Abstract
Easily reproducible animal models that allow for study of the biology of chronic lymphocytic leukemia (CLL) and to test new therapeutic agents have been very difficult to establish. We have developed a novel transplantable xenograft murine model of CLL by engrafting the CLL cell line MEC1 into Rag2−/−γc−/− mice. These mice lack B, T, and natural killer (NK) cells, and, in contrast to nude mice that retain NK cells, appear to be optimal recipient for MEC1 cells, which were successfully transplanted through either subcutaneous or intravenous routes. The result is a novel in vivo model that has systemic involvement, develops very rapidly, allows the measurement of tumor burden, and has 100% engraftment efficiency. This model closely resembles aggressive human CLL and could be very useful for evaluating both the biologic basis of CLL growth and dissemination as well as the efficacy of new therapeutic agents.
Introduction
Animal models of chronic lymphocytic leukemia (CLL) have been remarkably difficult to establish. At present, the most popular CLL model is the transgenic mouse obtained by inserting the human Tcl-1 gene under the control of the immunoglobulin heavy chain variable region promoter and immunoglobulin heavy chain enhancer (EμTcl-1) that develops a CLL-like disease after a long period of time (13-18 months).1 Xenotransplantation of human malignant cells into immunodeficient mice has been successfully used for the study of acute leukemias,2-4 chronic myeloid leukemia,5 and multiple myeloma.6 The development of xenograft models in CLL has been severely limited by inefficient7 or short-term8 engraftment. The infusion of human primary CLL cells into immunodeficient nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice by combined intravenous and intraperitoneal injection9 has resulted in highly localized splenic and peritoneal engraftment, but the recovery of leukemic cells from bone marrow (BM) or peripheral blood (PB) has been substantially low.9
We reasoned that a profound immunosuppression such as that observed in Rag2−/−γc−/− mice, which lack not only B cells and T cells but also natural killer (NK) cells, might help overcome part of the limitations so far encountered. In addition, we used an established CLL cell line, MEC1,10 to improve the model reproducibility.
We here report the successful establishment of a novel xenograft model that closely resembles aggressive disseminated human CLL, recapitulates the temporal and spatial distribution of the disease, and is conceivably very useful for investigating the biologic basis of CLL growth and for testing the efficacy of new therapeutic agents.
Methods
Mice, tumor cell lines, and reagents
Rag2−/−γc−/− and nu/nu mice on BALB/c background were maintained in a specific pathogen–free animal facility, treated in accordance with the European Union guidelines and with the approval of the Institutional Ethical Committee of the Istituto Scientifico San Raffaele. MEC1 cell line was obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DMSZ) and cultured in RPMI 1640 medium (Invitrogen) with 10% fetal bovine serum and gentamicin (15 μg/mL; Sigma-Aldrich). Fludarabine was obtained from Bayer and cyclophosphamide from Baxter, and they were used at the concentrations reported.
Generation of a stable CLL cell line expressing GFP
We used BLOCK-iT Pol II miR-RNAi Expression Vector Kits (pcDNA 6.2-GW/EmGFP-miR vector) for vector-based expression of selected miRNA (Invitrogen) according to the manufacturer's instructions. MEC1 cells were transfected with Nucleofactor technology (AMAXA) using program X-001, V solution, cultured under antibiotic selection (blasticidin; Invitrogen), and subsequently cell sorted for green fluorescent protein (GFP) expression to obtain a stable cell line, using a cell-sorted FACSVantage SE Cell Sorter (BD Biosciences).
In vivo studies
Eight-week-old Rag2−/−γc−/− male mice were challenged intravenously or subcutaneously in the left flank with 10 × 106 MEC1 cells (with or without GFP expression) in 0.1 mL of saline through a 27-gauge needle. In selected experiments, 21 days later, mice bearing subcutaneous tumor were intraperitoneally injected, every 2 weeks, with fludarabine (34 mg/kg) for 5 days or fludarabine (0.625 mg/kg) and cyclophosphamide (6.25 mg/kg) for 3 days or with phosphate buffer solution (PBS-saline), as control.11
Animals injected subcutaneously were monitored twice a week for weight and tumor growth (measuring 3 perpendicular diameters) and killed when the mean tumor volume reached 1000 mm3 or larger before reaching clinical signs and symptoms, to avoid unnecessary pain and discomfort according to the ethical guidelines. Subcutaneous tumor, PB, and organs (spleen, lymph nodes, kidneys, liver, lungs, and femoral bone marrow) were analyzed. After blocking fragment crystallizable receptors for 10 minutes at room temperature to avoid nonspecific binding of antibodies, cells from PB, BM, peritoneal exudates, and spleen were stained with phycoerythrin–cyanin 7–labeled antihuman CD19 antibody (Beckman Coulter) and analyzed with a Beckman Coulter FC500 flow cytometer. Organs were formalin-fixed, paraffin-embedded, cut at 5-μm–thick sections, and stained with hematoxylin and eosin. Histologic sections were evaluated in a double-blinded fashion by one of us (M.P.). Immunophenotyping was carried out using anti–human CD19, CD20, CD23 (Novocastra), and Ki-67 (Dako) monoclonal antibodies after antigen retrieval, using an automatized immunostainer.
Slides of different organs containing GFP-expressing MEC1 cells were fixed with 4% paraformaldehyde and permeabilized with Triton-X 100 0.1% (Sigma-Aldrich). The slides were incubated with primary antibody rabbit anti-GFP (1:200 dilution; Molecular Probes) and stained with secondary antibody anti–rabbit Alexa Fluor 488 (1:500 dilution). We used TO-PRO3 (Invitrogen) for the nuclear staining. Cells were acquired using confocal microscopy (Radiance 2100; Bio-Rad) dual laser (excitation wavelengths, 488 and 633 nm) with an inverted ×40 objective. Fluorescent signals from single optical sections were sequentially acquired and analyzed by Paint Shop Pro 7.02 (Jasc Software).
In vitro cytotoxicity assay
MEC1 cells were seeded in 48-well plates at a concentration of 5 × 105 cells/mL in 0.2 mL of RPMI. Increasing concentrations of fludarabine (0-100 μg/mL) were added, and, 72 hours later, cell viability was assessed by Celltiter-Glo Luminescent assay (Promega).
Statistical analysis
Statistical analyses were performed using the Student t test. Data were expressed as the mean plus or minus SD, and comparison of growth curves was considered statistically significant for P values less than .05. Half maximal effective concentration (EC50) was calculated using Z-Prism (GraphPad Software).
Results
Establishment of a Rag2−/−γc−/− CLL xenograft model
To determine MEC1 tumorigenicity, 10 × 106 cells were injected subcutaneously into the left flank of adult Rag2−/−γc−/− mice (at least 4 animals/experiment repeated twice). One week after injection, rapidly growing tumors occurred in both male (Figure 1A) and female mice, with no differences between the sexes. In all injected animals, the cells grew as a solid discoid subcutaneous mass that could show macroscopically evident central areas of necrosis. Due to ethical requirements, all injected mice were killed when the tumor volume reached 1000 mm3, usually before 45 days after injection, in all cases before the appearance of symptoms and signs of disease. Upon killing, a macroscopic analysis of different tissues showed an enlargement of spleen, liver, lung, kidney, and the tumor-draining axillary lymph node (Figure 1B). Immunofluorescence analysis confirmed that tumor cells substantially localized in all the tissues (Figure 2A-F). Mice also presented a distinct expansion of CD19+ leukemic cells in the PB (Figure 1C) and the peritoneal cavity (Figure 1D).
MEC1 cells injected subcutaneously grow and substantially localize in several organs of Rag2−/−γc−/− mice. (A) Rag2−/−γc−/− male mice received a transplant subcutaneously in the left flank of MEC1 cells with or without GFP expression (10 × 106 cells/mouse; n = 4, repeated twice), showing no significant difference in tumor engraftment. Tumor size was evaluated by measuring perpendicular diameters by a caliper. Animals were killed when the tumor volume reached 1000 mm3. (B) Organs from these animals were collected and analyzed macroscopically. (C) Peripheral blood was extracted from Rag2−/−γc−/− male mice injected subcutaneously with MEC1 cells expressing GFP and killed when the tumor volume reached a dimension of 1000 mm3. The dot plot analysis shows the intensity of the staining with monoclonal antibody (mAb) against human CD19, after gating on the GFP+ leukemic B-cell population. (D) Peritoneal exudate was extracted from Rag2−/−γc−/− male mice injected subcutaneously with MEC1 cells expressing GFP and killed when the tumor volume reached a dimension of 1000 mm3. The dot plot analysis shows the intensity of the staining with mAb against human CD19, after gating on the GFP+ leukemic B-cell population.
MEC1 cells injected subcutaneously grow and substantially localize in several organs of Rag2−/−γc−/− mice. (A) Rag2−/−γc−/− male mice received a transplant subcutaneously in the left flank of MEC1 cells with or without GFP expression (10 × 106 cells/mouse; n = 4, repeated twice), showing no significant difference in tumor engraftment. Tumor size was evaluated by measuring perpendicular diameters by a caliper. Animals were killed when the tumor volume reached 1000 mm3. (B) Organs from these animals were collected and analyzed macroscopically. (C) Peripheral blood was extracted from Rag2−/−γc−/− male mice injected subcutaneously with MEC1 cells expressing GFP and killed when the tumor volume reached a dimension of 1000 mm3. The dot plot analysis shows the intensity of the staining with monoclonal antibody (mAb) against human CD19, after gating on the GFP+ leukemic B-cell population. (D) Peritoneal exudate was extracted from Rag2−/−γc−/− male mice injected subcutaneously with MEC1 cells expressing GFP and killed when the tumor volume reached a dimension of 1000 mm3. The dot plot analysis shows the intensity of the staining with mAb against human CD19, after gating on the GFP+ leukemic B-cell population.
MEC1 cells injected subcutaneously localize in several organs of Rag2−/−γc−/− mice. Organs from Rag2−/−γc−/− male mice injected subcutaneously with MEC1 cells expressing GFP and killed when the tumor volume reached a dimension of 1000 mm3 were collected and analyzed by confocal microscopy (spleen, kidney, liver, axillary tumor-draining lymph node, bone marrow, and tumor represented in panels A, B, C, D, E, and F, respectively). The extent and pattern of invasion is demonstrated by the presence of green GFP+ leukemic cells. Cell nuclei were stained with TO-PRO3 showing in red.
MEC1 cells injected subcutaneously localize in several organs of Rag2−/−γc−/− mice. Organs from Rag2−/−γc−/− male mice injected subcutaneously with MEC1 cells expressing GFP and killed when the tumor volume reached a dimension of 1000 mm3 were collected and analyzed by confocal microscopy (spleen, kidney, liver, axillary tumor-draining lymph node, bone marrow, and tumor represented in panels A, B, C, D, E, and F, respectively). The extent and pattern of invasion is demonstrated by the presence of green GFP+ leukemic cells. Cell nuclei were stained with TO-PRO3 showing in red.
Histopathologic evaluation of the tumors in each examined organ showed either a diffuse pattern or focal, discrete aggregates composed of medium-to-large lymphocytes, with clumped chromatin, and round and evident nucleoli (Figure 3A-D). The tumoral lymphocytes disseminated in the organs, and in particular in the kidney, had a homogeneous distinctive immunoreactivity for human CD19 (Figure 3C) and CD20 (Figure 3D) and were CD23+ (Figure 3E), with a substantial proliferation index (Figure 3F).
Histopathologic analysis of organ localizations. Histopathologic analysis of the kidney from Rag2−/−γc−/− male mice injected subcutaneously with MEC1 cells showed discrete collections of tumoral lymphocytes (A and B: H&E staining, magnification ×200 and ×400, respectively). These neoplastic nodules displayed homogeneous distinctive immunoreactivity for human CD19 (C), CD20 (D), and CD23 (E), with a substantial proliferation index (F: Ki67).
Histopathologic analysis of organ localizations. Histopathologic analysis of the kidney from Rag2−/−γc−/− male mice injected subcutaneously with MEC1 cells showed discrete collections of tumoral lymphocytes (A and B: H&E staining, magnification ×200 and ×400, respectively). These neoplastic nodules displayed homogeneous distinctive immunoreactivity for human CD19 (C), CD20 (D), and CD23 (E), with a substantial proliferation index (F: Ki67).
As it was previously reported that the CLL cell line MEC2 failed to engraft into nude mice12 and to confirm the relevance of the recipient, we injected subcutaneously MEC1 cells into 8-week-old nu/nu male mice (n = 5). No measurable tumor growth was observed (data not shown).
Influence of inoculation route on tumor engraftment
To address the potential influence of the inoculation route, 10 × 106 MEC1 cells expressing GFP (Figure 4A) were injected intravenously into Rag2−/−γc−/− male mice (n = 3). Mice were monitored daily and killed after 2 weeks. Flow cytometric studies confirmed the presence of a leukemic expansion of CD19+/GFP+ cells in the spleen, BM, PB, and peritoneal exudates (Figure 4B-E), as well as in the liver, lungs, and kidneys (data not shown). As also shown in tumor cells from mice injected subcutaneously (data not shown), the leukemic B cells recovered from animals challenged intravenously consistently and reproducibly showed downmodulation of CD19 expression, at variable levels, in all tumor localizations (Figure 4B-E). In addition, CD20 and CD23 expression was also modulated at variable extent in all organs examined compared with the initial levels (data not shown).
MEC1 cells injected intravenously localize in several organs of Rag2−/−γc−/− mice. MEC1 cells expressing GFP (A) were injected intravenously into Rag2−/−γc−/− male mice (n = 3) and killed 2 weeks after injection. Cells collected from spleen (B), peripheral blood (C), bone marrow (D), and peritoneal cavity (E) were analyzed by flow cytometry after staining with mAb against human CD19 to identify the GFP+ leukemic B-cell population.
MEC1 cells injected intravenously localize in several organs of Rag2−/−γc−/− mice. MEC1 cells expressing GFP (A) were injected intravenously into Rag2−/−γc−/− male mice (n = 3) and killed 2 weeks after injection. Cells collected from spleen (B), peripheral blood (C), bone marrow (D), and peritoneal cavity (E) were analyzed by flow cytometry after staining with mAb against human CD19 to identify the GFP+ leukemic B-cell population.
Characterization of the Rag2−/−γc−/− xenograft model of CLL as a preclinical tool for drug testing
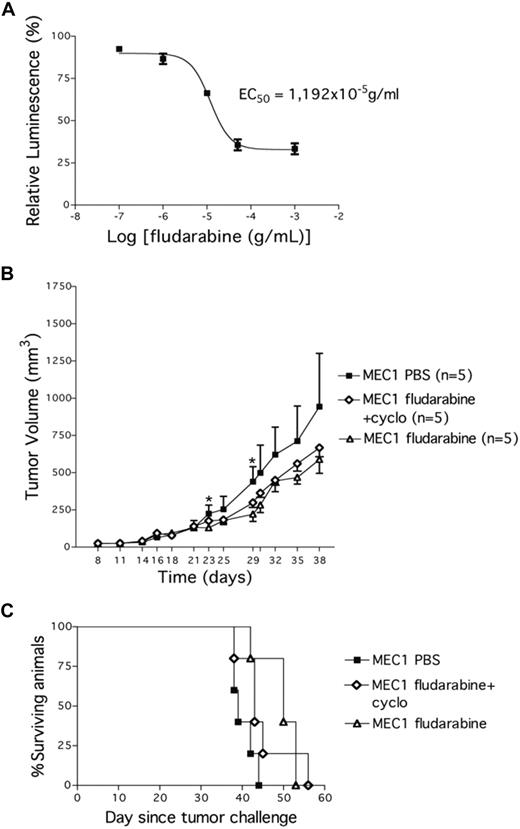
We next investigated whether this CLL xenograft model could be a reliable preclinical tool for drug investigation. First, we cultured MEC1 cells in vitro to study sensitivity to fludarabine, a well-established agent for the treatment of CLL. Leukemic cells were killed in vitro in a dose-related manner, with a half maximal effective concentration of approximately 11.9 μg/mL (Figure 5A). To then test the in vivo sensitivity to fludarabine, MEC1 cells were injected subcutaneously into the left flank of Rag2−/−γc−/− male mice. Twenty-one days later, when tumors had reached a mean volume of 137.2 (± 49 mm3), the animals were randomly assigned to one of the following systemic treatments: PBS, fludarabine alone (for 5 days), or fludarabine in combination with cyclophosphamide (for 3 days). Each treatment was repeated every 2 weeks and animals were monitored for tumor growth. Both fludarabine alone and the combined fludarabine/cyclophosphamide treatment significantly reduced MEC1 tumor growth compared with control mice (PBS vs fludarabine: P = .012; PBS vs fludarabine/cyclophosphamide: P = .03; day 29); fludarabine alone was effective earlier (day 23: P = .017; n = 5; Figure 5B). Repeated treatments with fludarabine alone or in combination did not modify the animal weight compared with control PBS-injected animals thereby excluding drug-related toxicity. All animals were killed when the tumor volume reached 1000 mm3. We observed a significant survival extension in mice treated with fludarabine compared with PBS-treated animals (PBS vs fludarabine: P = .047; Figure 5C), closely resembling the response to fludarabine treatment reported in Tcl-1 transgenic mice treated in a similar manner.11
Fludarabine impacts on the growth of MEC1 cells in vitro and in vivo. (A) MEC1 cells were plated in vitro in 48-well plates with increasing concentrations of fludarabine and a luminescent assay was performed 72 hours later to demonstrate MEC1 cells' sensitivity to the drug. EC50 indicates half maximal effective concentration (11.9 g/mL). (B) Tumor growth curves obtained in Rag2−/−γc−/− male mice that received a transplant subcutaneously in the left flank of MEC1 cells (10 × 106). Twenty-one days later, mice bearing MEC1 tumor were randomly assigned to one of the following intraperitoneal treatments (5 animals/group): saline solution (PBS), fludarabine alone (34 mg/kg) daily on days 21 to 25, or fludarabine (0.625 mg/kg) + cyclophosphamide (6.25 mg/kg) daily on days 21 to 23. The treatment was repeated 3 times every 2 weeks. Tumor size was evaluated by measuring perpendicular diameters by a caliper. Animals were killed when the tumor volume reached 1000 mm3. Measurements were stopped when 75% of originally treated mice were still surviving. *Statistically significant differences calculated using the Student t test: day 23, PBS versus fludarabine, P = .01; day 29, PBS versus fludarabine, P = .012, and PBS versus fludarabine + cyclophosphamide, P = .038. Data are representative of at least 2 independent experiments. (C) Kaplan-Meier survival curves for Rag2−/−γc−/− male mice challenged subcutaneously in the left flank with MEC1 cells. Twenty-one days later, mice bearing MEC1 tumor were randomly assigned to one of the following intraperitoneal treatments (5 animals/group): PBS, fludarabine alone (34 mg/kg) daily on days 21 to 25, or fludarabine (0.625 mg/kg) + cyclophosphamide (6.25 mg/kg) daily on days 21 to 23. The treatment was repeated 3 times every 2 weeks. Tumor size was evaluated by measuring perpendicular diameters by a caliper. Animals were killed when the tumor volume reached 1000 mm3.
Fludarabine impacts on the growth of MEC1 cells in vitro and in vivo. (A) MEC1 cells were plated in vitro in 48-well plates with increasing concentrations of fludarabine and a luminescent assay was performed 72 hours later to demonstrate MEC1 cells' sensitivity to the drug. EC50 indicates half maximal effective concentration (11.9 g/mL). (B) Tumor growth curves obtained in Rag2−/−γc−/− male mice that received a transplant subcutaneously in the left flank of MEC1 cells (10 × 106). Twenty-one days later, mice bearing MEC1 tumor were randomly assigned to one of the following intraperitoneal treatments (5 animals/group): saline solution (PBS), fludarabine alone (34 mg/kg) daily on days 21 to 25, or fludarabine (0.625 mg/kg) + cyclophosphamide (6.25 mg/kg) daily on days 21 to 23. The treatment was repeated 3 times every 2 weeks. Tumor size was evaluated by measuring perpendicular diameters by a caliper. Animals were killed when the tumor volume reached 1000 mm3. Measurements were stopped when 75% of originally treated mice were still surviving. *Statistically significant differences calculated using the Student t test: day 23, PBS versus fludarabine, P = .01; day 29, PBS versus fludarabine, P = .012, and PBS versus fludarabine + cyclophosphamide, P = .038. Data are representative of at least 2 independent experiments. (C) Kaplan-Meier survival curves for Rag2−/−γc−/− male mice challenged subcutaneously in the left flank with MEC1 cells. Twenty-one days later, mice bearing MEC1 tumor were randomly assigned to one of the following intraperitoneal treatments (5 animals/group): PBS, fludarabine alone (34 mg/kg) daily on days 21 to 25, or fludarabine (0.625 mg/kg) + cyclophosphamide (6.25 mg/kg) daily on days 21 to 23. The treatment was repeated 3 times every 2 weeks. Tumor size was evaluated by measuring perpendicular diameters by a caliper. Animals were killed when the tumor volume reached 1000 mm3.
Discussion
Animal models and in particular xenotransplant models are an invaluable tool in cancer research. CLL is a distinct exception as, despite remarkable efforts,1,7-9 suitable animal models have been difficult to establish. In the present study we have explored the ability of Rag2−/−γc−/− mice, which lack B, T, and NK cells, to support the growth of the MEC1 CLL cell line. Through this approach, we have generated a reproducible in vivo model of aggressive human CLL that develops rapidly, displays complete engraftment efficiency, allows measurement of tumor, and closely recapitulates its human counterpart by spreading systemically. The animal model described in this study has 100% engraftment efficiency with measurable subcutaneous tumors developing in the injection site of all mice that underwent transplantation. In addition, tumor-bearing Rag2−/−γc−/− mice respond to fludarabine treatment as TCL1 transgenic mice do.11
The relevance of this model is 3-fold. First, MEC1-bearing Rag2−/−γc−/− mice underscore the influence of the immunodeficient microenvironment in inducing resistance to human tumor growth. Numerous studies on CLL xenografts used SCID7 or NOD/SCID8,9 mice. The differences in the present study are a more profound immunodeficiency of Rag2−/−γc−/− mice and, notably, the absence of NK cells, whose potential importance in preventing CLL engraftment has been previously hypothesized.12
Second, this model can be valuable to study the microenvironmental interactions that occur in vivo between CLL cells and the different tissue components. It is well known that CLL cells retain the capacity to respond to microenvironmental signals by increasing survival and proliferation,13 suggesting the possibility that different tissue microenvironments may differentially influence the growth of leukemic cells within individual organs. Along this line of reasoning, the observation that the intensity of expression of several CLL markers, and in particular of CD19, is significantly and consistently downmodulated in all tissues, strongly indicates that transplanted leukemic cells do interact with other yet-undefined tissue elements. The latter likely differ in different organs, as suggested by a great heterogeneity in terms of modifications depending on the involved tissue. Of potential interest is the downmodulation of CD19 molecule in the spleen compared with other organs (Figure 4B-E). Although it is not unreasonable to hypothesize that the loss of some markers might also correlate with the acquisition of a more aggressive behavior, the overall aggressive course of the leukemia in our model prevented us from testing this hypothesis. Likewise, considering the fact that MEC1 cell line lacks the expression of CD5, our model cannot address the role of this molecule specifically expressed on primary CLL cells.
Finally, MEC1-bearing Rag2−/−γc−/− mice appear to be a simple in vivo system that can be used to test novel drugs or new therapeutic approaches potentially useful in CLL, with the significant advantage of giving rather rapid results.
In conclusion, the novel Rag2−/−γc−/− CLL mouse model here presented appears to be very useful for evaluating both the biologic basis of CLL growth and dissemination and the efficacy of new therapeutic agents.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are indebted to Dr Kawahata (Central Institute for Experimental Animals, Kawasaki, Japan) who kindly provided Rag2−/−γc−/− mice on BALB/c background.
This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC), Fondazione Italiana per la Ricerca sul Cancro (FIRC), CLL Global Research Foundation, Progetti di Ricerca di Interesse Nazionale (PRIN), Fondo per gli Investimenti della Ricerca di Base (FIRB), Progetto Integrato Oncologia (PIO), Fondazione Ferrero, and Fondazione Anna Villa e Felice Rusconi.
Authorship
Contribution: M.T.S.B. designed research, performed experiments, analyzed data, and wrote the paper; C.S. and M.P. performed experiments and analyzed data; G.S., B.A., C.F., and M.R. performed experiments; L.S. analyzed data; M.M. and F.C.-C. analyzed data and wrote the paper; and P.G. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Federico Caligaris-Cappio, Istituto Scientifico San Raffaele, Dipartimento di Oncologia e Divisione di Oncologia Molecolare, Via Olgettina 60, 20132 Milan, Italy; e-mail: caligaris.federico@hsr.it.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal