrate, and platelets were labeled with 1 μg/mL calcein for 10 minutes.
Perfusion.
Whole blood, in the presence of 10μM SB203580 or vehicle (dimethyl sulfoxide [DMSO]), was perfused in a parallel-plate flow chamber over glass slides coated with bovine type I collagen (200 μg/mL; Chrono-log) at a shear rate of 1200 seconds. Platelet adhesion was visualized with a Nikon Ti-U inverted microscope (Nikon Instruments) equipped with a Retiga EXL monochrome camera (QImaging). Images were analyzed using Nikon NIS Elements software. The kinetics of thrombus formation was evaluated by plotting the integrated fluorescence intensity of all pixels in the image, regardless of their intensity, as a function of time. The experiment was repeated 4 to 6 times for each condition.
Platelet transfusion and survival in mice
Platelets from mouse PRP were washed in modified Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer and labeled with 2 μg/mL CFSE at 37°C for 30 minutes under agitation. A total of 108 platelets/10 g of body weight was infused into the retro-orbital plexus of C57BL/6J recipient mice (4-8 weeks old). The percentage of labeled platelets was determined by flow cytometry in blood samples that were collected at different time points after transfusion. The time point t = 1 hour was chosen as a readout for the rapid posttransfusion clearance of platelets, whereas measurements at t = 24 hours, 48 hours, 72 hours, and 96 hours were used to determine the half-life of the transfused cells.
In vivo thrombosis model
Thrombus formation in vivo was studied as described previously.9 Briefly, platelets were labeled for 10 minutes with calcein-green (1 μg/mL) or calcein-red/orange (1 μg/mL) and infused into 3- to 5-week-old anesthetized male mice. The mesentery was exposed through a midline abdominal incision, and injury was induced by application of FeCl3. Vessels were monitored until cessation of blood flow lasted longer than 30 seconds. It is important to note that mice were injected with 5 × 107 SB203580-treated stored platelets or 1.5 × 108 vehicle-treated stored platelets per 10 g of body weight to achieve approximately 5% labeled platelets in circulation.
Platelet transfusion and tail bleeding time
Platelets were washed in modified Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and 108 platelets/10 g of body weight was infused into the retro-orbital plexus of IL4Rα/GPIb-α transgenic recipient mice. After 1 hour, the tail bleeding time was determined by removing 3 mm of the distal mouse tail and immediately immersing the tail in 37°C phosphate-buffered saline. A complete cessation of bleeding for more than 120 seconds was defined as the bleeding time. Bleeding time measurements exceeding 900 seconds were stopped by cauterization of the tail.
Statistics
Values are expressed as mean plus or minus SEM. The statistical significance was assayed using the 1-way analysis of variance followed by the Bonferroni multiple comparisons test or the 2-tailed unpaired Student t test. A P value less than .05 was considered statistically significant.
Results
TACE activity mediates GPIb-α shedding and affects the posttransfusion recovery of in vitro stored platelets
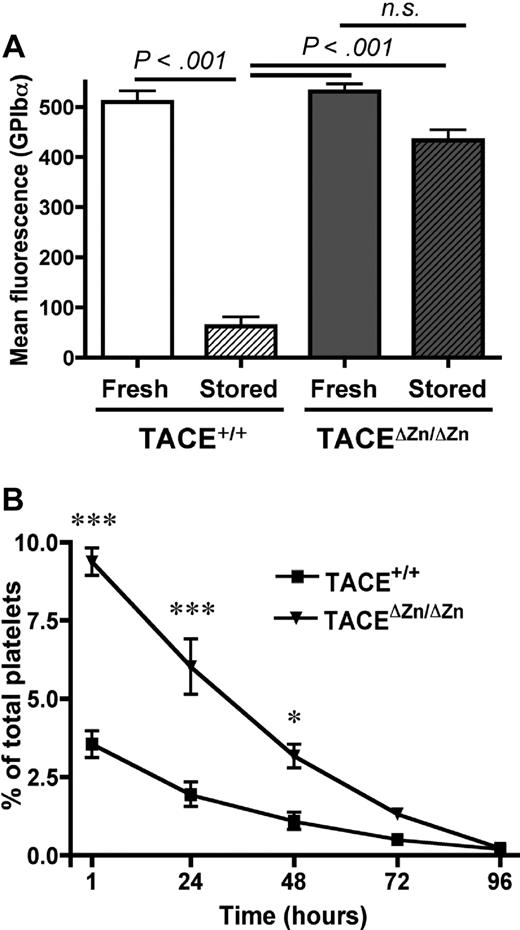
One of the main characteristics of PSL is the down-regulation of platelet surface receptors. TACE has been shown to be the major enzyme responsible for shedding of GPIb-α and GPV from activated platelets,5,6 but its role in the proteolysis of these glycoproteins in the setting of PSL has not been demonstrated. To address whether TACE is activated during platelet storage, we studied storage-induced changes in the expression levels of GPIb-α on the surface of wild-type mouse platelets and platelets expressing inactive TACE (TACEΔZn/ΔZn). Our studies were carried out at 37°C to accelerate the formation of PSL.1 We found that GPIb-α was markedly down-regulated from the surface of stored TACE+/+ platelets. In contrast, shedding was not significant in TACEΔZn/ΔZn platelets (Figure 1A). Similar results were obtained when the expression of GPV was measured (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article), suggesting that storage-induced TACE activation results in the cleavage of both GPIb-α and GPV. To test whether TACE activation during storage affects the immediate posttransfusion recovery and/or the life span of transfused platelets in circulation, we studied the survival of stored TACEΔZn/ΔZn platelets and TACE+/+ platelets transfused into recipient mice. Compared with stored TACE+/+ platelets, stored TACEΔZn/ΔZn platelets showed a significantly increased posttransfusion survival as we observed approximately 60% more circulating TACEΔZn/ΔZn platelets at t = 1 hour after infusion. In contrast, impaired TACE function did not affect the life span of these cells in circulation (Figure 1B).
TACEΔZn/ΔZn platelets are protected from storage-induced shedding of GPIb-α and increased posttransfusion clearance. (A) Surface expression of GPIb-α was determined by flow cytometry on freshly isolated or 15-hour stored platelet-rich plasma (PRP) from TACE+/+ and TACEΔZn/ΔZn mice. Results are mean ± SEM; n = 6. n.s. indicates not significant. (B) Platelets from stored PRP from TACE+/+ and TACEΔZn/ΔZn mice were washed in Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer and labeled with CFSE. A total of 108 platelets/10 g of body weight was infused intravenously into TACE+/+ mice. Blood was drawn at the indicated time points, and platelets were immediately analyzed by flow cytometry. Results are mean percentage CFSE-labeled platelets ± SEM; n = 4. ***P < .001. *P < .05.
TACEΔZn/ΔZn platelets are protected from storage-induced shedding of GPIb-α and increased posttransfusion clearance. (A) Surface expression of GPIb-α was determined by flow cytometry on freshly isolated or 15-hour stored platelet-rich plasma (PRP) from TACE+/+ and TACEΔZn/ΔZn mice. Results are mean ± SEM; n = 6. n.s. indicates not significant. (B) Platelets from stored PRP from TACE+/+ and TACEΔZn/ΔZn mice were washed in Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer and labeled with CFSE. A total of 108 platelets/10 g of body weight was infused intravenously into TACE+/+ mice. Blood was drawn at the indicated time points, and platelets were immediately analyzed by flow cytometry. Results are mean percentage CFSE-labeled platelets ± SEM; n = 4. ***P < .001. *P < .05.
p38 MAPK controls the shedding of GPIb-α and GPV during storage
Several signaling pathways, including PKC, caspases, and various members of the MAP kinase family, have been implicated in the TACE-mediated shedding of membrane proteins.23-25 We used pharmacologic inhibitors to assess the role of these pathways in the release of GPIb-α during platelet storage. Storage-induced shedding of GPIb-α was not affected by the broad range PKC inhibitor, Ro 31-8220 (2 μg/mL) or the ERK MAPK inhibitor, PD98059 (20μM; Table 1). Furthermore, pretreatment of platelets with Z-VAD (20μM), a pan-caspase inhibitor, did not affect storage-induced shedding of GPIb-α, indicating that apoptosis is not implicated in this process.
Effect of pharmacologic inhibitors on GPIb-α shedding during platelet storage
| Mouse platelets . | GPIb-α mean fluorescence (± SEM) . | P from stored PRP . |
|---|---|---|
| Fresh PRPr | 421.9 (± 12.6) | < 0.001 |
| Stored PRP (Vehicle) | 34.1 (± 5.5) | |
| Stored PRP/PKC inhibitor (Ro 31-8220 2 μg/mL) | 28.5 (± 10.9) | n.s. |
| Stored PRP/ERK inhibitor (PD98059 20μM) | 28.2 (± 8.9) | n.s. |
| Stored PRP/Caspases inhibitor (Z-VAD 20μM) | 38.7 (± 9.1) | n.s. |
| Mouse platelets . | GPIb-α mean fluorescence (± SEM) . | P from stored PRP . |
|---|---|---|
| Fresh PRPr | 421.9 (± 12.6) | < 0.001 |
| Stored PRP (Vehicle) | 34.1 (± 5.5) | |
| Stored PRP/PKC inhibitor (Ro 31-8220 2 μg/mL) | 28.5 (± 10.9) | n.s. |
| Stored PRP/ERK inhibitor (PD98059 20μM) | 28.2 (± 8.9) | n.s. |
| Stored PRP/Caspases inhibitor (Z-VAD 20μM) | 38.7 (± 9.1) | n.s. |
Surface expression of GPIbα was measured by flow cytometry on fresh platelets or platelets stored in presence of inhibitors of PKC (Ro 31-8220), ERK mitogen-activated protein kinase (PD98059), caspases (Z-VAD), or dimethyl sulfoxide (Vehicle). Results are given as mean ± SEM, n = 8.
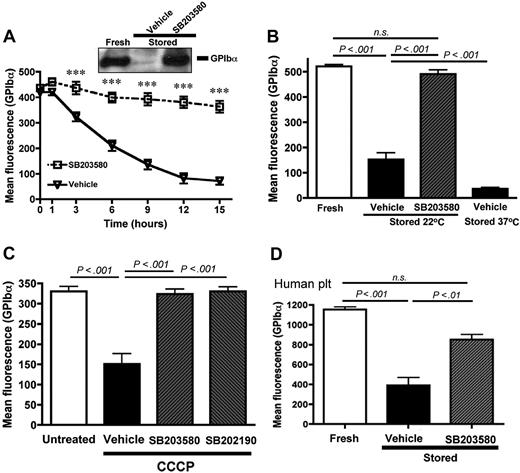
In contrast, storage-induced shedding of GPIb-α (Figure 2A) and GPV (supplemental Figure 1B) was prevented by the p38 MAPK inhibitor SB203580 (IC50 ∼ 20μM). Similar results were observed with another p38 MAPK inhibitor, SB202190 (not shown). Because p38 MAPK inhibitors are reported to block COX-1 activity,17 we stored platelets in the presence of the COX inhibitor acetylsalicylic acid (aspirin, 1mM). Aspirin did not significantly inhibit the storage-induced shedding of GPIb-α (not shown), indicating that the effect of SB203580 and SB202190 resulted from a specific p38 MAPK inhibition. In blood banking conditions, platelets are stored at 22°C to reduce the risk of bacterial contamination and also because platelets seem to age more slowly at 22°C compared with 37°C.1 Figure 2B shows that, after 15 hours at 22°C, GPIb-α was shed from the mouse platelet surface, but to a lesser extent than if stored at 37°C. The presence of SB203580 during storage at 22°C completely inhibited the shedding of GPIb-α. Similar results were obtained when the expression of GPV was measured (supplemental Figure 1C).
p38 MAPK inhibition prevents the shedding of GPIb-α from stored mouse and human platelets. GPIb-α surface expression was assessed by flow cytometry. (A) Wild-type mouse PRP was stored 1, 3, 6, 9, 12, and 15 hours at 37°C in the presence of DMSO (vehicle) or the p38 MAPK inhibitor SB203580 (40μM); n = 8. ***P < .001. (Inset) Western blot for GPIb-α in lysates from freshly isolated platelets and platelets stored for 16 hours in the presence or absence of SB203580. The results are representative of 3 independent experiments. (B) PRP was analyzed immediately (fresh) or after storage at 22°C or 37°C in the presence of DMSO (vehicle) or SB203580 (40μM); n = 10. (C) Washed platelets were treated for 60 minutes at 37°C with DMSO (untreated) or 100μM CCCP in the presence of DMSO (vehicle), SB203580 (40μM), or SB202190 (40μM); n = 9. (D) PRP isolated from human blood was analyzed immediately (fresh) or after 72 hours of storage at 37°C in the presence of DMSO (vehicle) or SB203580 (40μM); n = 8. Results are mean ± SEM. n.s. indicates not significant.
p38 MAPK inhibition prevents the shedding of GPIb-α from stored mouse and human platelets. GPIb-α surface expression was assessed by flow cytometry. (A) Wild-type mouse PRP was stored 1, 3, 6, 9, 12, and 15 hours at 37°C in the presence of DMSO (vehicle) or the p38 MAPK inhibitor SB203580 (40μM); n = 8. ***P < .001. (Inset) Western blot for GPIb-α in lysates from freshly isolated platelets and platelets stored for 16 hours in the presence or absence of SB203580. The results are representative of 3 independent experiments. (B) PRP was analyzed immediately (fresh) or after storage at 22°C or 37°C in the presence of DMSO (vehicle) or SB203580 (40μM); n = 10. (C) Washed platelets were treated for 60 minutes at 37°C with DMSO (untreated) or 100μM CCCP in the presence of DMSO (vehicle), SB203580 (40μM), or SB202190 (40μM); n = 9. (D) PRP isolated from human blood was analyzed immediately (fresh) or after 72 hours of storage at 37°C in the presence of DMSO (vehicle) or SB203580 (40μM); n = 8. Results are mean ± SEM. n.s. indicates not significant.
Treating platelets with the cyanide derivative CCCP damages the mitochondria and induces morphologic and functional changes that resemble PSL.9 We investigated whether p38 MAPK plays a role in TACE activation and platelet damage observed on CCCP treatment. Washed platelets were incubated with CCCP (100μM) for 1 hour in the presence or absence of SB203580 or SB202190. Both p38 MAPK inhibitors prevented the CCCP-induced shedding of GPIb-α (Figure 2C). Furthermore, SB203580 prevented storage-induced changes in GPIb-α expression on human platelets stored at 37°C for 72 hours (Figure 2D). This suggests that p38 MAPK signaling is important for the development of PSL, both in murine and human platelets.
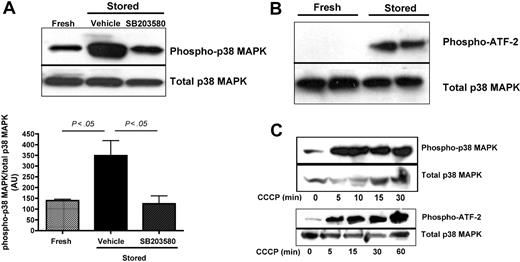
Western blot analyses confirmed that phosphorylation of p38 MAPK was significantly increased in stored mouse platelets. As expected, addition of SB203580 reduced the p38 MAPK phosphorylation back to baseline (Figure 3A). Corroborating these results, we could demonstrate phospho-p38 activity, measured by the phosphorylation of an ATF-2 fusion protein, in lysates of stored but not fresh platelets (Figure 3B). We also found that p38 MAPK was rapidly (within 5 minutes) activated in CCCP-treated platelets, and this activation increased over time (Figure 3C). Together, these results suggest that the p38 MAPK pathway is the main intracellular signaling pathway involved in TACE activation in stored or mitochondria-injured platelets.
Phosphorylation and activity of p38 MAPK are increased during in vitro storage of platelets. Mouse platelets were isolated from fresh PRP or PRP stored in the presence of SB203580 (40μM) or DMSO (vehicle), washed in Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer, and lysed immediately. (A) Immunoprecipitated phospho-p38 MAPK was detected by Western blot analysis using a specific antibody for p38 MAPK and quantified (mean ± SEM); n = 4. (B) The activity of phosphorylated p38 MAPK was assessed in lysates from 2 different preparations of freshly isolated or stored platelets by its ability to phosphorylate the substrate fusion protein ATF-2. (C) Phosphorylation of p38 and ATF-2 in platelets that were treated with 100μM CCCP for the indicated times. Total p38 MAPK was assessed in platelet lysates by Western blot analysis using a specific antibody for p38 MAPK.
Phosphorylation and activity of p38 MAPK are increased during in vitro storage of platelets. Mouse platelets were isolated from fresh PRP or PRP stored in the presence of SB203580 (40μM) or DMSO (vehicle), washed in Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer, and lysed immediately. (A) Immunoprecipitated phospho-p38 MAPK was detected by Western blot analysis using a specific antibody for p38 MAPK and quantified (mean ± SEM); n = 4. (B) The activity of phosphorylated p38 MAPK was assessed in lysates from 2 different preparations of freshly isolated or stored platelets by its ability to phosphorylate the substrate fusion protein ATF-2. (C) Phosphorylation of p38 and ATF-2 in platelets that were treated with 100μM CCCP for the indicated times. Total p38 MAPK was assessed in platelet lysates by Western blot analysis using a specific antibody for p38 MAPK.
Effect of p38 MAPK inhibition on platelet activation and adhesion
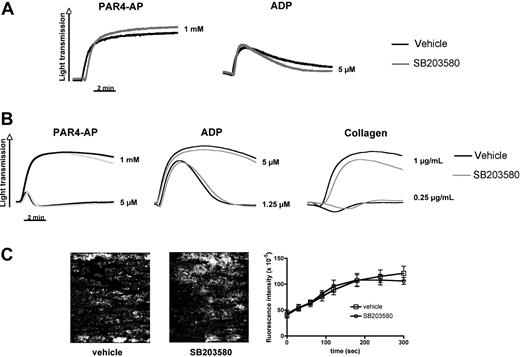
Consistent with previous reports,2,8 stored platelets showed signs of activation, including a small but significant increase in surface expression of the α-granule protein P-selectin, phosphatidylserine exposure, and microparticle release (Table 2). The increase in P-selectin expression was dependent on p38 MAPK signaling (Table 2) but occurred in both TACE+/+ and TACEΔZn/ΔZn platelets (not shown), suggesting that TACE is not important for this process. Inhibition of p38 MAPK signaling prevented phosphatidylserine exposure or microparticle formation only partially in the stored platelets (Table 2), demonstrating that various signaling pathways contribute to the development of PSL. Importantly, murine platelets stored in the presence or absence of SB203580 showed a robust aggregation response to stimulation by PAR4-AP or ADP (Figure 4A), suggesting that p38 MAPK inhibition does not affect the hemostatic function of these cells. To confirm this hypothesis, we also studied the effect of SB203580 on the adhesive function of freshly isolated murine platelets. The presence of SB203580 did not markedly reduce their aggregation in response to ADP, collagen, or PAR4-AP (Figure 4B). Platelet adhesion to a collagen matrix under arterial blood flow conditions was comparable in the presence or absence of SB203580 (Figure 4C). Similar results were observed with freshly isolated human platelets. We observed only a minor effect of p38 MAPK inhibition on fresh human platelet aggregation at threshold concentration of agonists. SB203580-treated human platelet adhesion and aggregation in collagen-coated flow chambers were indistinguishable from vehicle controls (supplemental Figure 2A-B).
Effect of storage with or without p38 MAPK inhibitor on platelet parameters
| . | Fresh PRP . | Stored PRP (vehicle) . | Stored PRP/SB203580 . |
|---|---|---|---|
| P-selectin, mean (± SEM) | 4.3 (± 0.1) | 12.9 (± 2.2)** | 6.3 (± 0.4) |
| GPIV, mean (± SEM) | 20.3 (± 0.5) | 17.6 (± 2.0) | 19.0 (± 0.8) |
| CD9, mean (± SEM) | 1133 (± 35.6) | 1034 (± 123.8) | 1024 (± 45.0) |
| Phosphatidylserine, mean (± SEM) | 9.3 (± 0.3) | 40.8 (± 7.9)*** | 28.2 (± 3.8)* |
| Microparticles, mean (± SEM) | 29.4 (± 2.9) | 201.0 (± 16.5)*** | 169.6 (± 12.0)*** |
| . | Fresh PRP . | Stored PRP (vehicle) . | Stored PRP/SB203580 . |
|---|---|---|---|
| P-selectin, mean (± SEM) | 4.3 (± 0.1) | 12.9 (± 2.2)** | 6.3 (± 0.4) |
| GPIV, mean (± SEM) | 20.3 (± 0.5) | 17.6 (± 2.0) | 19.0 (± 0.8) |
| CD9, mean (± SEM) | 1133 (± 35.6) | 1034 (± 123.8) | 1024 (± 45.0) |
| Phosphatidylserine, mean (± SEM) | 9.3 (± 0.3) | 40.8 (± 7.9)*** | 28.2 (± 3.8)* |
| Microparticles, mean (± SEM) | 29.4 (± 2.9) | 201.0 (± 16.5)*** | 169.6 (± 12.0)*** |
Surface expressions of P-selectin, GPVI, CD9, phosphatidylserine and microparticle counts were determined by flow cytometry. Microparticles are defined as particles smaller than platelets that are double positive for GPIX and GPIIb. Results are shown as mean ± SEM. Microparticles released per 1000 platelets, n = 6.
MAPK indicates p38 mitogen-activated kinase; and PRP, platelet-rich plasma.
Statistically different to Fresh PRP at p < 0.001
Statistically different to Fresh PRP at p < 0.01
Statistically different to Fresh PRP at p < 0.05
p38 MAPK inhibition does not affect integrin-mediated aggregation and adhesion to collagen under flow of freshly isolated and stored murine platelets. (A) PRP stored in the presence of DMSO (vehicle) or SB203580 (40μM) was examined in standard aggregometry after addition of 5μM ADP or 1mM PAR4-AP. (B) Freshly isolated murine PRP was activated with the indicated agonists in the presence or absence of SB203580. Results are representative of 3 separate experiments. (C) Platelets in freshly isolated murine blood were labeled by the addition of an Alexa488-labeled antibody to GPIX. Whole blood, in the presence or absence of SB203580, was perfused over collagen at a shear rate of 1200 seconds. Adhesion and thrombus formation of fluorescently labeled platelets were monitored over time. (Left) Representative images. (Right) Fluorescence intensity (platelet adhesion) measured over time. Results are mean ± SEM; n = 6 (3 different blood preparations).
p38 MAPK inhibition does not affect integrin-mediated aggregation and adhesion to collagen under flow of freshly isolated and stored murine platelets. (A) PRP stored in the presence of DMSO (vehicle) or SB203580 (40μM) was examined in standard aggregometry after addition of 5μM ADP or 1mM PAR4-AP. (B) Freshly isolated murine PRP was activated with the indicated agonists in the presence or absence of SB203580. Results are representative of 3 separate experiments. (C) Platelets in freshly isolated murine blood were labeled by the addition of an Alexa488-labeled antibody to GPIX. Whole blood, in the presence or absence of SB203580, was perfused over collagen at a shear rate of 1200 seconds. Adhesion and thrombus formation of fluorescently labeled platelets were monitored over time. (Left) Representative images. (Right) Fluorescence intensity (platelet adhesion) measured over time. Results are mean ± SEM; n = 6 (3 different blood preparations).
p38 MAPK inhibition and platelet function in vivo
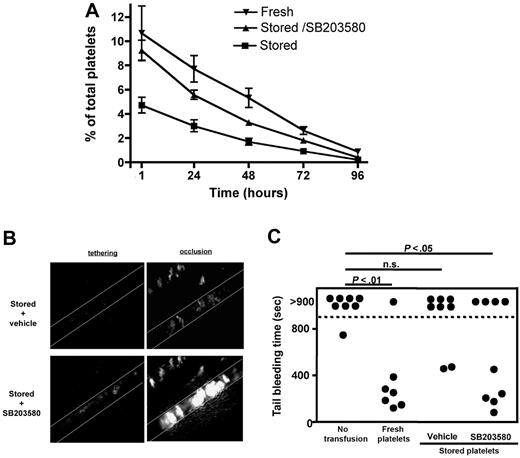
We next tested whether p38 MAPK inhibition during storage affects the posttransfusion survival and hemostatic function of platelets. Consistent with our studies on the survival of stored TACEΔZn/ΔZn platelets (Figure 1), we found that inhibition of p38 MAPK signaling during platelet storage resulted in a significantly increased posttransfusion recovery of these cells (Figure 5A). To study the impact of p38 MAPK inhibition on the hemostatic function of stored platelets in vivo, we performed intravital microscopy studies in a model of arterial thrombosis.26 To compare the adhesion of both platelet preparations in the same animal, mice were infused with 0.5 × 108 SB203580/stored platelets labeled with calcein-red/orange and 1.5 × 108 vehicle/stored platelets labeled with calcein-green. This resulted in comparable numbers of labeled circulating platelets. As shown in Figure 5B, platelets stored in the presence of SB203580 showed a markedly improved adhesion to the damaged vascular wall and a better incorporation into the growing thrombus. In addition, p38 MAPK inhibition during storage significantly improved the function of human platelets when we studied their adhesion to a collagen-coated surface under flow at arterial shear rate (supplemental Figure 2C).
Inhibition of p38 MAPK during storage improves the posttransfusion recovery and hemostatic function of platelets. (A) Platelets from fresh PRP or PRP stored in the presence of DMSO (stored) or SB203580 (40μM; stored/SB203580) were washed and labeled with 2 μg/mL CFSE. A total of 108 platelets/10 g of body weight was infused intravenously into a wild-type mouse. At the indicated time points, blood was drawn and analyzed by flow cytometry. Results are mean percentage of labeled platelets ± SEM; n = 6. (B) FeCl3-induced thrombosis was studied in mesenteric arterioles of mice transfused with 0.5 × 108 SB203580/stored platelets labeled with calcein-red/orange and 1.5 × 108 vehicle/stored platelets labeled with calcein-green. Platelet adhesion (tethering) and thrombus growth were monitored until complete vessel occlusion occurred (occlusion). Representative images are shown. (C) IL4Rα/GPIb-α-tg mice were transfused with the indicated platelet preparations (108 platelets/10 g of body weight). The tail bleeding time was measured 1 hour after platelet transfusion. n.s. indicates not significant.
Inhibition of p38 MAPK during storage improves the posttransfusion recovery and hemostatic function of platelets. (A) Platelets from fresh PRP or PRP stored in the presence of DMSO (stored) or SB203580 (40μM; stored/SB203580) were washed and labeled with 2 μg/mL CFSE. A total of 108 platelets/10 g of body weight was infused intravenously into a wild-type mouse. At the indicated time points, blood was drawn and analyzed by flow cytometry. Results are mean percentage of labeled platelets ± SEM; n = 6. (B) FeCl3-induced thrombosis was studied in mesenteric arterioles of mice transfused with 0.5 × 108 SB203580/stored platelets labeled with calcein-red/orange and 1.5 × 108 vehicle/stored platelets labeled with calcein-green. Platelet adhesion (tethering) and thrombus growth were monitored until complete vessel occlusion occurred (occlusion). Representative images are shown. (C) IL4Rα/GPIb-α-tg mice were transfused with the indicated platelet preparations (108 platelets/10 g of body weight). The tail bleeding time was measured 1 hour after platelet transfusion. n.s. indicates not significant.
We next tested the ability of transfused platelets to rescue bleeding in mice engineered to lack the extracellular, ligand binding domain of GPIb-α (IL4Rα/GPIb-α).21 These mice present a severe bleeding phenotype despite having normal platelet size and a platelet count of approximately 65% to 70% of wild-type mice.21 A total of 200 million wild-type platelets that were freshly isolated or stored in the presence or absence of SB203580 was transfused into IL4Rα/GPIb-α recipient mice, and their tail bleeding times were determined 1 hour later (Figure 5C). Without infusion of exogenous platelets, the bleeding time of IL4Rα/GPIb-α mice was 881 (± 18) seconds and exceeded 900 seconds in 7 of 8 mice. The infusion of fresh but not stored platelets significantly lowered the bleeding time in recipient mice. Importantly, transfusion with platelets stored in the presence of a p38 MAPK inhibitor significantly reduced the bleeding time of transfused IL4Rα/GPIb-α mice (532 ± 120 seconds).
Discussion
In previous works, we established a mouse model for platelet storage lesion,9 and we demonstrated that TACE activity is responsible for the reduced posttransfusion recovery and hemostatic function of these mitochondria-injured platelets in mice.5 In the present study, we confirm that TACE is activated during platelet storage, leading to a reduced posttransfusion recovery of these cells. In an attempt to identify new means to improve platelet storage conditions, we thus focused our attention on the mechanism(s) regulating TACE activation in stored platelets. Surprisingly, the PKC pathway, which has been considered the major signaling pathway mediating TACE activation in platelets,5,6,27 was not critical for TACE activation during platelet storage (Table 1). Based on the effect of pharmacologic inhibitors, our studies suggest that TACE-mediated receptor shedding in stored platelets depends on signaling by p38 MAPK (Figures 2–3) but is independent of ERK MAPK (Table 1). We cannot be entirely sure that some of the effects of the p38 MAPK inhibition are not the result of off-target effects, the classic reservation about the use of inhibitors. Interestingly, our group recently noticed that TACE is also activated through p38 MAPK in platelets stimulated with H2O2 or serotonin.28,29 These results indicate that the p38 MAPK pathway regulates TACE activity in platelets not only during storage but also in response to other stresses and physiologic stimuli.
The molecular mechanisms of TACE activation by p38 MAPK during platelet storage remain to be characterized. Downstream targets of p38 MAPK, such as MAPKAP-K216 and heat shock protein 27 (HSP27),30 have been detected in platelets. One possible mechanism could be that MAPKAP-K2 directly activates TACE, as was reported for cPLA2 when platelets are stimulated with H2O2.31 Alternatively, p38 MAPK may directly phosphorylate TACE and thereby modulate its activity. Such a direct effect on TACE was shown in vitro for ERK MAPK.24 The dissociation of GPIb-IX-V/calmodulin complexes was also shown to prime the receptor for TACE-mediated proteolysis.6 Therefore, one could consider that p38 MAPK activation leads to the dissociation of calmodulin from GPIb-IX-V by as-yet-undefined mechanisms. Interestingly, although platelet clearance was linked to the apoptotic pathway,32 our studies show that caspase inhibition does not interfere with TACE activation (Table 1).
TACE is involved in the shedding of many plasma membrane proteins.33 In platelets, TACE has been shown to regulate the surface expression of GPIb-α, GPV, and semaphorin 4D.5,6,27 So far, which substrate of TACE provides the survival or clearance signal for transfused platelets has not been addressed. One potential candidate is GPIb-α, the platelet receptor for von Willebrand factor. GPIb-α has also been implicated in the clearance of platelets that were stored at 4°C. As shown by Hoffmeister et al,34 refrigeration of platelets for 1 hour followed by rewarming leads to the clustering of GPIb-α on the cell surface and clearance by Mac-1 (CD11b/18)–bearing hepatic macrophages, suggesting different mechanisms of clearance for platelets stored at room temperature or 4°C. Interestingly, however, prolonged refrigeration (48 hours) before rewarming caused shedding of GPIb-α and GPV, and receptor shedding and the clearance of these cells were prevented by adding metalloproteinase inhibitors during storage.35 Thus, the clearance mechanisms of platelets stored at 4°C or room temperature for an extended period of time seem to be very similar. There are several potential explanations for how GPIb-α shedding may cause platelet clearance. First, the cleavage of the GPIb-α moiety may expose a novel ligand (the clearance signal) on the GPIb-V-IX complex. This signal could be clusters of the remaining truncated subunits of the VWF receptor or a neoepitope on the truncated receptor that was not accessible before. Alternatively, the clearance signal may derive from the loss of repulsive negative charges provided by the heavily O-glycosylated extracellular domain of GPIb-α. Further investigations are needed to identify the relevant substrate(s) of TACE and the pathways of recognition of the platelets after TACE activation during storage.
Our studies demonstrate that p38 MAPK plays a key role in the generation of PSL and the clearance of stored platelets (Figure 5A). Platelets stored in the presence of a p38 MAPK inhibitor showed a markedly improved hemostatic function in vivo. Inhibition of p38 MAPK increased both the incorporation of stored platelets into a growing thrombus in wild-type mice and the efficacy of these platelets to arrest hemorrhaging in mice with a severe bleeding phenotype (Figure 5B-C). It is well known that p38 MAPK signaling regulates cellular functions other than receptor shedding. Thus, infusion of platelet concentrates containing p38 inhibitors may lead to unwanted side effects in patients. However, there are advantages to the use of inhibitors in platelet concentrates that will limit these complications. First, p38 MAPK inhibitors would be used at optimal doses to inhibit PSL in platelet concentrates. On transfusion into patients, the p38 MAPK inhibitor would be diluted and its concentration in the circulation would be markedly lower. In addition, although the inhibitor seems to be active over days in stored platelets, its half-life in vivo should be much shorter because of the rapid clearance of such compounds from the circulation.36
In conclusion, our data show that p38 MAPK signaling is not a central component in platelet integrin activation and thus thrombus formation. We suggest that inhibition of p38 MAPK signaling during platelet storage may be a powerful means to improve the efficacy of platelet transfusions. This conclusion is supported by our findings that platelets stored in the presence of p38 MAPK inhibitors (1) show a markedly improved posttransfusion survival and (2) have a significantly improved hemostatic function on infusion in mice.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
M.C. received a postdoctoral grant from the Fondation pour la Recherche Médicale (Paris, France).
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grant P01 HL056949; D.D.W.) and the American Heart Association (Scientist Development grant 0630044N; W.B.).
National Institutes of Health
Authorship
Contribution: M.C. designed the study, performed most of the experiments, analyzed the results, and wrote the manuscript; D.D. helped with study design and performed the aggregation study and the experiments on human platelets; A.B., L.S., D.S., and S.M.C. helped perform the experiments; and W.B. and D.D.W. participated in the study design, analysis of results, and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.C. is Inserm U626, Faculté de Médicine Timone, Marseille, France. The current affiliation for D.D. is University Hospital of Freiburg, Department of Cardiology and Angiology, Freiburg, Germany.
Correspondence: Denisa D. Wagner, Immune Disease Institute, 3 Blackfan Cir, Boston, MA 02115; e-mail: wagner@idi.harvard.edu; or Wolfgang Bergmeier, Thomas Jefferson University, 1015 Walnut St, 702 Curtis Bldg, Philadelphia, PA 19131; e-mail: wolfgang.bergmeier@jefferson.edu.