Abstract
Mantle cell lymphoma (MCL) has a heterogeneous clinical course. The recently proposed Mantle Cell Lymphoma International Prognostic Index (MIPI) predicted the survival of MCL better than the International Prognostic Index in MCL patients treated with conventional chemotherapy, but its validity in MCL treated with more intensive immunochemotherapy has been questioned. Applied here to 158 patients of the Nordic MCL2 trial of first-line intensive immunochemotherapy followed by high-dose chemotherapy and autologous stem cell transplantation, the MIPI and the simplified MIPI (s-MIPI) predicted survival significantly better (P < .001) than the International Prognostic Index (P > .004). Both the MIPI and the s-MIPI mainly identified 2 risk groups, low and intermediate versus high risk, with the more easily applied s-MIPI being just as powerful as the MIPI. The MIPIB (biological), incorporating Ki-67 expression, identified almost half of the patients as high risk. We suggest that also a simplified MIPIB is feasible. This trial was registered at www.isrctn.org as #ISRCTN 87866680.
Introduction
Mantle cell lymphoma (MCL) is a non-Hodgkin lymphoma subtype with a heterogeneic prognosis often sought predicted by the International Prognostic Index (IPI),1-5 although this was derived from aggressive non-Hodgkin lymphoma data.6 Treatment options range from deferred therapy to intensive up-front treatment and autologous stem cell transplantation (ASCT).7-9 Recently, based on a multivariate analysis of 455 prospectively studied MCL patients who received mainly conventional therapy and immunotherapy, Hoster et al10 proposed the MCL International Prognostic Index, the MIPI, which segregated MCL patients better into risk groups than the IPI. In addition to the formula-based, computed MIPI, the authors proposed a simplified score-based index, the simplified MIPI (s-MIPI). Both still await external validation on independent patient cohorts treated with intensive immunochemotherapy and ASCT. We here apply the IPI, the MIPI, and the s-MIPI to the patients of the Nordic Lymphoma Group MCL2 protocol.
Methods
The Nordic Lymphoma Group MCL2 study accrued 160 newly diagnosed, untreated stage II to IV MCL patients from 2000 to 2006.9 Briefly, the treatment consisted of induction by augmented CHOP (cytoxan, hydroxyrubicin, oncovin, prednisone) alternating with high-dose cytarabine to a total of 6 cycles given with 3-week intervals, with rituximab from cycle 4. Responders with a sufficient stem cell harvest proceeded to high-dose chemotherapy consisting of BEAM (BCNU, etoposide, cytarabine, melphalan) or BEAC (BEAM with cytoxan instead of melphalan) plus ASCT. All patients fulfilled the World Health Organization diagnostic criteria of MCL11 with overexpression of cyclinD1 or a documented t(11;14) at the central pathology review. When possible, Ki-67 was analyzed by a semiquantitative assessment (“eyeballing”) of the proportion of MIB1-α–positive cells in representative areas of the lymphoma. Full clinical and laboratory workup, including computed tomography scans of the chest and abdomen, bone marrow trephine biopsies, and aspirates with histologic, cytologic, and flow cytometric assessment, was done at study entry and repeated at response evaluation after induction cycle 5, 2 months after transplantation, and subsequently every 6 months for 5 years, followed by annual clinical examination and blood tests until relapse. Standard response criteria were used.12 Overall and event-free survival rates were calculated from the date of inclusion in the protocol to the date of death from any cause, or to any event leading to exit from the protocol, including lymphoma and toxicity, respectively, by the Kaplan-Meier method13 and differences between subgroups analyzed by the log-rank test. All P values were 2-tailed. For the present analyses, the database was closed on March 12, 2008. The protocol was approved by the institutional or regional science ethics committees in all Nordic countries, and informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
The IPI was applied as previously described,6 and the MIPI and s-MIPI as described by Hoster et al.10 The MIPI as the sum of the products of the independent prognostic factors age, performance, leukocyte count, and lactate dehydrogenase (LDH)/upper limit normal, and their regression coefficients, and risk scored according to the following cutpoints: low (< 5.70), intermediate (5.70-6.20), and high risk (≥ 6.20). The s-MIPI as the sum of increasing points for grouped values of age, performance status, LDH/upper limit normal, and leukocyte count, and scored as low (0-3 points), intermediate (4-5 points), and high risk (> 5 points). In addition to the MIPI, Hoster et al10 proposed a biological MIPI (MIPIB), adding to the MIPI the percentage of Ki-67+ lymphoma cells multiplied by its regression coefficient, changing the cutpoints slightly (high risk ≥ 6.50). This was highly discriminative, but a simplified MIPIB was not proposed. We therefore explored the feasibility of a simplified MIPIB, by the addition of 1 point to the s-MIPI for patients with Ki-67 expression more than or equal to 30%.14
Results and discussion
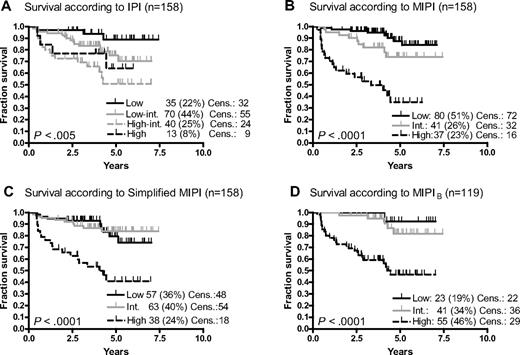
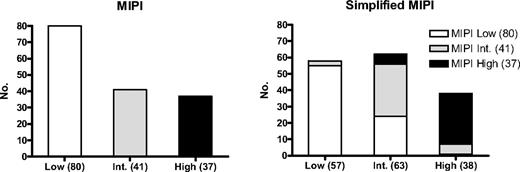
Of the 160 patients in the MCL2 study, 158 were evaluable for MIPI, 19% with the blastoid variant and 41% with Ki-67 expression more than or equal to 30% of 119 evaluable. The 5-year overall survival was 69%. IPI, MIPI, and s-MIPI (Figure 1A-C) all gave significant results, but the IPI segregated poorly between the high-intermediate and high categories, with the projected 5-year survival being higher than 50% in both. In contrast, both MIPI and s-MIPI identified high-risk groups, with 35% and 38% 5-year survival, respectively, but segregated the low- and intermediate-risk patients poorly (P = .07 and .86, respectively). The transformation from MIPI to s-MIPI did not lead to identical subgroups as 31% of MIPI low-risk patients were redistributed to the s-MIPI-intermediate group, mainly resulting from high LDH and leukocyte counts, whereas fewer (15%) of the high-risk patients changed category (Figure 2). The MIPIB (Figure 1D) and s-MIPIB (not shown) clearly allocated more than one-third of the patients to the high-risk group with 5-year survival rates of 46% and 39%, respectively, but, like the MIPI and s-MIPI, could not segregate significantly between low- and intermediate-risk groups with 5-year survival rates of 78% or higher. Regarding event-free survival (not shown), the MIPI and s-MIPI, but not the IPI, segregated the risk groups significantly.
Overall survival. (A) International Prognostic Index (IPI). (B) Mantle Cell Lymphoma International Prognostic Index (MIPI). (C) Simplified MIPI (s-MIPI). (D) Biological MIPI (MIPIB). Int. indicates intermediate; and Cens., censored.
Overall survival. (A) International Prognostic Index (IPI). (B) Mantle Cell Lymphoma International Prognostic Index (MIPI). (C) Simplified MIPI (s-MIPI). (D) Biological MIPI (MIPIB). Int. indicates intermediate; and Cens., censored.
The transformation from MIPI to s-MIPI moved 25 of the 80 MIPI-low patients (31%) to the s-MIPI-intermediate risk group. Only minor changes of the MIPI-intermediate and the MIPI–high-risk patients occurred.
The transformation from MIPI to s-MIPI moved 25 of the 80 MIPI-low patients (31%) to the s-MIPI-intermediate risk group. Only minor changes of the MIPI-intermediate and the MIPI–high-risk patients occurred.
Hoster et al10 constructed the MIPI on 2 cohorts of mostly conventionally treated patients but considered it valid also in patients receiving high-dose therapy and ASCT because this treatment modality has not yet been shown to improve survival. Applied to retrospective series of MCL patients receiving widely heterogeneic first-line immunochemotherapy regimens and some also ASCT, the MIPI had predictive value,15,16 whereas Shah et al17 and van't Veer et al18 found no predictive value of the s-MIPI in prospective cohorts homogeneously treated with intensive immunochemotherapy without and with ASCT, respectively, and suggested that the intensive treatment had overcome the high-risk features of the MIPI. Here we show that, applied to our prospective MCL2 cohort, homogeneously treated with intensive immunochemotherapy including ASCT, both the MIPI and the s-MIPI clearly identify high-risk patients better than the IPI, mainly resulting from their quantitative weighing of LDH and WBC, by which the MIPI identifies half of the IPI high-intermediate and even some IPI low-intermediate as having a poor prognosis.
What should be the purpose of a prognostic index on a cohort already offered high-dose therapy? For the low-risk patients of our cohort, one would obviously hesitate to weaken the intensity of a highly effective, maybe even curative, treatment with acceptable toxicity. In contrast, for the one-fourth of the patients with high-risk MCL with a 5-year survival of less than 40%, attempts to improve the treatment are clearly justified. Because both the MIPI and the s-MIPI qualified as tools for such up-front treatment selection, we advocate the more easily applied s-MIPI. Ki-67 can predict outcome irrespective of various clinical and therapeutic characteristics, including those of the IPI and MIPI.4,5,19-21 The MIPIb, combining MIPI and Ki-67 expression, assigned almost half of our patients to the high-risk category, possibly reflecting a somewhat higher proportion of cases with high Ki-67 expression in our cohort than in other.5 Some degree of selection of high-risk patients in multicenter protocols of highly intensive therapy is probably unavoidable but may even enhance the strength of the MIPI, s-MIPI, and MIPIb. Because score-based systems may gain broader application than formula based, we explored and confirmed the feasibility of a simplified MIPIb. However, according to recent guidelines for Ki-67 assessment,22 the proper setting of the Ki-67 cutoffs may require a more exact method of Ki-67 assessment than our semiquantitative eyeballing method. Provided this, such a simplified MIPIb might become an important prognostic tool, more easily applicable than other biologic approaches.23,24
The online version of this article contains a data supplement.
Presented in part at the 11th annual meeting of the European Hematology Association, June 6, 2009, Berlin, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of all contributing departments and blood banks for their contribution and the patients for their willingness to participate.
This work was supported by grants from the Danish Cancer Society, Nordic Cancer Union, John and Birthe Meyer Foundation, NovoNordisk Foundation, and Anders Nielsen & Co.
Authorship
Contribution: C.H.G., A.K., A.L., and E.E. designed the protocol, collected and analyzed data, and wrote the paper; R.R., M.J., M. Eriksson, M.N., E.K., A.M.B., H.N.-E., O.K., and G.F.L. collected and analyzed data; E.R., M. Ehinger, C.S., J.D., and M.-L.K.-L. performed the pathology review and analyzed data; P.B. analyzed data and performed statistical analysis; and all authors participated in preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of Nordic Lymphoma Group participants appears in the supplemental Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Christian H. Geisler, Department of Hematology 4042, Rigshospitalet, DK 2100 Copenhagen, Denmark; e-mail: christian.geisler@rh.regionh.dk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal