Abstract
Acute promyelocytic leukemia (APL) is highly curable with the combination of all-trans retinoic acid (ATRA) and anthracycline-based chemotherapy (CT), but very long-term results of this treatment, when CT should be added to ATRA and the role of maintenance treatment, remain uncertain. In our APL93 trial that included 576 newly diagnosed APL patients, with a median follow-up of 10 years, 10-year survival was 77%. Maintenance treatment significantly reduced 10-year cumulative incidence of relapses, from 43.2% to 33%, 23.4%, and 13.4% with no maintenance, maintenance using intermittent ATRA, continuous 6 mercaptopurine plus methotrexate, and both treatments, respectively (P < .001). Maintenance particularly benefited patients with white blood cell (WBC) count higher than 5 × 109/L (5000/μL). Early addition of CT to ATRA significantly improved 10-year event-free survival (EFS), but without significant effect on overall survival (OS). The 10-year cumulative incidence of deaths in complete response (CR), resulting mainly from myelosuppression, was 5.7%, 15.4%, and 21.7% in patients younger than 55, 55 to 65, and older than 65 years, respectively, supporting the need for less myelosuppressive treatments, particularly for consolidation therapy. This study is registered at http://clinicaltrials.gov as NCT00599937.
Introduction
Acute promyelocytic leukemia (APL) is a distinct subtype of acute myeloid leukemia characterized by the morphology of leukemic blasts (abnormal promyelocytes),1,2 a life-threatening coagulopathy combining disseminated intravascular coagulation and fibrinolysis, specific reciprocal translocation t(15;17)3,4 fusing the promyelocytic leukemia gene (PML) to the retinoic acid receptor alfa (RARΑ), specific sensitivity to the differentiating effect of all-trans retinoic acid (ATRA), and the proapoptotic effect of arsenical derivatives.5
In APL91 trial,6 we had shown that treatment with ATRA followed by anthracycline-arabinosylcytosine (AraC) chemotherapy significantly decreased the incidence of relapse and improved survival in newly diagnosed APL, by comparison with chemotherapy alone, a finding subsequently confirmed by many studies.7-11 In the subsequent APL93 trial, conducted between 1993 and 1998, results of the second interim analysis (published in 1999 on the first 413 patients included) suggested that early addition of anthracycline-AraC chemotherapy to ATRA and maintenance treatment with continuous low-dose chemotherapy and intermittent ATRA further decreased the incidence of relapse,7 a strategy also successfully adopted by other groups.12-15
We present here the very long-term results of this study, particularly focusing on late events, with a 10-year median follow-up, the longest published to our knowledge in APL.
Methods
APL93 trial
Details of APL93 trial, approved by ethics committees in all participating countries, have been previously published.7 Inclusion criteria were (1) diagnosis of APL based on the French-American-British morphologic criteria,1,2 (2) age of 75 years or younger, and (3) written informed consent, in accordance with the Declaration of Helsinki. Diagnosis had to be subsequently confirmed by presence of t(15;17) or PML-RARα gene rearrangement.
Induction treatment was stratified by age and initial white blood cell (WBC) count. Patients 65 years or younger with WBC count lower than 5 × 109/L (5000/μL) were randomized to receive the reference treatment arm of our previous trial (APL91),6 that is, ATRA 45 mg/m2 per day followed by chemotherapy (CT), daunorubicin (DNR) 60 mg/m2 per day for 3 days, and AraC 200 mg/m2 per day as continuous infusion for 7 days (ATRA→CT group) or ATRA plus CT (ATRA+CT), where CT was started on day 3 of ATRA treatment. In the ATRA→CT group, however, course 1 was rapidly added to ATRA if the WBC count increased to greater than 6 × 109/L (6000/μL), 10 × 109/L (10 000/μL), or 15 × 109/L (15 000/μL) by days 5, 10, and 15 of ATRA treatment, respectively, because, from our previous experience patients were at risk of ATRA syndrome above those thresholds.6 After achieving complete remission (CR), patients received 2 consolidation courses of DNR and AraC (the first identical to the induction course, the second with DNR 45 mg/m2 per day for 3 days and AraC 1 g/m2 per 12 hours for 4 days).
Patients with WBC count higher than 5 × 109/L (5000/μL) at presentation (irrespective of their age) and patients aged 66 to 75 years with WBC count of 5 × 109/L (5000/μL) or lower were not randomized but received ATRA with addition of CT on day 1 of ATRA treatment (high WBC count group) and the same schedule as in the ATRA→CT group (elderly group), respectively. Patients in the elderly group received only the first consolidation course.
Hematologic CR was defined by normal bone marrow cellularity without abnormal promyelocytes, neutrophil count higher than 1.5 × 109/L (1500/μL) and platelet count higher than 100 × 109/L (100 000/μL), and no transfusion requirement. In this trial started in 1993, when reverse-transcription–polymerase chain reaction was not widely available, molecular CR was not considered. Early death was defined by death occurring during ATRA, before CR had been reached, or during or after chemotherapy, especially during the phase of aplasia, without evidence of leukemic resistance.
Patients who were still in CR after the second consolidation course were randomized to receive or not intermittent ATRA (45 mg/m2 per day, 15 days every 3 months) and to receive or not continuous CT with 6 mercaptopurine (6MP; 90 mg/m2 per day, orally) and methotrexate (MTX; 15 mg/m2 per week, orally), according to a 2-by-2 factorial design stratified on the initial induction treatment group. Maintenance treatment was scheduled for 2 years. In agreement with recommendations made for similar maintenance CT in acute lymphoblastic leukemia (ALL) for many years, blood counts were to be monitored very regularly, and investigators were asked to administer full dose of 6MP and MTX if WBC count and platelets were higher than 3.5 × 109/L (3500/μL) and 150 × 109/L (150 000/μL), respectively; to administer half dose for WBC count between 2.5 × 109/L (2500/μL) and 3.5 × 109/L (3500/μL) or platelet count between 100 × 109/L (100/μL) and 150 × 109/L (150 000/μL); and to temporarily discontinue maintenance CT if WBC count was lower than 2.5 × 109/L (2500/μL) or platelet count was lower than 100 × 109/L (100 000/μL). After a few fatal cases of sepsis were recorded, those recommendations were regularly repeated. Maintenance CT was also to be temporarily stopped if liver enzymes were more than 5 times normal values, or bilirubin was more than 2N. If liver dysfunction persisted, treatment was to be restarted at lower dose.
No systematic molecular follow-up using reverse-transcription–polymerase chain reaction analysis of PML-RAR alpha transcript was planned.
Statistical analysis
The present analysis was performed 10 years after the last patient inclusion (at the reference date of October 15, 2008), with a median follow-up of 121 months. Analysis was made on an intention-to-treat basis in the 576 patients with confirmed diagnosis of APL. For induction treatment, event-free survival (calculated from the date of initial randomization) was the primary end point, whereas for maintenance treatment the primary end point was time to relapse, measured from randomization to maintenance. Secondary end points were as follows: for induction treatment, time to relapse and survival (measured from trial inclusion); for maintenance treatment, survival.
Censored criteria, except the incidence of relapse, were analyzed with the Kaplan-Meier estimate, the log-rank test, and a Cox model.16-18 Incidence of relapse was analyzed taking into account the competing risks (ie, nonresponse and death in CR); cumulative incidences of relapse were estimated using the cmprsk package, compared across groups by the Gray test, then adjusted for confounders using the regression model proposed by Fine and Gray.19,20 A time-dependent Cox model was used to estimate the effect of maintenance therapy duration on the instantaneous hazard of relapse.
For each end point, treatment comparison was adjusted, using the appropriate regression model, on a predetermined subset of prognostic parameters including age, sex, WBC count, platelet count, absolute number of circulating blasts, APL subtype (classical vs microgranular variant APL), and fibrinogen level. Continuous covariates were entered into native form after checking for log linear relationships. We tested for interaction in the maintenance factorial design. In the absence of interaction, we decided to analyze separately each maintenance treatment, with the main analysis (adjusted or unadjusted for baseline covariates) being stratified on the other treatment. Hazard ratios (HRs) were estimated with 5% confidence intervals (95% CIs). All tests were 2 sided. All statistical analyses were carried out on SAS 9.1 (SAS Inc) and R 2.0.1 software packages.
Results
Initial characteristics of the population studied
Between April 1993 and October 1998, 576 newly diagnosed APL patients from 97 Europeans centers (listed in the supplemental Appendix, available on the Blood website; see the Supplemental Materials link at the top of the online article) were included in APL93 trial (Table 1). Median age was 46 years, including 31 children (< 18 years) and 67 patients older than 65 years included in the elderly group. A total of 215 patients with WBC count higher than 5 × 109/L (5000/μL) were included in the high WBC count group. The remaining 306 patients (randomized patients) were randomized between ATRA→CT (122 patients) and ATRA+CT (184 patients) groups. The imbalance between the 2 groups resulted from the fact that the ATRA→CT group was closed to inclusion after the second interim analysis (performed at the reference date of January 1, 1998, in the 413 patients included before January 1, 1997).7 In the ATRA→CT group, CT was added before CR achievement because of increasing WBC counts in 54% of the patients
Outcome according to initial stratification and randomization
| N (%) Median (Q1-Q3) . | Randomized patients . | Nonrandomized patients . | ||
|---|---|---|---|---|
| ATRA→CT . | ATRA+CT . | High WBC . | Elderly group . | |
| No. of patients | 122 | 184 | 215 | 55 |
| Male sex | 59 (48.4) | 87 (47.3) | 113 (52.6) | 31 (56.4) |
| Age, y (range) | 46 (35-54) | 44 (33-55) | 43 (28-54) | 69 (67-72) |
| WBC count, 109/L (range) | 1.3 (0.8-2.25) | 1.4 (0.9-2.5) | 14.8 (7.9-35.3) | 1.3 (0.9-1.9) |
| Platelet count, 109/L (range) | 40 (14-69) | 33 (18-60) | 24 (16-40) | 36 (20-71) |
| Circulating blasts, % (range) | 20 (2-48) | 17 (2-47) | 83 (65-90) | 19 (5-42.5) |
| Microgranular variant (range) | 6 (5.2) | 10 (5.7) | 62 (30.1) | 3 (5.6) |
| Fibrinogen level, g/L (range) | 19 (12-28) | 17 (12-25) | 14 (9-21) | 19 (13-33) |
| CR: N (%) | 113 (92.6) | 177 (96.2) | 195 (90.7) | 48 (87.3) |
| 10-year CI of relapse | 21.6% | 13.2% | 37.9% | 9.3% |
| 10-year CI of deaths in CR | 6.6% | 6.7% | 4.7% | 21.7% |
| 10-year EFS | 64.4% | 76.3% | 48.1% | 59.5% |
| 10-year survival | 81.8% | 85.0% | 63.1% | 58.1% |
| N (%) Median (Q1-Q3) . | Randomized patients . | Nonrandomized patients . | ||
|---|---|---|---|---|
| ATRA→CT . | ATRA+CT . | High WBC . | Elderly group . | |
| No. of patients | 122 | 184 | 215 | 55 |
| Male sex | 59 (48.4) | 87 (47.3) | 113 (52.6) | 31 (56.4) |
| Age, y (range) | 46 (35-54) | 44 (33-55) | 43 (28-54) | 69 (67-72) |
| WBC count, 109/L (range) | 1.3 (0.8-2.25) | 1.4 (0.9-2.5) | 14.8 (7.9-35.3) | 1.3 (0.9-1.9) |
| Platelet count, 109/L (range) | 40 (14-69) | 33 (18-60) | 24 (16-40) | 36 (20-71) |
| Circulating blasts, % (range) | 20 (2-48) | 17 (2-47) | 83 (65-90) | 19 (5-42.5) |
| Microgranular variant (range) | 6 (5.2) | 10 (5.7) | 62 (30.1) | 3 (5.6) |
| Fibrinogen level, g/L (range) | 19 (12-28) | 17 (12-25) | 14 (9-21) | 19 (13-33) |
| CR: N (%) | 113 (92.6) | 177 (96.2) | 195 (90.7) | 48 (87.3) |
| 10-year CI of relapse | 21.6% | 13.2% | 37.9% | 9.3% |
| 10-year CI of deaths in CR | 6.6% | 6.7% | 4.7% | 21.7% |
| 10-year EFS | 64.4% | 76.3% | 48.1% | 59.5% |
| 10-year survival | 81.8% | 85.0% | 63.1% | 58.1% |
WBC indicates white blood cells; CR, complete remission; EFS, event-free survival; and CI, cumulative incidence.
Overall long-term outcome
A total of 533 patients (92.5%) achieved CR, 42 (7.3%) had early death, and 1 (0.2%) had resistant leukemia. The CR rate was 87.3% (48/55) and 90.7% (195/215) in the elderly and the high WBC count groups, respectively, and did not significantly differ in randomized patients between the ATRA→CT (92.6%, 113/122) and ATRA+CT group (96.2%, 177/184; P = .19; Table 1).
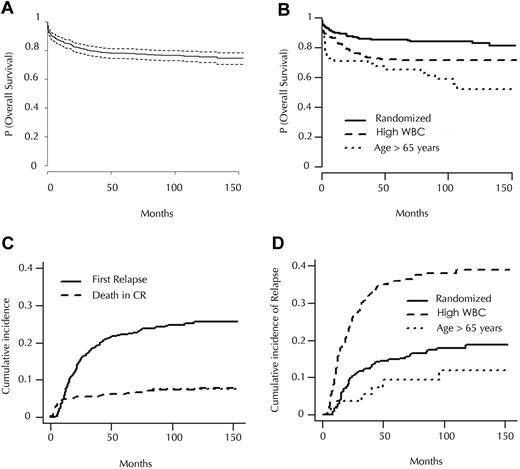
With a median follow-up of 121 months, 142 (26.6%) of the 533 patients who achieved CR had relapsed, 59 (11%) had died in CR, and 329 (61.7%) remained in first CR. Based on patient stratification at inclusion, the 10-year cumulative incidence of relapse (CIR) was 16.5% in the 306 randomized patients (patients < 65 years with WBC count < 5 × 109/L [5000/μL]), 37.9% in the high WBC count group, and 9.3% in the elderly group (P < .001). Estimated 10-year survival was 77% in the whole population, and 82%, 63.1%, and 58.1% in randomized, high WBC count, and elderly groups, respectively (P < .001; Table 1 and Figure 1).
Survival and incidence. (A) Overall survival of patients included in APL93 trial (with 95% confidence interval). (B) Overall survival according to initial stratification. (C) Cumulative incidence of relapse and of death in CR in patients included in APL93 trial who achieved CR. (D) Cumulative incidence of relapse according to initial stratification.
Survival and incidence. (A) Overall survival of patients included in APL93 trial (with 95% confidence interval). (B) Overall survival according to initial stratification. (C) Cumulative incidence of relapse and of death in CR in patients included in APL93 trial who achieved CR. (D) Cumulative incidence of relapse according to initial stratification.
Most relapses occurred before the arsenic era, and were treated with chemotherapy, combined with ATRA in 79% of the cases (only 4 relapsing patients received arsenic trioxide). As previously published by our group, 88% of the patients achieved a second CR, and 63% of them subsequently underwent autologous or allogeneic stem cell transplantation.21
Five of the first relapses (representing 3.5% of all relapses and 0.9% of the patients who achieved CR) occurred at extramedullary sites, 6 to 27 months after CR achievement (median 7), 3 with concomitant bone marrow relapse. Extramedullary relapse involved the central nervous system (n = 3), skin (n = 1), and orbit (n = 1). Baseline WBC count in those patients was 8.6 × 109/L (8600/μL), 15.5 × 109/L (15 500/μL), 31.7 × 109/L (31 700/μL), 140 × 109/L (140 000/μL), and 150 × 109/L (150 000/μL), respectively. Two of them, in whom relapse involved the central nervous system, achieved long-term salvage.
Eighteen of the first relapses occurred more than 4 years after CR achievement (“late relapses”), representing 12.7% of the relapses and 3.3% of the patients who achieved CR. Median time to late relapse was 72 months (range, 50-120 months). Nine relapses occurred after more than 6 years; 2, after more than 8 years; and the last, at 120 months. Of the 18 patients with late relapses, 17 (94%) achieved a second CR, and 4-year survival from late relapse was 77%.
Long-term outcome according to onset of chemotherapy and maintenance treatment
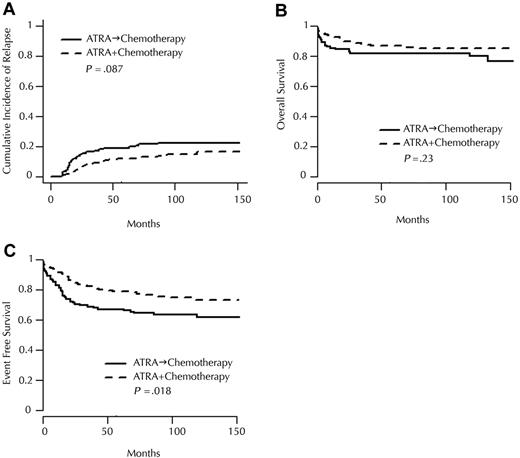
In the groups randomized at diagnosis, the 10-year CIR was 21.6% in the ATRA→CT group and 13.2% in the ATRA+CT group (P = .087; Figure 2A). The 10-year event-free survival (EFS) was 64.4% in the ATRA→CT group and 76.3% in the ATRA+CT group (P = .019; Figure 2C), and 10-year survival was 81.8% versus 85.0% in the ATRA→CT and ATRA+CT groups, respectively (P = .23; Figure 2B).
Incidence and randomization. Cumulative incidence of (A) relapse, (B) overall survival, and (C) event-free survival, according to randomization for induction treatment.
Incidence and randomization. Cumulative incidence of (A) relapse, (B) overall survival, and (C) event-free survival, according to randomization for induction treatment.
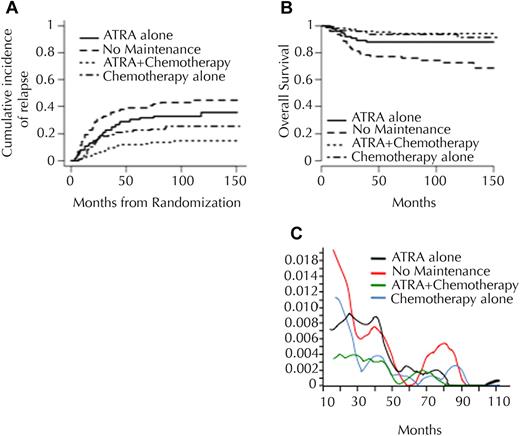
Of the 401 patients randomized for maintenance treatment, 79 were allocated to no maintenance; 76, to intermittent ATRA; 117, to continuous CT; and 129, to the combination of both (Table 2). Inclusion in groups without maintenance CT was stopped after the second interim analysis, causing the imbalance. The 10-year CIR was 43.2%, 33%, 23.4%, and 13.4%, after no maintenance, maintenance with ATRA alone, maintenance with CT alone, and both, respectively (P < .001; Table 2 and Figure 3). After adjustment on induction arm, a significant benefit of maintenance CT (P < .001) and maintenance ATRA (P = .012) was observed. Only 18 relapses were seen in the 129 patients randomized to receive both CT and ATRA maintenance. Maintenance with CT significantly improved survival (10-year survival: 85.2% vs 79.2%; P = .02) but survival was not significantly modified by maintenance with ATRA (10-year survival: 82.7% vs 79.4%; P = .44). Analysis of the instantaneous hazard of relapse over time showed that maintenance treatment, particularly with 6MP and MTX CT, reduced the incidence of relapse during the first 3 to 4 years of follow-up, without increasing the risk of later relapses (Figure 3D).
Long-term outcome according to randomization for maintenance treatment
| . | ATRA . | No maintenance . | ATRA+CT . | CT alone . | P . |
|---|---|---|---|---|---|
| No. of patients | 76 | 79 | 129 | 117 | |
| Relapse | |||||
| No. | 26 | 35 | 18 | 29 | < .001 |
| 10-year CI | 33.0% | 43.2% | 13.4% | 23.4% | |
| EFS | |||||
| Events | 28 | 40 | 22 | 33 | < .001 |
| 10-year EFS | 62.9% | 51.4% | 79.7% | 72.6% | |
| Survival | |||||
| Deaths | 9 | 22 | 7 | 8 | < .001 |
| 10-year OS | 88.0% | 74.4% | 94.4% | 93.4% |
| . | ATRA . | No maintenance . | ATRA+CT . | CT alone . | P . |
|---|---|---|---|---|---|
| No. of patients | 76 | 79 | 129 | 117 | |
| Relapse | |||||
| No. | 26 | 35 | 18 | 29 | < .001 |
| 10-year CI | 33.0% | 43.2% | 13.4% | 23.4% | |
| EFS | |||||
| Events | 28 | 40 | 22 | 33 | < .001 |
| 10-year EFS | 62.9% | 51.4% | 79.7% | 72.6% | |
| Survival | |||||
| Deaths | 9 | 22 | 7 | 8 | < .001 |
| 10-year OS | 88.0% | 74.4% | 94.4% | 93.4% |
Incidence and maintenance. Cumulative incidence of (A) relapse and (B) overall survival, according to second randomization (maintenance treatment). (C) Instantaneous hazard of relapse over time according to randomization for maintenance treatment.
Incidence and maintenance. Cumulative incidence of (A) relapse and (B) overall survival, according to second randomization (maintenance treatment). (C) Instantaneous hazard of relapse over time according to randomization for maintenance treatment.
In patients with WBC count higher than 5 × 109/L (5000/μL), the 10-year CIR was 68.4%, 53.1%, 32.8%, and 20.6% with no maintenance, ATRA alone, CT alone, and both, respectively (P < .001). In patients with WBC count less than 5 × 109/L (5000/μL), the 10-year CIR was 29.2%, 22.9%, 21.0%, and 11.5% with no maintenance, ATRA alone, CT alone, and both, respectively (P = .069). Survival after relapse was not influenced by previous maintenance treatment, with a 2-year overall survival after relapse of 50.4%, 38%, 51.5%, and 50% in patients randomized to no maintenance, ATRA alone, CT alone, or both, respectively (P = .86), although most relapses occurred before the arsenic era and therefore that patients were generally salvaged by anthracycline-based chemotherapy, with or without ATRA.21
Of the 322 patients allocated to a maintenance treatment arm (ATRA, CT, or both) 274 (85.1%) actually received at least one complete cycle of maintenance. Their median maintenance treatment duration was 24 months (Q1-Q3: 14-24); 41 of them received maintenance treatment for less than a year because of side effects or a patient or physician's decision. Sixty relapses were observed in the 274 patients, with hazard of relapse significantly decreased in those who received maintenance treatment for more than a year (HR = 0.16, 95% CI: 0.10-0.28; P < .001) compared with less than 1 year. Results were similar when the analysis was restricted to patients who received maintenance low-dose chemotherapy (with or without ATRA maintenance).
Side effects of treatment and long-term complications
Of the 533 patients who achieved CR, 59 (11%) died in first CR. Causes of death in CR are listed in Table 3: 23 deaths occurred during consolidation treatment; 10, during maintenance treatment; and 26, after the end of maintenance treatment. Sepsis was the leading cause of death in CR (n = 23), followed by secondary tumors (n = 10, including 1 myelodysplastic syndrome [MDS]). Among the 23 fatal infections, 17 occurred during the phase of myelosuppression that followed consolidation courses, but 6 occurred during maintenance treatment. In the last patients 2 were receiving maintenance with CT alone and 4, maintenance with CT and ATRA, constituting 2.5% of the patients randomized to maintenance CT (with or without ATRA). Those 6 patients had been included during the first 2 years of the trial, and were all profoundly neutropenic when fatal infection occurred.
Causes of death in CR in patients included in APL93 trial
| . | n . | Cause . | % . |
|---|---|---|---|
| During consolidation courses | 23 | Sepsis, n = 16 | 70 |
| Bleeding, n = 1 | 4 | ||
| Heart failure, n = 1 | 4 | ||
| Unknown, n = 4 | 18 | ||
| Tumor unrelated to APL,* n = 1 | 4 | ||
| During the maintenance treatment phase | 10 | Sepsis, n = 6 | 60 |
| Heart failure, n = 2 | 20 | ||
| Sudden death, n = 1 | 10 | ||
| Unknown, n = 1 | 10 | ||
| After maintenance | 26 | Sepsis, n = 1 | 4 |
| Other tumor, n = 8 | 30 | ||
| Heart failure, n = 2 | 8 | ||
| Sudden death, n = 2 | 8 | ||
| Liver cirrhosis, n = 1 | 4 | ||
| MDS, n = 1 | 4 | ||
| Unrelated to APL, n = 11 | 42 |
| . | n . | Cause . | % . |
|---|---|---|---|
| During consolidation courses | 23 | Sepsis, n = 16 | 70 |
| Bleeding, n = 1 | 4 | ||
| Heart failure, n = 1 | 4 | ||
| Unknown, n = 4 | 18 | ||
| Tumor unrelated to APL,* n = 1 | 4 | ||
| During the maintenance treatment phase | 10 | Sepsis, n = 6 | 60 |
| Heart failure, n = 2 | 20 | ||
| Sudden death, n = 1 | 10 | ||
| Unknown, n = 1 | 10 | ||
| After maintenance | 26 | Sepsis, n = 1 | 4 |
| Other tumor, n = 8 | 30 | ||
| Heart failure, n = 2 | 8 | ||
| Sudden death, n = 2 | 8 | ||
| Liver cirrhosis, n = 1 | 4 | ||
| MDS, n = 1 | 4 | ||
| Unrelated to APL, n = 11 | 42 |
Relapse of a carcinoma which was in complete remission at the time of inclusion.
If one excludes MDSs, 13 patients developed secondary tumors (non-Hodgkin lymphoma, n = 2; esophageal carcinoma, n = 2; lung carcinoma, n = 2; liver carcinoma, n = 2; others, n = 5), 1 to 9.6 years (median, 6.5 years) after diagnosis of APL. Cumulative incidence of secondary tumors was 1.4% at 5 years and 2.7% at 10 years, similar to the incidence expected in the French general population (Institut National de Veille Sanitaire, data from year 2000). Only 1 of the 3 patients who died from liver carcinoma or cirrhosis had received maintenance treatment with 6MP and MTX. Ten patients developed MDS, 13 to 74 months (median, 46 months) after the diagnosis of APL. The cumulative incidence of MDS was 0.2% at 5 years, and 1.1% at 10 years.
Deaths of cardiac origin were reported in 5 patients, 1 during consolidation treatment, 2 during maintenance treatment, and 2 after the end of maintenance. Their median age was 59.1 years (range, 43-73 years). No details about cardiovascular risk factors were available in those patients. No case of late cardiac failure unexplained by usual causes (coronary artery disease, hypertension, valvular disease, etc) was reported, but no systematic analysis of cardiac function was made during follow-up. In addition, 3 unexplained “sudden deaths” were reported in the trial or after the end of treatment (Table 3).
If one excludes deaths in CR that clearly appeared unrelated to APL or its treatment (considering that treatment, in addition to deaths from sepsis or bleeding, was also potentially responsible for the occurrence of solid tumors, MDS, cardiac failure, and liver cirrhosis), the 10-year cumulative incidence of death in CR was 5.7%, 15.4%, and 21.7% in patients younger than 55 years, between 55 and 65 years, and older 65 years, respectively.
Main side effects of maintenance treatment were cytopenias and liver enzyme elevation. In patients who received maintenance CT alone or maintenance with ATRA+CT, 36% had to reduce and/or transiently stop maintenance treatment because of cytopenias. Increases in liver enzymes leading to dose reduction of chemotherapy and/or ATRA were seen in 25% of the cases with ATRA, 35% with CT, and 34% with both maintenance treatments. Overall, 45% of the patients experienced either liver and/or hematologic toxicity during the maintenance phase, leading to dose reduction of chemotherapy. In addition to the 6 fatal cases of sepsis during maintenance, hospitalization for febrile neutropenia was reported in 6 patients, including 3 cases of nonfatal Pneumocystis carinii pneumonia that occurred 5, 13, and 19 months after starting maintenance CT. Cytopenias were not observed with maintenance using ATRA alone. Finally, 1 case of myositis with ATRA was reported.
Discussion
Results of APL93 trial have been published after the second interim analysis only, in 1999, on the first 413 patients included.7 Updated results of the US intergroup study and of the PETHEMA group LPA 99 study have since confirmed, with a 5.2-year and 5.6-year follow-up, respectively, the long-term high antileukemic efficacy of ATRA combined with chemotherapy in newly diagnosed APL.8,22 Our updated results of APL93 trial, with a median follow-up of 10 years, confirmed those favorable results, but also results of randomized end points of this trial regarding onset of chemotherapy and maintenance treatment.
We indeed confirmed that early addition of anthracycline-AraC chemotherapy to ATRA significantly improved EFS. This benefit appeared to result from the combination of a trend toward smaller 10-year CIR (21.6% in the ATRA→CT group vs 13.2% in the ATRA+CT group) and a slight increase in CR rate, from 92.6% with ATRA→CT to 96.2% with ATRA+CT. Those findings suggest that the combined effect of ATRA and CT on the leukemic clone may be optimal when the 2 treatments are administered together. This better EFS and the lower incidence of the leukocyte activation syndrome (“ATRA syndrome”) we previously reported in the ATRA+CT group in APL93 trial23 strongly argue for early addition of chemotherapy to ATRA, even in patients with low WBC counts. On the other hand, this benefit did not translate into a significant survival improvement.
We also confirmed with much longer follow-up the beneficial effect of maintenance treatment with intermittent ATRA and continuous 6MP+MTX, with an additive effect of the 2 modalities. Longer follow-up demonstrated that maintenance treatment reduced the incidence of early relapses, without increasing that of late relapses. Patients with WBC count higher than 5 × 109/L (5000/μL) particularly benefited from maintenance therapy, the relapse rate dropping from 68.4% with no maintenance to 20.6% with combined (ATRA+CT) maintenance. In patients with WBC counts lower than 5 × 109/L (5000/μL), the long-term benefit appeared less important, with a 10-year CIR reduced from 29.2% without maintenance to 11.5% with combined maintenance (P = .069). Three other published studies, with shorter follow-up, randomized maintenance treatment in APL. The US intergroup study showed that maintenance treatment with continuous ATRA during one year significantly reduced relapses and improved survival.7,12 On the other hand, the Japan Adult Leukemia Study Group (JALSG) suggested that, after induction treatment with ATRA, idarubicin, and AraC, and consolidation treatment with 3 intensive cycles of chemotherapy, intensified maintenance therapy with 6 courses combining behenoyl AraC and (as alternating agents) DNR and 6MP, mitoxantrone, VP 16 and vindesine, aclarubicin and 6MP, respectively, did not improve DFS. Recently updated Italian GIMEMA group results, using the same randomization design as in APL93 for maintenance treatment (ie, no maintenance vs 6MP+MTX versus ATRA versus both), also found no difference in relapses among maintenance arms.24,25 The benefit of maintenance treatment may in fact depend on prior induction and consolidation therapy. For example, both GIMEMA and JALSG groups used idarubicin as anthracycline for induction and consolidation chemotherapy, whereas our study and the US intergroup study, which showed a large benefit for maintenance, used DNR, an anthracycline that may be less effective than idarubicin in APL, although this remains disputed.26
Our results also showed a significant increase in the hazard of relapse in patients who received maintenance treatment for less than 1 year (for reasons other than relapse). Although duration of maintenance was not randomized, this result suggests that maintenance treatment should be administered for longer than 1 year. Those findings may be reminiscent of what is observed in ALL, especially in children, where sufficiently prolonged maintenance treatment with 6MP and MTX is an important component of the treatment protocol.27
A major complication of our treatment approach was the 11% deaths in CR (if causes that were unrelated to APL or its treatment were excluded). The major cause of death was sepsis secondary to myelosuppression, especially after consolidation courses and in elderly patients, although they received only one consolidation course beyond the age of 65 years. To reduce the incidence of deaths in CR, several groups have reduced the intensity of consolidation chemotherapy, especially in older patients. In PETHEMA group LPA 99 study, in which patients received ATRA and anthracyclines without AraC, the incidence of deaths in CR was reduced to 1.3%.26 This was not associated with an increase in relapse in standard-risk APL, whereas in high-risk APL (with WBC count > 10 × 109/L [10 000/μL]) the CIR was higher than of comparative patients treated with AraC, suggesting that AraC may be useful in high-risk patients.26 Introduction of arsenic compounds, which are not myelosuppressive, during first-line treatment of APL, may allow for reduction of the total amount of chemotherapy while limiting the risk of relapse, especially in patients with high WBC counts, as supported by recent results of a US intergroup study.28
Also of note is that 6 deaths due to sepsis occurred during maintenance treatment with 6MP and MTX, all during the first 2 years of the trial and in patients who were profoundly neutropenic, confirming that treatment with this combination requires close monitoring and dose adjustments in case of cytopenias. After those fatal cases, recommendations for close monitoring of blood counts, especially during the first months of treatment, were regularly repeated, and no more fatal neutropenic episodes were seen. Finally, we observed 3 cases of P carinii pneumonia in patients receiving 6MP+MTX maintenance chemotherapy and no Pneumocystis carinii pneumonia prophylaxis. Those patients, like ALL patients who receive the same maintenance with 6MP+MTX, may be at risk for this infection, possibly justifying systematic prophylaxis.
With long-term follow-up, no late cardiac toxicity was seen despite the relatively high cumulative dose of anthracyclines used (495 and 315 mg/m2 in patients younger than and older than 65 years). However no systematic evaluation of cardiac function was performed during follow-up. Furthermore, 3 patients had sudden death mainly during follow-up, and some of those deaths may have been due to cardiac complications. Finally, the cumulative incidence of MDS we observed was lower than that initially reported by the GIMEMA group,29 but similar to what was subsequently reported by most groups,22,30 suggesting that MDS is not a frequent complication of APL.
In conclusion, with a median follow-up of 10 years, our results confirm that the combination of ATRA and chemotherapy can cure at least three-quarters of APL patients. The results also show the long-term usefulness of sufficiently prolonged maintenance treatment, particularly in patients with initial WBC counts higher than 5 × 109/L (5000/μL) and, in patients with WBC counts lower than 5 × 109/L (5000/μL), the probable benefit of early addition of chemotherapy to ATRA. Very few long-term complications were seen. However, reduction of the incidence of deaths in CR in this highly curative disease is mandatory and requires reduction in the use of myelosuppressive drugs. Arsenic derivatives appear to have a growing place in this situation, either alone or, more frequently, combined with ATRA, with or without limited doses of chemotherapy. Several recent studies using such approaches have yielded, in the first-line treatment of APL, similar results as more classical ATRA chemotherapy protocols (such as the present APL93 trial).28,31,32 On the other hand, some of those recent studies were performed mainly in experienced centers, and their results may have to be confirmed on a large multicenter basis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by the Programme Hospitalier de Recherche Clinique and the Centre Hospitalier Universitaire (CHU) of Lille, France, the Association pour la Recherche sur le Cancer, and the Ligue contre le cancer (Comité du Nord).
Authorship
Contribution: L.D., S. Chevret, and P.F. conceived the study; L.A., S. Chevret, and P.F. collected the data; L.A. and P.F. wrote the paper; S. Chevret performed the statistical analysis; and L.A., A.G., E.R., M.S., P.C., S.L., C. Recher, X.T., C. Rayon, S. Castaigne, O.T., S.d.B., N.I., J.-Y.C., E.S., C.G., N.F. D.B., A.F., S.M.-M., N.V., and H.D. included the patients and collected the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of European APL Group participants appears in the supplemental Appendix.
Correspondence: Pierre Fenaux, Hôpital Avicenne–Service d'hématologie clinique-Paris 13 university, Assistance Publique-Hopitaux de Paris (AP-HP), 125 rue de Stalingrad, 93009 Bobigny, France; e-mail: pierre.fenaux@avc.aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal