Abstract
Both total nucleated cell (TNC) dose and human leukocyte antigen (HLA)–match affect the outcome of cord blood (CB) transplantation. However, how to prioritize these characteristics in unit selection is not established. Therefore, we analyzed the outcomes of 1061 patients who received single-unit myeloablative CB transplantation for leukemia or myelodysplasia. TNC dose and HLA-match each affected survival via their effect on transplant-related mortality (TRM); neither was associated with relapse. Therefore, TRM was the focus of multivariate analyses combining dose and HLA-match. Compared with our 1 HLA-mismatch (MM) reference group with TNC 2.5 to 4.9 × 107/kg, recipients of 0 MM units had the lowest TRM regardless of dose (relative risk [RR] = 0.4, P = .019). TRM for recipients of 1- or 2-MM units with TNC 5.0 × 107/kg or greater was similar to the reference group (RR = 0.8, P = .391 and RR = 1.0, P = .847) despite their greater dose. Recipients of 2 MM units with TNC 2.5 to 4.9 × 107/kg had a greater TRM (RR = 1.5, P = .014), and those with 1 or 2 MM and TNC less than 2.5 × 107/kg or 3 MM did substantially worse. These findings support new unit selection criteria that take into account both TNC dose and HLA-match and have important implications for the size of the global CB inventory needed to find an optimum CB graft.
Introduction
Cord blood (CB) is increasingly used as an alternative hematopoietic stem cell (HSC) source and has the advantages of rapid availability and less-stringent requirements for human leukocyte antigen (HLA) match compared with HSC from adult unrelated volunteer donors.1-4 However, because of the high transplant-related mortality (TRM) and poor survival associated with CB units providing a low total nucleated cell (TNC) dose, cell dose frequently is given priority over donor-recipient HLA match in CB unit selection.5-7 However, HLA match also affects outcome after CB transplantation (CBT) and should be considered in selecting an optimal graft.1,5,8 Although it is common practice to select the unit with the greatest TNC within a given HLA-match grade, how to select between a better-matched unit with a lower TNC and a less well-matched unit with a greater TNC is not established.
Therefore, we analyzed the combined impact of prefreeze TNC dose and HLA match upon CBT outcome in recipients of 0 to 3 HLA-A, -B antigen, and -DRB1 allele-mismatched CB units. To limit the number of patient and treatment variables that might confound the results, the study included only patients who received myeloablative conditioning in single-unit CBT for the treatment of leukemia or myelodysplasia. In addition, all patients received a transplant with units provided by a single bank, the National Cord Blood Program of the New York Blood Center (NYBC), eliminating another potential source of variability. We hypothesized that better HLA-match would compensate for lower TNC dose. Our findings support new criteria for unit selection that prioritize HLA match over TNC dose for many patients and, therefore, have significant implications for the size of the global CB inventory required to meet patient needs.
Methods
All sequential patients were eligible for this study if they received myeloablative conditioning and single-unit grafts from the NYBC during 1993 to 2006 for the treatment of leukemia or myelodysplasia. Mismatch (MM) for HLA-A and -B was defined at an intermediate level of resolution. Thus, mismatched “splits” of broad antigens (such as B62, B63, B75, B76, and B77 for B15) were considered mismatched. MM at HLA-DRB1 was defined at the high-resolution allele level. Patients were excluded if they had reduced intensity or nonmyeloablative conditioning, received a graft with more than 3-MMs, or if splits of broad class I or high-resolution HLA-DRB1 typing were not available. Outcome data were provided by transplant centers on 1061 (89%) of 1198 eligible patients. Patients from centers outside the US were more likely to lack follow-up (23%, compared with 6% for US centers). Follow-up reports were reviewed for completeness and consistency, and centers were contacted for resolution of ambiguities. Among survivors, the median follow-up was 29 months, with 70% having outcome data for at least 1 year after transplantation. Mothers signed institutional review board–approved informed consent to donate their babies' cord blood to the NYBC program. Patients signed informed consent for CBT at their respective transplant centers, which reported outcome data to the NYBC under an investigational new drug exemption from the US Food and Drug Administration in accordance with the Declaration of Helsinki.
Neutrophil engraftment was defined as a sustained absolute neutrophil count greater than 0.5 × 109/L with the time to neutrophil engraftment defined as the first of 3 consecutive days that an absolute neutrophil count of 0.5 was achieved. Data on neutrophil engraftment were reported or evaluable in all but 57 patients. Platelet engraftment was defined as achievement of an absolute platelet count of greater than 50 × 109/L without transfusion support for 7 days and was reported or evaluable in all but 98 patients. Patients who had autologous recovery or received a backup graft were considered to have graft failure and were censored at the time of these events. Acute graft-versus-host disease (aGVHD) grade was analyzed according to transplant center assignment, and, when organ-specific grades were provided, also was assigned according to International Bone Marrow Transplant Registry criteria.9 The probability and relative risk (RR) for aGVHD was analyzed in patients who engrafted and for chronic GVHD (cGVHD) in patients who engrafted and survived at least 100 days after transplantation.
We combined limited and extensive disease for analyses of cGVHD. Data on aGVHD were reported on all but 69 patients who engrafted and for cGVHD were reported on all but 131 patients who engrafted and survived to day 100. The time to relapse after transplantation was determined on the basis of the date of first documented disease progression. TRM was defined as any death not caused by relapse or persistence of malignancy. Disease-free survival (DFS) was defined as survival without relapse or disease progression. Treatment failure was the inverse of DFS. Overall mortality was defined as death from any cause. Cause of death was reported for all but 26 of 669 deaths. The primary cause of death was assigned to mutually exclusive categories according to the algorithm of Copelan et al.10 Deaths attributed to GVHD were cause either by acute or chronic disease.
For comparisons between categorical variables, we used the χ2 statistic, whereas we used the Student t test was used to compare means of continuous variables, both 2-tailed. Probabilities of neutrophil and platelet engraftment, aGVHD, cGVHD, TRM, and relapse rates were estimated by cumulative incidence methods, whereas DFS, treatment failure, and overall mortality were estimated by Kaplan-Meier.11,12 Analysis of relapse and survival end points was limited to the first 3 years after transplantation, with 83% of relapse events and 90% of all reported deaths occurring within the first year after transplantation. The competing event for engraftment was early death, and the competing events for GVHD were graft failure or death before that end point. A transplantation-related death was the competing event for relapse, whereas relapse was the competing event for TRM. In analyses of variables related to the primary cause of death, death from an alternate cause was the competing event. Patients with competing events were censored at the time of the event.
Cox regression was used to estimate RRs, 95% confidence intervals, and statistical significance for outcome end points in multivariate analyses.11,12 Variables previously reported to be significant predictors of the outcome of CBT (patient age, ancestry, disease stage, recipient cytomegalovirus antibody status, and GVHD prophylaxis) as well as year of transplantation and center experience (US centers with > 50 patients given NYBC CB units, US centers with < 50 such patients, and non-US centers) were examined. Because patient age was highly correlated with recipient weight and TNC dose, age was excluded from multivariate analyses of survival. In multivariate analyses of outcome end points, all variables that had univariate P values of .10 or less were entered into the initial model and then eliminated stepwise in order of significance until all remaining variables had P values of .05 or less. Analyses were performed with SPSS (Version 16.0; SPSS) software.
Results
Characteristics of patients and grafts
Patient and graft characteristics are described in Table 1. Although most patients (median age, 9.3 years; range, 2 months to 64 years) were children, 39% were adults or adolescents at least 12 years of age, and nearly one-half had nonwhite ancestry. Most patients were transplanted for treatment of acute lymphoblastic (44%) or myelogenous (33%) leukemia. Overall, 671 (63%) patients were in remission, 254 (24%) were in relapse, 65 (6%) had unknown disease status, and 71 (7%) had MDS without further classification by the transplant center. Most transplantations (75%) were performed in US centers, with nearly one-third in the 6 most experienced CBT centers. Most CB grafts were HLA-mismatched (88% had 1- or 2-MM). For the entire group the geometric mean (GM) cryopreserved TNC dose was 4.3 × 107/kg recipient body weight at the time of transplantation (median, 4.1; range, 0.7-28). For patients given grafts with 0-MM, the GM cryopreserved TNC dose was 4.4 × 107/kg (median, 4.0; range, 0.7-19.4). For recipients of 1-MM units, it was 4.7 × 107/kg (median, 4.5; range, 0.7-26.1), for 2-MM units it was 4.1 × 107/kg (median, 3.8; range, 0.9-28.0), and for 3-MM units it was 3.7 × 107/kg (median, 3.5; range, 1.4-16.5). The difference in GM TNC dose was not significant for 0 versus 1-MM (P = .425), 2-MM (P = .533), or 3-MM (P = .148) recipient but was for 1- versus 2-MM recipients (P = .002) and 1- versus 3-MM recipients (P = .008). TNC dose correlated strongly with patient age. Thus, 80% of patients who were younger than 6 years of age received a unit providing a TNC dose 5.0 × 107/kg or greater compared with 36% of 6- to 11-year-old patients and only 5% of patients 12 years of age or older (P < .001).
Characteristics of 1061 study patients and CB grafts
| Characteristic . | n (%) . |
|---|---|
| Sex | |
| Male | 588 (55) |
| Female | 473 (45) |
| Age, y | |
| 0-1 | 137 (13) |
| 2-9 | 426 (40) |
| 10-19 | 261 (25) |
| 20-39 | 147 (14) |
| 40 or more | 90 (8) |
| Ancestry* | |
| Asian | 41 (4) |
| African | 180 (17) |
| Hispanic | 218 (21) |
| Native American | 8 (1) |
| Middle Eastern | 29 (3) |
| White | 555 (54) |
| Other | 3 (< 1) |
| Diagnosis | |
| ALL | 463 (44) |
| AML | 350 (33) |
| CML | 126 (12) |
| JCML | 31 (3) |
| Other leukemia | 20 (2) |
| Myelodysplasia | 71 (7) |
| Disease risk† | |
| Early-stage disease | 240 (27) |
| Intermediate-stage disease | 403 (46) |
| Advanced-stage disease | 242 (27) |
| Conditioning | |
| TBI-based | 672 (63) |
| Chemotherapy only | 300 (28) |
| Unknown | 89 (8) |
| Immunosuppression | |
| CSA/corticosteroids | 530 (50) |
| Methotrexate-based | 166 (16) |
| Other | 139 (13) |
| Unknown | 226 (21) |
| Year of transplant | |
| 1993-1996 | 192 (18) |
| 1997-1999 | 384 (36) |
| 2000-2002 | 213 (20) |
| 2003-2006 | 272 (26) |
| Center | |
| US center, > 50 NYBC CBT | 330 (31) |
| US center, < 50 NYBC CBT | 461 (43) |
| Non-US center | 270 (25) |
| HLA match | |
| 0 mismatch | 56 (5) |
| 1 mismatch | 352 (33) |
| 2 mismatch | 588 (55) |
| 3 mismatch | 65 (6) |
| Cryopreserved TNC (geometric mean) | |
| 0.7-2.4 × 107 TNC/kg (1.8) | 239 (23) |
| 2.5-4.9 × 107 TNC/kg (3.5) | 408 (38) |
| 5.0-9.9 × 107 TNC/kg (6.9) | 280 (26) |
| More than 10.0 × 107 TNC/kg (13.6) | 134 (13) |
| Characteristic . | n (%) . |
|---|---|
| Sex | |
| Male | 588 (55) |
| Female | 473 (45) |
| Age, y | |
| 0-1 | 137 (13) |
| 2-9 | 426 (40) |
| 10-19 | 261 (25) |
| 20-39 | 147 (14) |
| 40 or more | 90 (8) |
| Ancestry* | |
| Asian | 41 (4) |
| African | 180 (17) |
| Hispanic | 218 (21) |
| Native American | 8 (1) |
| Middle Eastern | 29 (3) |
| White | 555 (54) |
| Other | 3 (< 1) |
| Diagnosis | |
| ALL | 463 (44) |
| AML | 350 (33) |
| CML | 126 (12) |
| JCML | 31 (3) |
| Other leukemia | 20 (2) |
| Myelodysplasia | 71 (7) |
| Disease risk† | |
| Early-stage disease | 240 (27) |
| Intermediate-stage disease | 403 (46) |
| Advanced-stage disease | 242 (27) |
| Conditioning | |
| TBI-based | 672 (63) |
| Chemotherapy only | 300 (28) |
| Unknown | 89 (8) |
| Immunosuppression | |
| CSA/corticosteroids | 530 (50) |
| Methotrexate-based | 166 (16) |
| Other | 139 (13) |
| Unknown | 226 (21) |
| Year of transplant | |
| 1993-1996 | 192 (18) |
| 1997-1999 | 384 (36) |
| 2000-2002 | 213 (20) |
| 2003-2006 | 272 (26) |
| Center | |
| US center, > 50 NYBC CBT | 330 (31) |
| US center, < 50 NYBC CBT | 461 (43) |
| Non-US center | 270 (25) |
| HLA match | |
| 0 mismatch | 56 (5) |
| 1 mismatch | 352 (33) |
| 2 mismatch | 588 (55) |
| 3 mismatch | 65 (6) |
| Cryopreserved TNC (geometric mean) | |
| 0.7-2.4 × 107 TNC/kg (1.8) | 239 (23) |
| 2.5-4.9 × 107 TNC/kg (3.5) | 408 (38) |
| 5.0-9.9 × 107 TNC/kg (6.9) | 280 (26) |
| More than 10.0 × 107 TNC/kg (13.6) | 134 (13) |
ALL indicates acute lymphoblastic leukemia; AML, acute myeloblastic leukemia; CBT, cord blood transplant; CML, chronic myelogenous leukemia; CSA, cyclosporine-A; HLA, human leukocyte antigen; JCML, juvenile chronic myelogenous leukemia; NYBC, New York Blood Center; TBI, total body irradiation; TNC, total nucleated cell; and US, United States.
Ancestry was unknown in 27 patients.
Applies to patients with ALL, AML, or CML only and was unknown in 54 patients (including 13 in remission, but the number of remissions was unknown).
Neutrophil and platelet engraftment, GVHD, and relapse
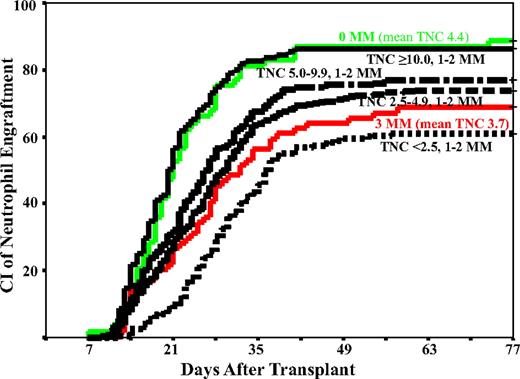
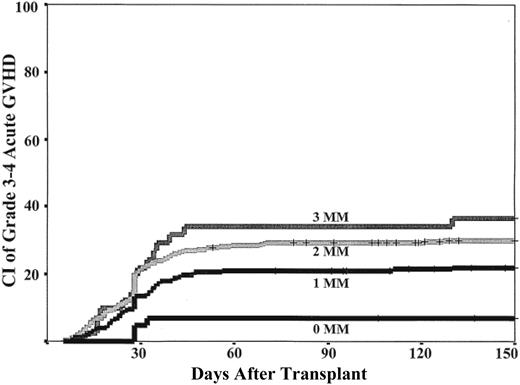
The cumulative incidence of neutrophil engraftment was 74% (95% confidence interval, 71%-77%) by day 77 and platelet engraftment to greater than 50 × 109/L was 46% (95% confidence interval, 43%-49%) by 9 months after transplantation. The cumulative incidences of grades 2 to 4 and grades 3 to 4 aGVHD were 49% (95% confidence interval, 45%-53%) and 26% (95% confidence interval, 23%-29%) by day 150, respectively, and 37% (95% confidence interval, 32%-42%) for chronic GVHD by 3 years after transplantation. TNC dose was associated with neutrophil and platelet engraftment in a dose-response relationship with progressively faster and greater engraftment rates as the dose increased (Table 2; Figure 1). However, TNC dose had no relationship to aGVHD, cGVHD, or relapse.
Multivariate analyses of neutrophil engraftment (ANC ≥ 0.5 × 109/L), platelet engraftment (platelets > 50 × 109/L), aGVHD, and cGVHD
| Variable . | n . | RR (95% Confidence interval) . | P . |
|---|---|---|---|
| Neutrophil engraftment* | |||
| 0.7-2.4 × 107/kg | 233 | 0.7 (0.6-0.8) | < .001¶ |
| 2.5-4.9 × 107/kg | 383 | Reference | |
| 5.0-9.9 × 107/kg | 262 | 1.2 (1.0-1.5) | .026¶ |
| >10.0 × 107/kg | 126 | 1.8 (1.4-2.2) | < .001¶ |
| 0-MM | 53 | 1.8 (1.3-2.5) | < .001¶ |
| 1-MM | 331 | Reference | |
| 2-MM | 556 | 1.0 (0.9-1.2) | .896 |
| 3-MM | 64 | 0.8 (0.6-1.1) | .162 |
| Platelet engraftment* | |||
| 0.7-2.4 × 107/kg | 226 | 0.7 (0.5-0.9) | .006¶ |
| 2.5-4.9 × 107/kg | 358 | Reference | |
| 5.0-9.9 × 107/kg | 256 | 1.4 (1.1-1.7) | .008¶ |
| More than 10.0 × 107/kg | 123 | 1.8 (1.4-2.4) | < .001¶ |
| 0-MM | 49 | 1.8 (1.2-2.5) | .002¶ |
| 1-MM | 316 | Reference | |
| 2-MM | 534 | 0.8 (0.6-0.9) | .027¶ |
| 3-MM | 64 | 0.5 (0.3-0.8) | .006¶ |
| Grade 3-4 aGVHD†‡ | |||
| 0-MM | 43 | 0.3 (0.1-0.9) | .030¶ |
| 1-MM | 229 | Reference | |
| 2-MM | 382 | 1.4 (1.0-2.0) | .039¶ |
| 3-MM | 41 | 1.8 (1.0-3.3) | .050¶ |
| cGVHD§‖ | |||
| 0-MM | 30 | 0.9 (0.4-1.8) | .781 |
| 1-MM | 150 | Reference | |
| 2-MM | 213 | 1.3 (0.9-1.9) | .184 |
| 3-MM | 19 | 2.4 (1.2-4.6) | .011¶ |
| Variable . | n . | RR (95% Confidence interval) . | P . |
|---|---|---|---|
| Neutrophil engraftment* | |||
| 0.7-2.4 × 107/kg | 233 | 0.7 (0.6-0.8) | < .001¶ |
| 2.5-4.9 × 107/kg | 383 | Reference | |
| 5.0-9.9 × 107/kg | 262 | 1.2 (1.0-1.5) | .026¶ |
| >10.0 × 107/kg | 126 | 1.8 (1.4-2.2) | < .001¶ |
| 0-MM | 53 | 1.8 (1.3-2.5) | < .001¶ |
| 1-MM | 331 | Reference | |
| 2-MM | 556 | 1.0 (0.9-1.2) | .896 |
| 3-MM | 64 | 0.8 (0.6-1.1) | .162 |
| Platelet engraftment* | |||
| 0.7-2.4 × 107/kg | 226 | 0.7 (0.5-0.9) | .006¶ |
| 2.5-4.9 × 107/kg | 358 | Reference | |
| 5.0-9.9 × 107/kg | 256 | 1.4 (1.1-1.7) | .008¶ |
| More than 10.0 × 107/kg | 123 | 1.8 (1.4-2.4) | < .001¶ |
| 0-MM | 49 | 1.8 (1.2-2.5) | .002¶ |
| 1-MM | 316 | Reference | |
| 2-MM | 534 | 0.8 (0.6-0.9) | .027¶ |
| 3-MM | 64 | 0.5 (0.3-0.8) | .006¶ |
| Grade 3-4 aGVHD†‡ | |||
| 0-MM | 43 | 0.3 (0.1-0.9) | .030¶ |
| 1-MM | 229 | Reference | |
| 2-MM | 382 | 1.4 (1.0-2.0) | .039¶ |
| 3-MM | 41 | 1.8 (1.0-3.3) | .050¶ |
| cGVHD§‖ | |||
| 0-MM | 30 | 0.9 (0.4-1.8) | .781 |
| 1-MM | 150 | Reference | |
| 2-MM | 213 | 1.3 (0.9-1.9) | .184 |
| 3-MM | 19 | 2.4 (1.2-4.6) | .011¶ |
aGVHD indicates acute graft-versus-host disease; ANC, absolute neutrophil count; CBT, cord blood transplant; cGVHD, chronic graft-versus-host disease; CSA, cyclosporine-A; JCML, juvenile chronic myeloid leukemia; MM, mismatch; RR, relative risk; TNC, total nucleated cells; TRM, transplant-related mortality; and US, United States.
Other predictive variables, such as more recent year of transplantation (2003-2006) and transplantation in more-experienced US centers, were associated with improved neutrophil and platelet engraftment. GVHD prophylaxis with methotrexate (compared with CSA and corticosteroids) was associated with an inferior engraftment rate.
Other predictive variables, such as GVHD prophylaxis with methotrexate (compared with CSA and corticosteroids), were associated with less aGVHD, whereas older patient age and transplantation in less-experienced US centers were associated with a greater incidence of severe aGVHD. TNC dose and patient ethnicity had no association with aGVHD.
Analysis was performed among patients who engrafted according to transplant center assignment. Reanalysis of severe acute GVHD according to International Bone Marrow Transplant Registry grade with reported organ involvement yielded similar results (data not shown).
Other predictive variables, such as older patient age, nonwhite ancestry, and transplantation in less experienced US centers, were associated with a greater risk of cGVHD. TNC dose and GVHD prophylaxis had no association with cGVHD.
Analysis was performed among patients who engrafted and survived ≥ 100 days after transplant.
Statistically significant P value.
The cumulative incidence (CI) of neutrophil engraftment by day 77 in patients given units with 0-MM (mean TNC, 4.4) or 3-MM (mean TNC, 3.9), or according to the TNC ×107/kg in patients with 1- or 2-MM CB grafts. Note that the 4 TNC categories in this figure exclude those that had either 0 or 3 mismatches, accounting for the lower number of cases in each TNC group in the figure compared with Table 2.
The cumulative incidence (CI) of neutrophil engraftment by day 77 in patients given units with 0-MM (mean TNC, 4.4) or 3-MM (mean TNC, 3.9), or according to the TNC ×107/kg in patients with 1- or 2-MM CB grafts. Note that the 4 TNC categories in this figure exclude those that had either 0 or 3 mismatches, accounting for the lower number of cases in each TNC group in the figure compared with Table 2.
HLA-match (0-MM) was associated with improved neutrophil engraftment (Table 2; Figure 1). However, there was no difference in the neutrophil engraftment rates between recipients of units with 1- or 2-MM. In contrast, HLA-match was associated with progressively greater rates of platelet engraftment as the match improved (Table 2). HLA-match level also was associated with the incidence of grades 3 to 4 aGVHD (Table 2; Figure 2). Recipients of 0-MM units had significantly less grade 3 to 4 aGVHD compared with recipients of 1-MM units, and there was a progressively greater risk in recipients of units with 2-MM or 3-MM. Worse HLA match was also associated with a greater incidence of cGVHD but was only significant in 3-MM recipients (Table 2).
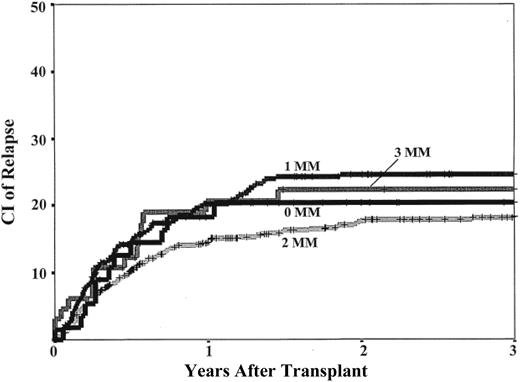
The cumulative incidence of relapse was 21% (95% confidence interval, 18%-24%) at 3 years after transplantation. Notably, there was no association between HLA-match and the incidence of relapse (Table 3; Figure 3). Analyses in subgroups that included only patients transplanted in relapse, only those in remission, or only those who engrafted (Table 3) also failed to demonstrate an association with HLA-match.
Multivariate analyses of relapse
| Variable . | n . | RR (95% Confidence interval) . | P . |
|---|---|---|---|
| All patients* | |||
| 0-MM | 56 | 0.7 (0.4-1.3) | .211 |
| 1-MM | 352 | Reference | |
| 2-MM | 588 | 0.9 (0.6-1.2) | .364 |
| 3-MM | 65 | 1.2 (0.7-2.2) | .458 |
| Patients in relapse† | |||
| 0-MM | 7 | 1.0 (0.2-4.4) | .977 |
| 1-MM | 77 | Reference | |
| 2-MM | 142 | 0.9 (0.5-1.5) | .693 |
| 3-MM | 28 | 1.4 (0.6-3.1) | .394 |
| Patients in remission† | |||
| 0-MM | 40 | 0.7 (0.3-1.5) | .359 |
| 1-MM | 228 | Reference | |
| 2-MM | 371 | 0.9 (0.6-1.4) | .662 |
| 3-MM | 32 | 1.5 (0.6-3.8) | .411 |
| Patients who engrafted | |||
| 0-MM | 47 | 0.7 (0.4-1.4) | .350 |
| 1-MM | 254 | Reference | |
| 2-MM | 418 | 1.0 (0.7-1.4) | .804 |
| 3-MM | 45 | 1.7 (0.9-3.4) | .108 |
| Variable . | n . | RR (95% Confidence interval) . | P . |
|---|---|---|---|
| All patients* | |||
| 0-MM | 56 | 0.7 (0.4-1.3) | .211 |
| 1-MM | 352 | Reference | |
| 2-MM | 588 | 0.9 (0.6-1.2) | .364 |
| 3-MM | 65 | 1.2 (0.7-2.2) | .458 |
| Patients in relapse† | |||
| 0-MM | 7 | 1.0 (0.2-4.4) | .977 |
| 1-MM | 77 | Reference | |
| 2-MM | 142 | 0.9 (0.5-1.5) | .693 |
| 3-MM | 28 | 1.4 (0.6-3.1) | .394 |
| Patients in remission† | |||
| 0-MM | 40 | 0.7 (0.3-1.5) | .359 |
| 1-MM | 228 | Reference | |
| 2-MM | 371 | 0.9 (0.6-1.4) | .662 |
| 3-MM | 32 | 1.5 (0.6-3.8) | .411 |
| Patients who engrafted | |||
| 0-MM | 47 | 0.7 (0.4-1.4) | .350 |
| 1-MM | 254 | Reference | |
| 2-MM | 418 | 1.0 (0.7-1.4) | .804 |
| 3-MM | 45 | 1.7 (0.9-3.4) | .108 |
MM indicates mismatch; and RR, relative risk.
Other predictive variables, such as advanced disease, diagnosis of JCML, and younger age (< 10 years), were associated with an increased risk of relapse. TNC, transplant center experience, year of transplant, and patient ethnicity were not predictive of relapse.
The subanalyses of those transplanted in relapse or remission excludes those with unknown disease status at time of transplantation and MDS.
Survival end points
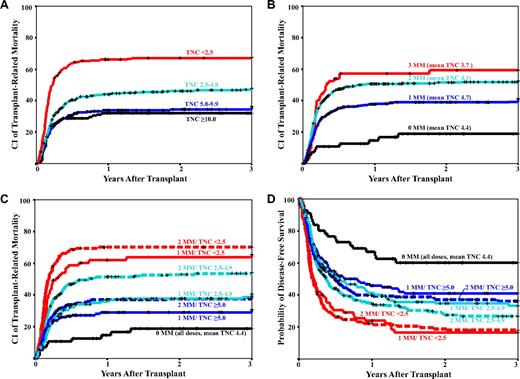
Consistent with previous reports, TNC dose and HLA-mismatch were independently predictive of posttransplantation TRM (Figure 4A-B), treatment failure, or overall survival.1,5,8 However, as seen in Figure 4B, the lowest TRM was seen, not in recipients of the greatest TNC, but rather in recipients of 0-MM units. Although there was a wide range of dose within the 0-MM group (< 2.5 × 107/kg, n = 14; 2.5-4.9 × 107/kg, n = 17; 5.0-9.9 × 107/kg, n = 17; and > 10.0 × 107/kg, n = 8), TNC dose had no discernable impact on survival. Thus, there was no significant difference in TRM between pediatric and adult recipients in the 0-MM group: the 3-year cumulative incidence of TRM in recipients of 0-MM units younger than 12 years of age was 18% compared with 22% for older children and adults (univariate RR = 1.1; 95% confidence interval, 0.3-4.4; P = .845).
CI of 3-year TRM. Data are shown by TNC dose (A), HLA-mismatch (B), TNC dose and HLA-mismatch combined (C), and the Kaplan-Meier probability of disease-free survival (D). Recipients of units with either 1 or 2 mismatches were analyzed by separate TNC dose categories, whereas recipients of 0-MM units and 3-MM units were not.
CI of 3-year TRM. Data are shown by TNC dose (A), HLA-mismatch (B), TNC dose and HLA-mismatch combined (C), and the Kaplan-Meier probability of disease-free survival (D). Recipients of units with either 1 or 2 mismatches were analyzed by separate TNC dose categories, whereas recipients of 0-MM units and 3-MM units were not.
Given that the majority of patients cannot find a matched CB graft, transplantation physicians frequently have to choose between a unit with a lower TNC and better HLA-match versus a less well-matched unit with greater TNC. To address this issue, we combined the TNC dose and HLA-match for recipients of 1- and 2-MM units in multivariate analyses of survival end points in which both unit characteristics contribute. The 3-MM group was not subdivided by TNC dose because of the small sample size and because few patients now have to resort to such poorly matched units. Because there was no difference in TRM (Figure 4A) or survival for TNC doses greater than 5.0 × 107/kg, the greatest TNC dose categories were combined in the 1-MM and 2-MM recipients. Because few adults and larger children can find a unit 5.0 × 107/kg or greater, we chose recipients of 1-MM units with an intermediate TNC dose of 2.5 to 4.9 × 107/kg as the reference group. TRM was the major focus of the analyses because it was the major determinant of survival, given neither TNC dose nor HLA-match was associated with relapse risk.
In the combined analysis, compared with the reference group (1-MM, TNC 2.5-4.9 × 107/kg), recipients of 0-MM units had a significantly lower TRM (Table 4; Figure 4C). TRM did not differ for patients given CB units with a TNC dose greater than 5 × 107/kg and either 1- or 2-MM compared with the reference group despite a TNC dose that was more than 2-fold greater (GM TNC was 9.2 × 107/kg and 8.2 × 107/kg for 1- and 2-MM high TNC dose recipients, respectively, compared with 3.6 × 107/kg in the intermediate TNC dose recipients, P < .001). Recipients of units with a high TNC dose with either 1- or 2-MM also did not differ from each other in TRM risk (1- vs 2-MM, RR = 0.8; 95% confidence interval, 0.5-1.2; P = .223). However, patients given units with 2-MM and a TNC dose in the same range (2.5-4.9 × 107/kg) as the 1-MM reference group had a significantly greater TRM (Table 4; Figure 4C). Those who received 1- or 2-MM units with a lower TNC dose of less than 2.5 × 107/kg, or 3 mismatches regardless of TNC dose, did substantially worse. Analyses of TRM in the subsets of patients who engrafted (Table 4) or those transplanted in remission (data not shown) also showed similar findings. Similar relationships also were seen with overall mortality (Table 4), treatment failure (Table 4), and DFS (Figure 4D).
Multivariate analyses of 3-year survival end points
| Variable . | n . | RR (95% Confidence interval) . | P . |
|---|---|---|---|
| Transplant-related mortality | |||
| 0-MM | 56 | 0.4 (0.2-0.9) | .019* |
| 1-MM > 5.0 × 107/kg | 155 | 0.8 (0.6-1.3) | .391 |
| 2-MM > 5.0 × 107/kg | 212 | 1.0 (0.7-1.5) | .847 |
| 1-MM 2.5-4.9 × 107/kg | 130 | Reference | |
| 2-MM 2.5-4.9 × 107/kg | 240 | 1.5 (1.1-2.1) | .014* |
| 1-MM < 2.5 × 107/kg | 67 | 1.9 (1.3-2.9) | .002* |
| 2-MM < 2.5.0 × 107/kg | 136 | 2.4 (1.7-3.4) | < .001* |
| 3-MM | 65 | 1.7 (1.1-2.6) | .020* |
| Transplant-related mortality (engrafted patients only) | |||
| 0-MM | 47 | 0.5 (0.2-1.1) | .072 |
| 1-MM > 5.0 × 107/kg | 120 | 0.8 (0.5-1.4) | .518 |
| 2-MM > 5.0 × 107/kg | 165 | 1.2 (0.8-1.9) | .452 |
| 1-MM 2.5-4.9 × 107/kg | 94 | Reference | |
| 2-MM 2.5-4.9 × 107/kg | 171 | 1.6 (1.0-2.5) | .032* |
| 1-MM < 2.5 × 107/kg | 40 | 1.9 (1.1-3.3) | .029* |
| 2-MM < 2.5.0 × 107/kg | 81 | 2.3 (1.4-3.7) | .001* |
| 3-MM | 45 | 2.1 (1.2-3.6) | .011* |
| Overall mortality | |||
| 0-MM | 56 | 0.5 (0.3-0.8) | .005* |
| 1-MM > 5.0 × 107/kg | 155 | 0.8 (0.6-1.2) | .295 |
| 2-MM > 5.0 × 107/kg | 212 | 1.0 (0.7-1.3) | .769 |
| 1-MM 2.5-4.9 × 107/kg | 130 | Reference | |
| 2-MM 2.5-4.9 × 107/kg | 240 | 1.2 (0.9-1.6) | .195 |
| 1-MM < 2.5 × 107/kg | 67 | 1.6 (1.1-2.2) | .013* |
| 2-MM < 2.5.0 × 107/kg | 136 | 1.8 (1.3-2.4) | < .001* |
| 3-MM | 65 | 1.4 (1.0-2.1) | .048* |
| Treatment failure (reciprocal of disease-free survival) | |||
| 0-MM | 56 | 0.5 (0.3-0.9) | .011* |
| 1-MM > 5.0 × 107/kg | 155 | 0.9 (0.7-1.3) | .693 |
| 2-MM > 5.0 × 107/kg | 212 | 1.0 (0.8-1.4) | .822 |
| 1-MM 2.5-4.9 × 107/kg | 130 | Reference | |
| 2-MM 2.5-4.9 × 107/kg | 240 | 1.2 (0.9-1.6) | .120 |
| 1-MM < 2.5 × 107/kg | 67 | 1.5 (1.1-2.2) | .018* |
| 2-MM < 2.5.0 × 107/kg | 136 | 1.7 (1.3-2.3) | < .001* |
| 3-MM | 65 | 1.5 (1.0-2.1) | .039* |
| Variable . | n . | RR (95% Confidence interval) . | P . |
|---|---|---|---|
| Transplant-related mortality | |||
| 0-MM | 56 | 0.4 (0.2-0.9) | .019* |
| 1-MM > 5.0 × 107/kg | 155 | 0.8 (0.6-1.3) | .391 |
| 2-MM > 5.0 × 107/kg | 212 | 1.0 (0.7-1.5) | .847 |
| 1-MM 2.5-4.9 × 107/kg | 130 | Reference | |
| 2-MM 2.5-4.9 × 107/kg | 240 | 1.5 (1.1-2.1) | .014* |
| 1-MM < 2.5 × 107/kg | 67 | 1.9 (1.3-2.9) | .002* |
| 2-MM < 2.5.0 × 107/kg | 136 | 2.4 (1.7-3.4) | < .001* |
| 3-MM | 65 | 1.7 (1.1-2.6) | .020* |
| Transplant-related mortality (engrafted patients only) | |||
| 0-MM | 47 | 0.5 (0.2-1.1) | .072 |
| 1-MM > 5.0 × 107/kg | 120 | 0.8 (0.5-1.4) | .518 |
| 2-MM > 5.0 × 107/kg | 165 | 1.2 (0.8-1.9) | .452 |
| 1-MM 2.5-4.9 × 107/kg | 94 | Reference | |
| 2-MM 2.5-4.9 × 107/kg | 171 | 1.6 (1.0-2.5) | .032* |
| 1-MM < 2.5 × 107/kg | 40 | 1.9 (1.1-3.3) | .029* |
| 2-MM < 2.5.0 × 107/kg | 81 | 2.3 (1.4-3.7) | .001* |
| 3-MM | 45 | 2.1 (1.2-3.6) | .011* |
| Overall mortality | |||
| 0-MM | 56 | 0.5 (0.3-0.8) | .005* |
| 1-MM > 5.0 × 107/kg | 155 | 0.8 (0.6-1.2) | .295 |
| 2-MM > 5.0 × 107/kg | 212 | 1.0 (0.7-1.3) | .769 |
| 1-MM 2.5-4.9 × 107/kg | 130 | Reference | |
| 2-MM 2.5-4.9 × 107/kg | 240 | 1.2 (0.9-1.6) | .195 |
| 1-MM < 2.5 × 107/kg | 67 | 1.6 (1.1-2.2) | .013* |
| 2-MM < 2.5.0 × 107/kg | 136 | 1.8 (1.3-2.4) | < .001* |
| 3-MM | 65 | 1.4 (1.0-2.1) | .048* |
| Treatment failure (reciprocal of disease-free survival) | |||
| 0-MM | 56 | 0.5 (0.3-0.9) | .011* |
| 1-MM > 5.0 × 107/kg | 155 | 0.9 (0.7-1.3) | .693 |
| 2-MM > 5.0 × 107/kg | 212 | 1.0 (0.8-1.4) | .822 |
| 1-MM 2.5-4.9 × 107/kg | 130 | Reference | |
| 2-MM 2.5-4.9 × 107/kg | 240 | 1.2 (0.9-1.6) | .120 |
| 1-MM < 2.5 × 107/kg | 67 | 1.5 (1.1-2.2) | .018* |
| 2-MM < 2.5.0 × 107/kg | 136 | 1.7 (1.3-2.3) | < .001* |
| 3-MM | 65 | 1.5 (1.0-2.1) | .039* |
CBT indicates cord blood transplant; CMV, cytomegalovirus; MM, mismatch; RR, relative risk; TNC, total nucleated cells; TRM, transplant-related mortality; and US, United States.
Other predictive variables, such as white patient ancestry, recipient seronegativity for CMV, US transplant centers with greater experience, and more recent year of transplantation (2003-2006), were associated with lower TRM, treatment failure, and mortality. High-risk leukemia was associated with a worse outcome for these end points.
Statistically significant P value.
Causes of death
Sixty-two patients had an early death within the first 20 days after transplantation (cumulative incidence, 5.8%). Only high-risk acute lymphoblastic leukemia, acute myeloid leukemia, or chronic myelogenous leukemia (RR = 2.7; 95% confidence interval, 1.6-4.5; P < .001), non-US center (RR = 2.8; 95% confidence interval, 1.3-5.8) versus more experienced US centers (P < .001), and lower TNC dose (RR = 0.5; 95% confidence interval, 0.3-0.8; P = .001) were predictive of early deaths. Among subsequent deaths, the most common primary causes were attributable to relapse or progressive disease (n = 158), graft failure (n = 130), and infection (n = 154). In addition, there were 81 deaths primarily attributable to GVHD and 59 to organ failure. In multivariate analysis of deaths after day 20, only high-risk acute lymphoblastic leukemia, acute myeloid leukemia, or chronic myelogenous leukemia was predictive of death attributable to relapse or progressive disease (RR = 2.5; 95% confidence interval, 1.8-3.5; P < .001). For deaths attributed primarily to graft failure, TNC dose was predictive (as a log-transformed continuous variable, RR for increasing dose was 0.4; 95% confidence interval, 0.3-0.6, P < .001) as were center experience and year of transplantation, but HLA-mismatch was not (data not shown). HLA-mismatch was predictive of GVHD deaths: compared with a 1-MM reference group, RR for 0-MM recipients was 0.6 (95% confidence interval, 0.1-2.8, P = .540); for 2-MM recipients was 2.3 (95% confidence interval, 1.3-4.1; P = .005); and for 3-MM recipients was 1.9 (95% confidence interval, 0.7-5.3, P = .223). GVHD prophylaxis, center experience, and patient age also were predictive of GVHD deaths (data not shown). HLA-mismatch also was predictive for deaths attributed primarily to infection: compared with 1-MM recipients, RR for 0-MM recipients was 0.3 (95% confidence interval, 0.1-0.8; P = .021); for 2-MM recipients was 1.0 (95% confidence interval, 0.7-1.4, P = .794); and for 3-MM recipients was 1.9 (95% confidence interval, 1.1-3.2, P = .028). TNC dose was predictive of infection deaths (as a continuous variable RR for increasing TNC dose was 0.8; 95% confidence interval, 0.6-0.9; P = .036) as was pretransplantation recipient CMV antibody positivity (data not shown).
Discussion
Our study of the combined impact of TNC dose and HLA-match demonstrated that the best outcome for neutrophil and platelet engraftment, aGVHD, TRM, treatment failure, and overall mortality was associated with the transplantation of 0-MM (“6/6” HLA-matched) units, regardless of the TNC dose. The next best survival outcomes (TRM, treatment failure, and overall mortality) were observed in recipients of 1-MM with a TNC dose of 2.5 × 107/kg or greater or 2-MM units with a TNC dose of 5.0 × 107/kg or greater. Importantly, there was no difference in survival outcomes between 1-MM units that provided a TNC dose 2.5 to 4.9 × 107/kg and 2-MM units that provided a dose of 5.0 × 107/kg or greater. Although the latter group had more rapid engraftment associated with their greater TNC dose, this benefit was not reflected in a lower TRM. This finding may be attributed to the greater risk among recipients of 2-MM units of severe aGVHD and death from GVHD or infection, the latter observation suggesting inferior immune recovery associated with the greater level of HLA-mismatch as previously demonstrated by Szabolcs and Niedzwiecki.13
The lower TRM and greater survival seen among 0 MM CBT recipients regardless of TNC dose, as well as the comparable TRM and survival between 1-MM recipients with a TNC dose of 2.5 to 4.9 × 107/kg and those receiving 2-MM units with a dose 5.0 × 107/kg or greater supports our hypothesis that better HLA-match can compensate for lower TNC dose, a finding that has not been widely recognized. Although the converse (high TNC dose > 5.0 × 107/kg can compensate for 2 mismatches) is also true, such doses can only be achieved in children and will be associated with an increased risk of severe or lethal GVHD and death from infection. However, the extent to which better HLA-match can compensate for low TNC dose has limits. For example, when the TNC dose was less than 2.5 × 107/kg, we detected no advantage in TRM for 1-MM units over 2-MM units. Nonetheless, that HLA-match is a critical determinant of transplantation outcome is consistent with reports in transplantation of bone marrow or peripheral blood from unrelated volunteer donors.14 In particular, the benefit associated with the HLA-A, -B antigen, and -DRB1 allele-matched CB units in adolescents and adults warrants independent confirmation in a larger study, which will likely require international collaboration. Such confirmation in adults is important given the report that such grafts may be a superior HSC source to 8/8 allele-matched unrelated volunteer donors for children with leukemia.4
Notably, lower TRM was achieved with better HLA-match without any increase in relapse risk in our study population. Moreover, we found no benefit among our CBT recipients in terms of reduced relapse risk with greater mismatch. This finding suggests that the graft-versus-leukemia effect of CB grafts is mediated by mechanisms that are independent of the number of HLA-mismatches as evaluated in this study. The lack of a relationship between HLA-match and the risk of relapse is in contrast to a previous report by Gluckman and Rocha1 on behalf of Eurocord. Although the reason for this discrepancy is unclear and will require further study, our results suggest HLA-mismatched CB units need not be chosen preferentially in hopes of protecting against leukemic relapse. Rather, HLA-match should be optimized to accelerate engraftment, reduce GVHD, protect against TRM, and improve overall survival.
Taken together the results of this study suggest new unit selection guidelines, which give first priority to 0-MM units, followed by either 1-MM units with TNC greater than 2.5 × 107/kg, or 2-MM units with TNC greater than 5.0 × 107/kg. These recommendations may prompt selection of a unit with lower TNC dose but better match over a lesser-matched unit with a greater TNC dose and represent a significant change in practice for many transplant centers that now prioritize TNC dose in unit selection. They also suggest that novel strategies, such as double-unit grafts, should be used to improve CBT outcomes for patients with poor unit selections such as units that are mismatched and have TNC dose less than 2.5 × 107/kg. Further, if strategies can be used that optimize engraftment, the better-matched units with lower dose may prove to be the superior choice given they are associated with a lower risk of death from GVHD and infection. Although it is not known how the findings from this study should influence unit selection for a double-unit graft, given that only one unit is responsible for sustained donor engraftment in the majority of double-unit CBT recipients,14 the unit selection principles from our analysis of single-unit CBT are likely also relevant to double-unit CBT. Moreover, if TNC dose mainly exerts its effect by augmenting engraftment and if HLA-match is a critical determinant of the postengraftment events of GVHD and infection, one possible strategy to improve CBT outcome could be to prioritize HLA-match in unit selection but to maximize the chance of engraftment by transplanting 2 units as a double-unit graft. What TNC dose threshold would be acceptable for each unit with such an approach is unknown but should be investigated.
However, we recognize that our unit selection recommendations have some limitations. First, our study was undertaken on the basis of units from a single CB bank. Although this strategy has the advantage of standardization among units, our proposed guidelines may not apply to units from all banks, especially ones that differ significantly in their processing and freezing methodologies. In addition, TNC dose is a continuous variable that is, moreover, a distant surrogate from the engrafting stem cell. Therefore, although transplant centers need guidelines as to how to distinguish between and select units, any TNC cutoff is approximate and somewhat arbitrary. Moreover, our sample sizes remain inadequate to establish absolute TNC cutoffs for each HLA-mismatch level. Although not detected in our study, there is likely a TNC dose “threshold” for units with 0-MM. Consequently, because our study included only 5 recipients of 0-MM units with TNC less than 1.5 × 107/kg, we do not recommend such units be used at this time. In addition, it is conceivable that improved peritransplantation care, such as fludarabine-based preparative regimens and/or more effective immunosuppression, might at least partially overcome the effects of graft characteristics. Indeed patients in our study transplanted in more experienced centers or since 2002 had greater engraftment, lower TRM, and lower overall mortality.
Novel treatment approaches such as use of double-unit grafts, ex vivo expansion, or measures to augment homing3,15 also may alter the guidelines we propose from this analysis. It is also possible that some mismatches may be more detrimental than others. The importance of matching class I HLA alleles, as well as the relative importance of mismatches at class I versus class II HLA loci are of great interest, but such analyses will require larger numbers of patients. Further, additional factors beyond cryopreserved TNC dose and HLA-match can affect the engraftment potential even for some seemingly “optimal” CB units. Graft quality can be adversely affected by events at any step between unit collection and infusion. Thus, additional strategies are required to assess graft quality. These strategies could include testing of progenitor cell viability or potency from an attached segment of the CB unit before shipment or postthaw testing before infusion.
In conclusion, the critical importance of HLA-match as well as TNC dose suggests that improved survival after CBT will depend on the ability to obtain units that are both of sufficient dose and better HLA-match. Although our data support the use of units with 2-MM (“4/6” match) when they provide a high TNC dose, such units should not be considered ideal because 2-MM is associated with an increased risk of severe aGVHD. Further, it is unclear that the protective effect of increased TNC dose is sufficient to counterbalance the increased risk of infection that is mediated by 2 mismatches. Further, despite our expansion of the definition of optimal CB units (0-MM, followed by 1-MM ≥ 2.5 × 107/kg or 2-MM ≥ 5.0 × 107/kg), many patients, especially adults and larger children, remain without a suitable graft. Thus, further improvement in single unit CBT will likely require a substantial increase in the global CB inventory of high-quality units of adequate dose and sufficient diversity so that most patients can find 0- to 1-MM units. On the basis of our study, both HLA-match and TNC dose should be taken into account in setting goals for national inventories such as the US National Cord Blood Inventory and in funding public CB banks to help achieve these goals. Because patients are most likely to find good HLA-matches within their own ethnic group, CB inventories must be appropriately balanced by donor ethnicity. Moreover, because few adolescents and adults can find a unit providing a TNC dose 5.0 × 107/kg or greater, goals must seek to provide these patients with units having either 0-MM or 1-MM with a TNC dose 2.5 × 107/kg or greater (ie, a unit TNC content of at least 175 × 107 for a 70-kg adult). These goals suggest that global inventories may need to be substantially larger than previously estimated.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.N.B. designed the study and interpreted the data; A.S. and C.E.S. analyzed and interpreted the data; and J.N.B., A.S., and C.E.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Juliet N. Barker, MBBS (Hons), FRACP, Assistant Professor, Allogeneic Bone Marrow Transplantation Service, Memorial Sloan-Kettering Cancer Center, Box 259, 1275 York Ave, New York, NY 10065; e-mail: barkerj@mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal