Abstract
We identified 18 patients with the distinct clinical phenotype of susceptibility to disseminated nontuberculous mycobacterial infections, viral infections, especially with human papillomaviruses, and fungal infections, primarily histoplasmosis, and molds. This syndrome typically had its onset in adulthood (age range, 7-60 years; mean, 31.1 years; median, 32 years) and was characterized by profound circulating monocytopenia (mean, 13.3 cells/μL; median, 14.5 cells/μL), B lymphocytopenia (mean, 9.4 cells/μL; median, 4 cells/μL), and NK lymphocytopenia (mean, 16 cells/μL; median, 5.5 cells/μL). T lymphocytes were variably affected. Despite these peripheral cytopenias, all patients had macrophages and plasma cells at sites of inflammation and normal immunoglobulin levels. Ten of these patients developed 1 or more of the following malignancies: 9 myelodysplasia/leukemia, 1 vulvar carcinoma and metastatic melanoma, 1 cervical carcinoma, 1 Bowen disease of the vulva, and 1 multiple Epstein-Barr virus+ leiomyosarcoma. Five patients developed pulmonary alveolar proteinosis without mutations in the granulocyte-macrophage colony-stimulating factor receptor or anti–granulocyte-macrophage colony-stimulating factor autoantibodies. Among these 18 patients, 5 families had 2 generations affected, suggesting autosomal dominant transmission as well as sporadic cases. This novel clinical syndrome links susceptibility to mycobacterial, viral, and fungal infections with malignancy and can be transmitted in an autosomal dominant pattern.
Introduction
Disseminated nontuberculous mycobacterial infections are associated with primary immunodeficiencies that involve defects in the interleukin-12 (IL-12)/IL-23/interferon-γ (IFN-γ) axis, Tyk2, or nuclear factor-κB essential modulator.1,2 Patients with these abnormalities also have variable susceptibility to other organisms, including Salmonella spp, certain viruses, and dimorphic fungi. These genetic and acquired susceptibilities to mycobacteria and other intracellular infections highlight the critical role of monocytes/macrophages. In contrast, invasive aspergillosis is rare in primary immunodeficiencies, mostly limited to chronic granulomatous disease and hyper-IgE recurrent infection syndrome or Job's syndrome. Except for lymphoma in hyper-IgE recurrent infection syndrome, none of these immunodeficiencies is significantly associated with malignancy.3 However, mice with defects in the genes of the IFN-γ/IL-12/IL-23 pathway have increased epithelial tumors, suggesting that IFN-γ–mediated immunity is important in the control of both chemically induced and spontaneous tumors in mice.4,5 Mutations in genes involved in the IFN-γ signal cascade also have been identified in primary human tumors,6 and 1 child with IFNGR1 deficiency developed human herpesvirus 8–associated Kaposi sarcoma.7 Disseminated mycobacterial infections have been reported in hairy cell leukemia and chronic myelogenous leukemia, as well as advanced HIV infection.8,9 Therefore, at least some of the pathways that mediate mycobacterial susceptibility also control susceptibility to other infections and malignancies.
As a result of recruiting patients with mycobacterial infections, we identified a syndrome characterized by disseminated nontuberculous mycobacterial and other opportunistic infections that was also associated with an increased incidence of myelodysplasia and malignancy. This syndrome is recognized primarily in adulthood and occurs in both sporadic and autosomal dominant familial cases. These patients were distinct from previous reported syndromes, were not infected with HIV, and did not have identifiable functional defects or mutations in the IL-12/IL-23/IFN-γ axis, STAT1, or nuclear factor-κB essential modulator. Most patients had severe or disseminated human papillomavirus (HPV) infection, whereas several also had disseminated histoplasmosis, invasive aspergillosis, or cryptococcal meningitis. Pulmonary alveolar proteinosis (PAP), a condition resulting from abnormalities in pulmonary alveolar macrophage metabolism of granulocyte-macrophage colony-stimulating factor (GM-CSF) or surfactant,10-14 developed in 5 patients with long-standing disease. All affected persons demonstrated persistent and profound peripheral monocytopenia, B-cell and NK-cell lymphocytopenia, with variable T-cell lymphocytopenia. Several developed trisomy 8, monosomy 7, or dicentric chromosome 6 accompanied by myelodysplasia or acute leukemia. This novel inherited and sporadic syndrome connects infection susceptibility, predisposition to myelodysplasia, and malignancy with multiple cytopenias.
Methods
Samples
Subjects were enrolled in appropriate approved natural history protocols of the National Institute of Allergy and Infectious Diseases. All participants or their guardians gave written informed consent in accordance with the Declaration of Helsinki. Whole blood was collected from each patient or normal healthy volunteer in sodium heparin tubes (BD Biosciences) and processed immediately. Whole blood was reconstituted in an equal volume of Hanks balanced salt solution without divalent cations, and leukocytes were separated by discontinuous gradient centrifugation through Hypaque-Ficoll. Peripheral blood mononuclear cells were harvested, washed twice in Hank's balanced salt solution, reconstituted in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum (Gemini BioProducts), and enumerated by hemocytometer. Neutrophils were harvested after erythrocyte sedimentation with 3% dextran. Two rounds of hypotonic lysis removed contaminating red blood cells, and neutrophils were enumerated on a hemocytometer. Both peripheral blood mononuclear cells and neutrophils were more than 95% viable as assessed by exclusion of trypan blue stain. Plasma was obtained from patients and normal donors by centrifugation of heparinized blood and was frozen at −80°C. Determination of the presence of anti–IFN-γ autoantibodies was performed as previously described.15
Lymphocyte phenotyping
Lymphocyte phenotyping was performed on indicated patients (Table 1) using whole blood with the red cell lysis technique. Samples anticoagulated with ethylenediaminetetraacetic acid were stained using flow cytometry and analyzed on a FACScan (BD Biosciences) using CellQuest software (BD Biosciences). The following lymphocytes and lymphocyte subsets were analyzed by the corresponding directly conjugated monoclonal antibodies: T cells and T-cell subsets (anti-CD3, anti-CD4, and anti-CD8, γδ, αβ, and CD57/CD8); B cells by anti-CD20; and NK cells by a combination of anti-CD16 and anti-CD56, evaluated on CD3− lymphocytes; T-cell activation markers by anti-CD25 and anti–human leukocyte antigen-DR; directly conjugated, murine IgG1 was used to ascertain background staining. All monoclonal antibodies were obtained from BD Biosciences, with the exception of anti-CD4, anti-CD8, and αβ TCR (Beckman-Coulter) and γδ TCR (Pierce Endogen). Lymphocytes were identified using anti-CD45/anti-CD14 (BD Biosciences), then gating to establish forward and side scatter. List mode parameters were collected for 106 lymphocytes. To calculate absolute numbers of each lymphocyte subset, the percentage of cells staining positive was multiplied by the absolute peripheral blood lymphocyte count, which was determined by a Celldyne 3500 (Abbott). Peripheral blood from healthy adult controls was stained and analyzed to establish 95% confidence interval normal ranges.
Peripheral blood immunophenotyping of the index cases of all kindreds
| . | Affected cell lineage (reference range, cells/μL) . | ||||||
|---|---|---|---|---|---|---|---|
| CD14/monocytes (210-660) . | Total lymphocytes (1320-3570) . | CD20/B cells (49-424) . | CD3− CD16+ NK cells (87-505) . | CD3/T cells (650-2108) . | CD4+ T cells (362-1275) . | CD8+ T cells (344-911) . | |
| Autosomal dominant cases | |||||||
| 1.II.1 | 0 | 402 | 2 | 4 | 396 | 124 | 234 |
| 1.II.5 | 20 | 646 | 8 | 69 | 569 | 260 | 273 |
| 2.II.3 | 4 | 179 | 0 | 0 | 179 | 84 | 81 |
| 4.II.1 | 10 | 759 | 4 | 3 | 752 | 246 | 494 |
| 5.II.1 | 25 | 633 | 4 | 5 | 624 | 396 | 205 |
| 13.I.2 | 19 | 1493 | 21 | 22 | 1450 | 301 | 1140 |
| 13.II.1 | 0 | 112 | 1 | 2 | 109 | 37 | 64 |
| Sporadic cases | |||||||
| 3.I.1 | 20 | 442 | 13 | 2 | 427 | 198 | 210 |
| 6.I.1 | 22 | 828 | 16 | 6 | 796 | 408 | 329 |
| 7.I.1 | 13 | 700 | 3 | 55 | 642 | 261 | 364 |
| 8.I.1 | 16 | 807 | 4 | 10 | 793 | 438 | 396 |
| 9.III.1 | 27 | 1987 | 30 | 24 | 1933 | 1021 | 864 |
| 10.I.1 | 21 | 520 | 4 | 1 | 515 | 233 | 246 |
| 11.II.1 | 4 | 1300 | 51 | 39 | 1210 | 538 | 613 |
| 12.I.1 | 0 | 280 | 1 | 0 | 279 | 193 | 80 |
| 14.II.1 | 29 | 1797 | 2 | 22 | 1773 | 808 | 964 |
| 15.II.1 | 9 | 999 | 5 | 24 | 970 | 489 | 432 |
| 16.II.1 | 0 | 747 | 0 | 0 | 747 | 345 | 330 |
| . | Affected cell lineage (reference range, cells/μL) . | ||||||
|---|---|---|---|---|---|---|---|
| CD14/monocytes (210-660) . | Total lymphocytes (1320-3570) . | CD20/B cells (49-424) . | CD3− CD16+ NK cells (87-505) . | CD3/T cells (650-2108) . | CD4+ T cells (362-1275) . | CD8+ T cells (344-911) . | |
| Autosomal dominant cases | |||||||
| 1.II.1 | 0 | 402 | 2 | 4 | 396 | 124 | 234 |
| 1.II.5 | 20 | 646 | 8 | 69 | 569 | 260 | 273 |
| 2.II.3 | 4 | 179 | 0 | 0 | 179 | 84 | 81 |
| 4.II.1 | 10 | 759 | 4 | 3 | 752 | 246 | 494 |
| 5.II.1 | 25 | 633 | 4 | 5 | 624 | 396 | 205 |
| 13.I.2 | 19 | 1493 | 21 | 22 | 1450 | 301 | 1140 |
| 13.II.1 | 0 | 112 | 1 | 2 | 109 | 37 | 64 |
| Sporadic cases | |||||||
| 3.I.1 | 20 | 442 | 13 | 2 | 427 | 198 | 210 |
| 6.I.1 | 22 | 828 | 16 | 6 | 796 | 408 | 329 |
| 7.I.1 | 13 | 700 | 3 | 55 | 642 | 261 | 364 |
| 8.I.1 | 16 | 807 | 4 | 10 | 793 | 438 | 396 |
| 9.III.1 | 27 | 1987 | 30 | 24 | 1933 | 1021 | 864 |
| 10.I.1 | 21 | 520 | 4 | 1 | 515 | 233 | 246 |
| 11.II.1 | 4 | 1300 | 51 | 39 | 1210 | 538 | 613 |
| 12.I.1 | 0 | 280 | 1 | 0 | 279 | 193 | 80 |
| 14.II.1 | 29 | 1797 | 2 | 22 | 1773 | 808 | 964 |
| 15.II.1 | 9 | 999 | 5 | 24 | 970 | 489 | 432 |
| 16.II.1 | 0 | 747 | 0 | 0 | 747 | 345 | 330 |
Total lymphocyte counts were obtained by summing B cells, NK cells, and CD3+ T cells.
Microarray analysis
RNA was extracted from isolated neutrophils of 3 patients (patients 4.II.1, 13.II.1, and 12.I.1) and 7 healthy control subjects using RNeasy Mini Kit (QIAGEN) according to the manufacturer's instructions. Microarray analysis was performed using HU133 + 2 human Affymetrix GeneChips as described previously.16 A separate GeneChip was used for each donor. Genes were defined as differentially expressed when changes in transcript levels were statistically significant by t test and/or analysis of variance as indicated, were at least 1.5-fold increased or decreased compared with cells from control subjects, and transcripts passed all quality filters. Complete microarray data are posted on the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo) under accession number GSE16020.
Results
Clinical reports
The 5 families with clear autosomal dominant patterns of inheritance are described and the sporadic cases listed in Table 1.
Kindred 1
Patient 1.II.1 was a Hispanic woman who had severe genital HPV infection and cervical intraepithelial neoplasia while in her 20s (Figure 1; patient 5 in Holland et al17 ). At 40 years, she presented with fevers, dyspnea, fatigue, and 4 months of weight loss. Pathology results from a left lower lobectomy suggested bronchiolitis obliterans organizing pneumonia, for which high-dose corticosteroids were initiated. Three months later, diffuse cutaneous nodules yielded Mycobacterium avium complex (MAC). Despite cessation of steroids and appropriate MAC therapy, infection progressed with persistent positive cultures from skin, blood, small bowel, and bone marrow. Herpetic esophagitis necessitated hospitalization.
Pedigrees of the kindreds with multiple affecteds demonstrating autosomal dominant pattern of transmission.
Pedigrees of the kindreds with multiple affecteds demonstrating autosomal dominant pattern of transmission.
Eighteen months after presentation, multiple skin lesions persisted with mild left cranial nerve VI palsy. Adjuvant IFN-γ with anti-MAC therapy led to clinical resolution of infection. Two years later, increased CD34+ cells and newly appearing monocytes in peripheral blood led to the diagnosis of chronic myleomonocytic leukemia (CMMoL) without karyotypic abnormality. A right posterior orbital Epstein-Barr virus–positive (EBV+) leiomyosarcoma was excised. Allogeneic bone marrow transplantation from a sibling was performed for blast crisis. She engrafted uneventfully, but subsequent sepsis with methicillin-resistant Staphylococcus aureus and Candida albicans was complicated by respiratory failure and death 85 days after transplantation. At autopsy, bone marrow showed recurrent CMMoL, and EBV+ leiomyosarcomas were found in the posterior orbit, liver, colon, and uterus. There was no evidence of granulomata or acid-fast bacilli, and mycobacterial cultures were negative.
Patient 1.II.5
Patient 1.II.5 is the youngest of 4 siblings of patient 1.II.1. She had recurrent, severe perineal HPV starting in adolescence, Bowenoid papulosis of the vulva, and 8 early spontaneous abortions without etiology. At age 37, she presented with fevers, night sweats, and weight loss. Small, tender nodules over the limbs and trunk showed panniculitis. Granulomata were found in marrow; biopsy of an enlarged retroperitoneal lymph node yielded MAC. Conventional drug therapy led to resolution of the lymphadenopathy and weight gain. She had episodes of bilateral Bell palsy at 40 and 41 years, progressive PAP-like lung disease at age 42, and parvovirus B19 infection at age 46. Recurrent refractory genital HPV disease with condylomata, cervical dysplasia, and Bowenoid papulosis of the vulva persists. At 47 years, she developed disseminated M fortuitum biov. fortuitum with severe pulmonary compromise.
Patient 1.I.2
The mother of patients 1.II.1 and 1.II.5 died before this study, but medical records and autopsy reports showed CD4+ lymphocytopenia and monocytopenia in the setting of severe disseminated mycobacterial infection (unknown species). She was initially diagnosed with CMMoL but developed refractory anemia with excess blasts leading to death at age 54. The other siblings in this kindred, 1.II.2, 1.II.3, and 1.II.4, were clinically well and had normal hemograms when tested in their fourth and fifth decades.
Kindred 2
Patient 2.II.3 was a white man with long-standing verrucae on the left arm, without dissemination. At 34 years, flu-like symptoms with pulmonary infiltrates, a subcarinal mass, and mediastinal lymphadenopathy were the result of disseminated Histoplasma capsulatum, successfully treated with prolonged itraconazole. Fourteen months after initial presentation, fever, chills, cough, night sweats, and weight loss were associated with pulmonary infiltrates, pleural effusions, new mediastinal lymphadenopathy, and significant hepatosplenomegaly. Granulomatous inflammation in a lymph node and pleural effusion yielded MAC. Bone marrow biopsy showed noncaseating epithelioid granulomata. Multiple, tender, violaceous skin nodules showed epithelioid and necrotizing granulomatous inflammation and grew MAC. While on IFN-γ, he developed progressive pancytopenia and hypogranular neutrophils with asymmetric distribution of primary and secondary granules in the myeloid precursors. Myelodysplasia with refractory anemia and thrombocytopenia led to death at 39 years.
Patient 2.I.1, the father of 2.II.3, died before this study, but medical records were reviewed. Constitutional symptoms and disseminated granulomata in his late 30s were associated with subcutaneous masses resulting from M scrofulaceum. During appropriate therapy, he developed disseminated nodular lung infiltrates with noncaseating granulomata. He subsequently developed cryptococcal meningitis and recurrent staphylococcal infections. Pancytopenia led to transfusion dependence and death at 42 from a “leukemic process.”
Kindred 4
Patient 4.II.1 had had 2 premature births (at 26 and 34 weeks of gestation) and 1 spontaneous abortion before 3 successful term deliveries. At 28 years, she developed a severe postpartum rash diagnosed histopathologically as discoid lupus. Recurrent upper and lower respiratory tract infections required antibiotics. Low to normal levels of IgG2 and IgG4 led to a diagnosis of common variable immunodeficiency and treatment with intravenous immunoglobulin. At 38 years, progressively worsening fevers, dyspnea, and weight loss with a right middle lobe infiltrate led to bronchoalveolar lavage and a mediastinal lymph node biopsy which grew MAC; bone marrow showed numerous granulomata. She was treated successfully and remained well for 2 years, when pneumonia recurred; lung biopsy showed granulomata but no organisms, and she was treated empirically for MAC. Bone marrow was hypocellular. At 39 years, clonal large granular lymphocytes were identified in peripheral blood. She has had multiple basal and squamous cell skin cancers of the head/neck region; however, the patient had fair skin and significant ultraviolet exposure in the southwestern United States. At 44 years, biopsy samples associated with granulomatous hepatitis and necrotizing granulomatous mesenteric and intraperitoneal lymphadenitis grew MAC. At 48 years, she developed Serratia marcescens pneumonia. At 49 years, H capsulatum grew from blood, right lower lobe, mediastinal, and retroperitoneal lymph nodes, and was successfully treated with liposomal amphotericin B followed by posaconazole. Lower leg lesions consistent with erythema nodosum continue to recur.
Patient 4.II.5, the youngest sister of 4.II.1, had severe genital HPV infection in her late teens treated with IFN-α. At age 27, myelodysplastic syndrome (MDS) with monosomy 7 was noted during her first pregnancy. Progression of her myelodysplasia led to a successful matched related bone marrow transplantation.
Kindred 5
Patient 5.II.1 is a white woman who presented at the age of 32 years with fever, weight loss, erythematous nodules on the legs, a positive tuberculin test, and interstitial infiltrates. Lung and bone marrow biopsies showed noncaseating granulomata leading to empiric treatment for M tuberculosis. Subsequently, prednisone treatment for presumptive sarcoidosis was associated with worsening pulmonary infiltrates, increased thoracic and abdominal lymphadenopathy, and cytopenias. Bronchoalveolar lavage, lymph node biopsies, and bone marrow biopsy yielded MAC. She had severe genital HPV infection during adolescence and developed cervical carcinoma requiring resection with hysterectomy at 19 years. She had chronic disseminated verruca plana (flat warts) of the hands and arms and verrucae vulgaris (common warts). Factor V Leiden and methylenetetrahydrofolate reductase deficiencies were identified after multiple lower extremity deep vein thromboses and pulmonary emboli, requiring an inferior vena cava filter. Two years after presentation, MDS with trisomy 8 was identified. Bilateral lung nodules and recurrent erythematous nodules of the leg yielded M abscessus, which was successfully treated with antimycobacterials and adjunctive IFN-γ. Massive lower gastrointestinal bleeding required urgent resection of the terminal ileum with right hemicolectomy. Pathology showed multifocal ulceration without pathogens. Recurrent erythematous nodular lesions have consistently shown panniculitis. Five years after presentation, she is transfusion-dependent, and bone marrow shows spontaneous resolution of trisomy 8 but new monodicentric chromosome 6.
Patient 5.III.1 was the son of 5.II.1. He had severe recalcitrant HPV infections as a child. At age 17, he developed acute myeloid leukemia (AML) in blast crisis. His illness was refractory to chemotherapy, and he died at age 19.
Kindred 13
Patient 13.II.1 is a white man who presented at age 31 with persistent cough. A lung biopsy was diagnosed as sarcoidosis, for which he received prednisone. After 18 months, he presented with fatigue, fevers, and pancytopenia. Bone marrow demonstrated histoplasmosis. He failed to respond clinically to amphotericin B and tapering of his steroids. He had had verrucae of the hands and feet for almost 20 years. For the 5 years before admission, he had pneumonias almost yearly, and 1 abscess in the neck had required drainage.
He was pancytopenic; bone marrow was normocellular with multiple nonnecrotizing granulomata. Hypogranular neutrophils were partially CD64+ and CD56+ and CD10−, with asynchronous maturation but no increase in blasts (< 0.2%). No monocytic cells had normal maturation. Cytogenetics identified both monosomy 7 and trisomy 8, confirming myelodysplasia. Skin biopsies, bone marrow, blood cultures, and pleural fluid yielded MAC. Extensive verruca plana and verruca vulgaris involved the forehead and extremities. Splenectomy for severe pancytopenia showed severe white pulp depletion and also grew MAC. A progressive pulmonary infiltrate yielded the hyaline septated mold Neosartorya udagawae. Six months later, despite combination therapy with voriconazole and caspofungin, N udagawae was still isolated from numerous respiratory specimens. Subsequently, multiple intracerebral masses with ring enhancement showed invasive mold, and he died of disseminated fungal infection.18
Patient 13.II.2 was the sister of 13.II.1. She had recurrent infections, including verrucae in childhood, and had been diagnosed with aplastic anemia resulting from leukopenia and thrombocytopenia. Disseminated primary varicella zoster virus (VZV) infection led to coagulopathy and death at age 17 years.
Patient 13.I.2 is the 60-year-old mother of 13.II.1 and 13.II.2. She had diffuse verrucae of the extremities beginning in adulthood but had never had significant infections or hematologic symptoms. She did have unilateral lymphedema of the left leg successfully managed with compression stockings. Hemograms showed severely reduced monocytes, B cells, and NK cells from peripheral blood. Bone marrow showed decreased monocytes (< 5%) but normal-appearing maturation and no aberrant antigen expression, a small population of CD5+ monoclonal B cells (1.3% of total lymphocytes) negative for CD23 and CD11c, less than 1% NK cells, a subset of plasma cells expressing CD56, and mildly atypical megakaryocytes that were small and hypo- or mono-lobulated.
To date, 11 sporadic cases have been additionally recognized. Their peripheral blood immunophenotyping results are summarized in Table 1, and their clinical features are summarized in Table 2.
Clinical features and salient complications of the syndrome
| Clinical feature . | Frequency overall, percentage (n = 18), no. (%) . | Autosomal dominant patients, percentage (n = 7), no. (%) . | Sporadic patients, percentage (n = 11), no. (%) . |
|---|---|---|---|
| Infection | |||
| Mycobacteria | 14/18 (78) | 6/7 (86) | 8/11 (73) |
| HPV | 14/18 (78) | 6/7 (86) | 8/11 (73) |
| Fungi | 5/18 (28) | 3/7 (43) | 2/11 (18) |
| Complication | |||
| PAP | 6/18 (33) | 2/7 (29) | 4/11 (36) |
| Panniculitis/erythema nodosum | 6/18 (33) | 2/7 (29) | 4/11 (36) |
| Myelodysplasia/acute myeloid leukemia | 9/18 (50) | 5/7 (71) | 4/11 (36) |
| Death during study | 5/18 (28) | 3/7 (43) | 2/11 (18) |
| Clinical feature . | Frequency overall, percentage (n = 18), no. (%) . | Autosomal dominant patients, percentage (n = 7), no. (%) . | Sporadic patients, percentage (n = 11), no. (%) . |
|---|---|---|---|
| Infection | |||
| Mycobacteria | 14/18 (78) | 6/7 (86) | 8/11 (73) |
| HPV | 14/18 (78) | 6/7 (86) | 8/11 (73) |
| Fungi | 5/18 (28) | 3/7 (43) | 2/11 (18) |
| Complication | |||
| PAP | 6/18 (33) | 2/7 (29) | 4/11 (36) |
| Panniculitis/erythema nodosum | 6/18 (33) | 2/7 (29) | 4/11 (36) |
| Myelodysplasia/acute myeloid leukemia | 9/18 (50) | 5/7 (71) | 4/11 (36) |
| Death during study | 5/18 (28) | 3/7 (43) | 2/11 (18) |
Clinical characteristics
A total of 18 patients (12 females, 6 males) with sufficient clinical information and laboratory investigations were identified, involving 16 kindreds (13 white, 3 Hispanic). There was no reported consanguinity. Five families had 2 or more first-degree relatives affected with similar syndromes (kindreds 1, 2, 4, 5, and 13; Figure 1), suggesting autosomal dominant transmission with variable expressivity. In kindreds 3, 9, and 11, there were incomplete clinical or laboratory data, but anecdotal evidence of variants of the syndrome in first-degree relatives, supporting autosomal dominant transmission and variable expressivity.
The cardinal laboratory and clinical features of this syndrome are provided in Tables 1 and 2. Of the 18 patients, 14 had developed disseminated mycobacterial disease at the time of this report, 12 of which were the result of slow-growing mycobacteria (9 MAC, 2 M kansasii, 1 M scrofulaceum) and 3 resulting from rapid-growing species (2 M fortuitum, 1 M abscessus); 1 patient had infections by both slow-growing and rapid-growing mycobacteria. One patient (13.I.2) had not had documented mycobacterial infection but had bilateral apical pulmonary scarring and left hilar lymph node calcification.
HPV affected 14 patients as disseminated or recalcitrant cutaneous and/or genital disease. The other major viral pathogens were members of Herpesviridae (2 herpes simplex virus, 2 VZV, 2 EBV). Cytomegalovirus (CMV) serologies were positive in all patients, but the CMV viral loads were never elevated and no patient had clinical evidence of CMV disease. In addition, parvovirus B19 was identified in 2 patients.
Three patients had disseminated H capsulatum infections and 3 had septated mold infections. Invasive fungal infections were reported in 2 other family members (1 with Aspergillus sp, 1 with Cryptococcus neoformans). Routine bacterial infections occurred in only 5 patients, none of which was difficult to treat.
Profound, persistent peripheral blood monocytopenia and B- and NK-lymphocytopenia were seen in all evaluated patients (Table 1). Despite the marked B-lympocytopenia, no patient had hypogammaglobulinemia, although IgA levels were decreased in 2 patients. Total circulating T lymphocyte numbers were abnormal in 9 patients. CD4+ T lymphocytes were less than 300 cells/μL in 9 patients; the CD8+ T-cell subset was reduced in 10 patients. Both CD4+ and CD8+ were depressed in 7 patients. Neutropenia was observed in 5 patients, 4 of whom were in advanced stages of illness.
PAP (Figure 2) developed in 6 patients (4 female, 2 male) with a median age of onset of 42 years (range, 25-60 years). Autoantibodies to GM-CSF were not detected, nor were mutations in the GM-CSF receptor α chain or common β chain. Neither subcutaneous nor aerosolized GM-CSF had significant effect. Periodic whole-lung lavages were moderately effective.
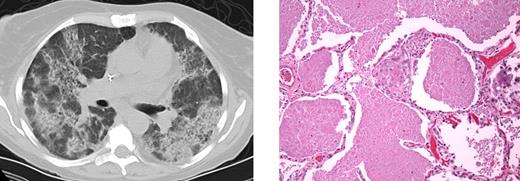
PAP in patient 3.I.1. Computed tomography (left) demonstrates significant bilateral airspace disease. Histopathology (right) demonstrates excessive accumulation of amorphous proteinaceous material in the alveolar spaces. Images were taken using an Olympus Bx41 microscope, objectives UPlanFI 40×/0.75 ∞/0.17, and UPlanFI 20×/05.0 ∞/0.17, with an adaptor U-TV0.5×C using a digital camera Q-imaging Micropublisher 5.0RTV. The images were captured using Q-Capture Version 3.1 and imported into Adobe Photoshop 7.0.
PAP in patient 3.I.1. Computed tomography (left) demonstrates significant bilateral airspace disease. Histopathology (right) demonstrates excessive accumulation of amorphous proteinaceous material in the alveolar spaces. Images were taken using an Olympus Bx41 microscope, objectives UPlanFI 40×/0.75 ∞/0.17, and UPlanFI 20×/05.0 ∞/0.17, with an adaptor U-TV0.5×C using a digital camera Q-imaging Micropublisher 5.0RTV. The images were captured using Q-Capture Version 3.1 and imported into Adobe Photoshop 7.0.
Multiple inflammatory nodules demonstrating panniculitis or granulomatous inflammation without microorganisms were observed in 6 patients. These lesions were tender erythematous nodules, primarily on the distal extremities, resembling erythema nodosum. Other cutaneous manifestations included erythematous papules, patches, and indurated plaques, which were sometimes tender and occasionally were accompanied by fever and arthralgia. Histologically, mixed inflammatory infiltrates were typically seen. Despite severely low circulating monocytes and B cells, CD68+ tissue macrophages and plasma cells were typically identified in biopsies (Figure 3).
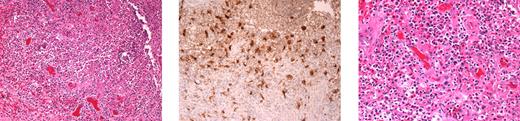
Skin biopsy demonstrating the presence of tissue macrophages and plasma cells, despite the virtual absence of circulating monocytes and B cells. Full-thickness skin biopsy from patient 1.II.1 demonstrating granulomatous inflammation within the dermis (left). Immunohistochemistry reveals the presence of macrophages, stained with monoclonal antibody to KP-1/CD68 (center). Plasmacytosis is also seen in the tissue (hematoxylin and eosin stain; right). Images were taken using an Olympus Bx41 microscope, objectives UPlanFI 40×/0.75 ∞/0.17, and UPlanFI 20×/05.0 ∞/0.17, with an adaptor U-TV0.5×C using a digital camera Q-imaging Micropublisher 5.0RTV. The images were captured using Q-Capture Version 3.1 and imported into Adobe Photoshop 7.0.
Skin biopsy demonstrating the presence of tissue macrophages and plasma cells, despite the virtual absence of circulating monocytes and B cells. Full-thickness skin biopsy from patient 1.II.1 demonstrating granulomatous inflammation within the dermis (left). Immunohistochemistry reveals the presence of macrophages, stained with monoclonal antibody to KP-1/CD68 (center). Plasmacytosis is also seen in the tissue (hematoxylin and eosin stain; right). Images were taken using an Olympus Bx41 microscope, objectives UPlanFI 40×/0.75 ∞/0.17, and UPlanFI 20×/05.0 ∞/0.17, with an adaptor U-TV0.5×C using a digital camera Q-imaging Micropublisher 5.0RTV. The images were captured using Q-Capture Version 3.1 and imported into Adobe Photoshop 7.0.
MDS or AML developed in 9 evaluated patients. Review of family histories identified an additional 5 cases. One patient (3.I.1) had a sibling who died at age 7 with “leuko-lymphosarcoma.” In 4 kindreds, more than 1 family member developed MDS or AML (kindreds 1, 2, 3, and 5). In kindred 13, the proband 13.II.1 had MDS and his sister, 13.II.2, had aplastic anemia and died of severe VZV infection. The median age of diagnosis of MDS or AML was 32 years (range, 7-54 years).
Three patients had abnormal cytogenetics: Patient 5.II.1 initially had trisomy 8, which resolved, and then developed dicentric chromosome 6. Patient 13.II.1 initially had 2 clonal populations (one with monosomy 7 and one with trisomy 8) that progressed over 3 months to pure monosomy 7. Patient 15.II.1 initially had trisomy 8 and subsequently gained a small submetacentric marker (47 XX +mar). Clonal or oligoclonal large granular cytotoxic T lymphocytes, detected by T-cell receptor PCR, were observed in 5 patients, but no patient developed large granular-cell leukemia.
The initial bone marrows of evaluated patients ranged in cellularity but were primarily hypocellular (Table 3). All consistently showed severely reduced monocyte precursors, B cells, and NK cells. In 4 cases, identified B cells displayed abnormal expression of different surface antigens. Granulocytes were variably affected, demonstrating morphologic dysplasia (n = 6) or abnormal granularity alone (n = 9) or in conjunction with abnormal surface antigen expression (n = 4). Involvement of the other hematopoietic lineages was also variable: megakaryocyte precursors were reduced in 6 patients and dysplastic in 12, whereas erythrocyte precursors were diminished in only 2 patients but dysplastic in 8. Thus, aberrant morphology and/or antigen expression affecting other lineages were variable.
Summary table of bone marrow findings and peripheral blood T-cell clonality (large granular lympohocytosis)
| Patient . | Cellularity . | Decreased cell types . | Lineage dysplasia . | Abnormal antigen expression . | Karyotyping . | Large granular lymphocytosis . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypo . | Normo . | Hyper . | Monocyte . | B cell . | NKcell . | Tcell . | Erythroid . | Megakaryocyte . | Erythrocyte . | Myeloid . | Megakaryocyte . | Abnormal neutrophil granules . | Neutrophil . | B cell . | Normal . | Abnormal . | ||
| 1.II.1 | + | + | + | |||||||||||||||
| 1.II.5 | + | |||||||||||||||||
| 2.II.3 | + | + | + | + | + | + | + | + | + | |||||||||
| 3.I.1 | + | + | + | + | + | |||||||||||||
| 4.II.1 | + | + | + | + | + | + | + | + | ||||||||||
| 5.II.1 | + | + | + | + | + | + | + | + | + | + | ||||||||
| 6.I.1 | + | + | + | + | + | + | + | + | + | |||||||||
| 7.I.1 | + | + | + | + | + | + | + | + | + | + | ||||||||
| 8.I.1 | + | + | + | + | + | + | + | + | + | |||||||||
| 9.III.1 | + | |||||||||||||||||
| 10.I.1 | + | + | + | + | ||||||||||||||
| 11.II.1 | + | + | + | + | + | + | + | |||||||||||
| 12.I.1 | + | + | + | + | + | + | + | + | ||||||||||
| 13.I.2 | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| 13.II.1 | + | + | + | + | + | + | + | |||||||||||
| 14.II.1 | + | + | + | + | + | + | + | + | ||||||||||
| 15.II.1 | + | + | + | + | + | + | ||||||||||||
| 16.II.1 | + | + | + | + | ||||||||||||||
| Total (%) | 10 (55.6) | 5 (29.4) | 2 (11.8) | 13 (72.2) | 13 (72.2) | 11 (64.7) | 0 | 2 (11.8) | 6 (35.3) | 8 (47) | 6 (35.3) | 12 (70.6) | 9 (52.9) | 4 (23.5) | 4 (23.5) | 8 (44.4) | 3 (17.6) | 5 (29.4) |
| Patient . | Cellularity . | Decreased cell types . | Lineage dysplasia . | Abnormal antigen expression . | Karyotyping . | Large granular lymphocytosis . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypo . | Normo . | Hyper . | Monocyte . | B cell . | NKcell . | Tcell . | Erythroid . | Megakaryocyte . | Erythrocyte . | Myeloid . | Megakaryocyte . | Abnormal neutrophil granules . | Neutrophil . | B cell . | Normal . | Abnormal . | ||
| 1.II.1 | + | + | + | |||||||||||||||
| 1.II.5 | + | |||||||||||||||||
| 2.II.3 | + | + | + | + | + | + | + | + | + | |||||||||
| 3.I.1 | + | + | + | + | + | |||||||||||||
| 4.II.1 | + | + | + | + | + | + | + | + | ||||||||||
| 5.II.1 | + | + | + | + | + | + | + | + | + | + | ||||||||
| 6.I.1 | + | + | + | + | + | + | + | + | + | |||||||||
| 7.I.1 | + | + | + | + | + | + | + | + | + | + | ||||||||
| 8.I.1 | + | + | + | + | + | + | + | + | + | |||||||||
| 9.III.1 | + | |||||||||||||||||
| 10.I.1 | + | + | + | + | ||||||||||||||
| 11.II.1 | + | + | + | + | + | + | + | |||||||||||
| 12.I.1 | + | + | + | + | + | + | + | + | ||||||||||
| 13.I.2 | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| 13.II.1 | + | + | + | + | + | + | + | |||||||||||
| 14.II.1 | + | + | + | + | + | + | + | + | ||||||||||
| 15.II.1 | + | + | + | + | + | + | ||||||||||||
| 16.II.1 | + | + | + | + | ||||||||||||||
| Total (%) | 10 (55.6) | 5 (29.4) | 2 (11.8) | 13 (72.2) | 13 (72.2) | 11 (64.7) | 0 | 2 (11.8) | 6 (35.3) | 8 (47) | 6 (35.3) | 12 (70.6) | 9 (52.9) | 4 (23.5) | 4 (23.5) | 8 (44.4) | 3 (17.6) | 5 (29.4) |
+ indicates the presence of the trait listed in the column, and a blank indicates normality for the column's trait.
Autoimmune phenomena were seen in 4 patients: 2 had “lupus”-like syndromes (4.II.1 and 10.I.1), 1 had a “primary biliary cirrhosis”–like pattern of liver injury (3.I.1), and 1 had “multiple sclerosis”–like syndrome (7.I.1). Interestingly, the daughter of patient 7.I.1 had typical aggressive multiple sclerosis.
Of the 18 patients evaluated, 5 have died (age range, 39-64 years). Family pedigrees identified another 7 persons who died from what was a similar syndrome. Thus, of 25 persons probably afflicted by the same disease, 12 (48%) died of causes ranging from malignancy to myelodysplasia (age at death: mean, 34.7 years; median, 36.5 years).
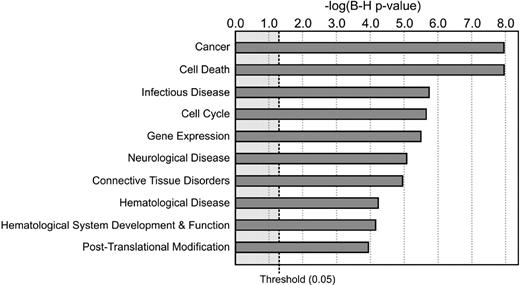
Immune functions were assessed by routine methods. Peripheral blood mononuclear cell cytokine production and proliferation in response to phytohemagglutinin were impaired; but on addition of normal monocytes, lymphocyte function was restored, suggesting that the defect was in the myeloid component (not shown). Polymorphonuclear cells (PMNs) were available for study in vitro and were subjected to routine testing, including nitro blue tetrazolium reduction and dihydrorhodamine oxidation, both of which were normal. Neutrophil granules and content were variably reduced (not shown). Chemotaxis of neutrophils and elutriated monocytes was within the normal range (not shown). PMNs from 3 patients were used for microarray analysis. We chose PMNs for analysis because they were abnormal and arose from the same precursor as monocytes and were accessible, whereas monocytes were not. Select key genes that were differentially expressed relative to healthy donors are listed in Table 4. By Ingenuity Pathways Analysis, the differentially expressed genes function principally in cancer/cell-cycle regulation, infectious diseases, and hematopoietic processes or pathophysiology, consistent with the clinical manifestations of the syndrome (Figure 4).
Selected transcripts differentially expressed in PMNs from affected persons
| Gene* . | Encoded protein . | Fold change . | P . |
|---|---|---|---|
| Increased in patients (selected) | |||
| CD40 | CD40 | +1.8 | < .001† |
| CYBB | gp91phox (NOX2) | +3.4 | .04† |
| FCER1G (n = 2) | CD23 | +2.3 | < .001† |
| ICAM1 (n = 3) | CD54 | +2.3 | < .001 |
| IL6ST (n = 5) | IL-6 signal transducer (gp130) | +2.4 | > .001 |
| ITGA2B (n = 2) | CD41 | +5.1 | .01† |
| ITGA7 (n = 2) | Integrin, α7 | Absent → present | NA† |
| ITGB3 (n = 3) | CD61 | +5.3 | .04† |
| Decreased in patients (selected) | |||
| AKT1 | AKT1 | −2.7 | < .001† |
| CSF1R | CD115 | Present → absent | NA† |
| CXCL6 | Granulocyte chemotactic protein 2 (GCP2) | −6.2 | .006 |
| IL13RA1 (n = 3) | IL-13 receptor, α1 | −2.0 | .03† |
| IL16 (n = 2) | IL-16 | −2.5 | < .001† |
| IL17RA | IL-17 receptor A | −1.5 | .005† |
| IL23A | IL-23, α subunit p19 | Present → absent | NA |
| IRF8 | IFN regulatory factor 8 | −2.8 | < .001 |
| JAK1 (n = 2) | Janus kinase 1 | −1.6 | < .001 |
| STAT6 | Signal transducer and activator of transcription 6 | −1.6 | .03 |
| TGFBR2 (n = 2) | Transforming growth factor, β receptor II | −1.6 | .01 |
| Not differentially expressed (selected) | |||
| CSF2RA (n = 2) | CD116, colony stimulating factor 2 receptor, α | −1.1 | .6 |
| CSF2RB | CD131, colony stimulating factor 2 receptor, β | −1.2 | .1 |
| IFNAR1 (n = 3) | IFN (α, β, and ω) receptor 1 | −1.3 | .4 |
| IFNGR2 | IFN-γ receptor 2 | 1.0 | .8 |
| STAT1 (n = 2) | Signal transducer and activator of transcription 1 | +1.2 | .5 |
| STAT3 (n = 4) | Signal transducer and activator of transcription 3 | 1.0 | > .999 |
| Gene* . | Encoded protein . | Fold change . | P . |
|---|---|---|---|
| Increased in patients (selected) | |||
| CD40 | CD40 | +1.8 | < .001† |
| CYBB | gp91phox (NOX2) | +3.4 | .04† |
| FCER1G (n = 2) | CD23 | +2.3 | < .001† |
| ICAM1 (n = 3) | CD54 | +2.3 | < .001 |
| IL6ST (n = 5) | IL-6 signal transducer (gp130) | +2.4 | > .001 |
| ITGA2B (n = 2) | CD41 | +5.1 | .01† |
| ITGA7 (n = 2) | Integrin, α7 | Absent → present | NA† |
| ITGB3 (n = 3) | CD61 | +5.3 | .04† |
| Decreased in patients (selected) | |||
| AKT1 | AKT1 | −2.7 | < .001† |
| CSF1R | CD115 | Present → absent | NA† |
| CXCL6 | Granulocyte chemotactic protein 2 (GCP2) | −6.2 | .006 |
| IL13RA1 (n = 3) | IL-13 receptor, α1 | −2.0 | .03† |
| IL16 (n = 2) | IL-16 | −2.5 | < .001† |
| IL17RA | IL-17 receptor A | −1.5 | .005† |
| IL23A | IL-23, α subunit p19 | Present → absent | NA |
| IRF8 | IFN regulatory factor 8 | −2.8 | < .001 |
| JAK1 (n = 2) | Janus kinase 1 | −1.6 | < .001 |
| STAT6 | Signal transducer and activator of transcription 6 | −1.6 | .03 |
| TGFBR2 (n = 2) | Transforming growth factor, β receptor II | −1.6 | .01 |
| Not differentially expressed (selected) | |||
| CSF2RA (n = 2) | CD116, colony stimulating factor 2 receptor, α | −1.1 | .6 |
| CSF2RB | CD131, colony stimulating factor 2 receptor, β | −1.2 | .1 |
| IFNAR1 (n = 3) | IFN (α, β, and ω) receptor 1 | −1.3 | .4 |
| IFNGR2 | IFN-γ receptor 2 | 1.0 | .8 |
| STAT1 (n = 2) | Signal transducer and activator of transcription 1 | +1.2 | .5 |
| STAT3 (n = 4) | Signal transducer and activator of transcription 3 | 1.0 | > .999 |
NA indicates not available because one of the sample values was 0.
n values (in parentheses) indicate number of probe sets on the microarray that gave concordant results; a representative is shown.
Also significant (P ≤ .01) by analysis of variance and correction for multiple comparisons using the false discovery rate (Partek Genomics Suite software).
Altered cell function and signal pathways in patients as assessed by microarray analysis of PMN transcripts. Ten most significant BioFunctions were identified using Ingenuity Pathways Analysis (Ingenuity Systems; www.ingenuity.com). Data are based on PMN transcripts differentially expressed in the patients compared with healthy control subjects. The P value indicates the likelihood that association of the specific set of transcripts and the indicated process or pathway is the result of random chance. B-H P value indicates P values after Benjamini-Hochberg correction for multiple comparisons.
Altered cell function and signal pathways in patients as assessed by microarray analysis of PMN transcripts. Ten most significant BioFunctions were identified using Ingenuity Pathways Analysis (Ingenuity Systems; www.ingenuity.com). Data are based on PMN transcripts differentially expressed in the patients compared with healthy control subjects. The P value indicates the likelihood that association of the specific set of transcripts and the indicated process or pathway is the result of random chance. B-H P value indicates P values after Benjamini-Hochberg correction for multiple comparisons.
Given the constellation of manifestations, the following candidate genes were sequenced from cDNA or genomic DNA in 3 or more patients without identified mutation: IL12Rβ1, IFNGR1, IFNGR2, STAT1, STAT2, JAK2, GNB2L1, CSF2, CSF2RB, C/EBPA, C/EBPB, C/EBPD, C/EBPE, RUNX1, IRF4, ICSBP1, PDGFRB, RhoH, HSP90AB1, CXCL14, CCR5, CXCR4, and CXCL12 (SDF-1).
Cases similar to those described here have been previously reported (Table 5). As well, 2 of the patients in the current report (patients 1.II.1 and 7.I.1) were previously described.17 This assembled cohort suggests that the previous individual case reports were consistent with this discrete syndrome. Further, our pedigrees prove that, although this syndrome occurs as a sporadic disease, it can be transmitted in an autosomal dominant fashion, suggesting that it is a single-gene defect with high penetrance and variable expressivity.
Summary of potentially related cases in the literature
| Reference . | Age at presentation, y/sex . | Infections . | Cytopenias . | Clinical features . | Outcome . |
|---|---|---|---|---|---|
| Ballas et al19 | 23/female (sister with acute leukemia; mother with genital cancer who died of progressive sarcoidosis) | Genital and periungual HPV | Monocytes, B cells, NK cells | Lymphadenitis, diffuse pulmonary disease | Not reported |
| Couderc et al20,21 | 32/female | Genital HPV, buccal herpes simplex virus, disseminated MAC | Neutrophils (mild), monocytes, lymphocytes | Pulmonary alveolar proteinosis | Died of respiratory failure |
| Wendland et al22 | 15/male | Salmonella enteritis, disseminated MAC, disseminated VZV | Monocytes, B cells, NK cells, T cells | Died of VZV-related multiorgan failure | |
| Khanjari et al23 | 19/male | Disseminated MAC | “idiopathic marrow aplasia,” monocytes, T cells | Pulmonary alveolar proteinosis | Not reported |
| Witzke et al24 | 38/female | Widespread vulvar HPV | Monocytes, B cells, NK cells | Pulmonary alveolar proteinosis, membranous nephropathy | Died of respiratory failure |
| Kobashi et al25 | 14/male | Pulmonary MAC | Monocytes, lymphocytes, platelets | Not reported | |
| Kobashi et al25 | 19/male (brother) | Disseminated MAC (age 22) | MDS (age 17) | Not reported |
| Reference . | Age at presentation, y/sex . | Infections . | Cytopenias . | Clinical features . | Outcome . |
|---|---|---|---|---|---|
| Ballas et al19 | 23/female (sister with acute leukemia; mother with genital cancer who died of progressive sarcoidosis) | Genital and periungual HPV | Monocytes, B cells, NK cells | Lymphadenitis, diffuse pulmonary disease | Not reported |
| Couderc et al20,21 | 32/female | Genital HPV, buccal herpes simplex virus, disseminated MAC | Neutrophils (mild), monocytes, lymphocytes | Pulmonary alveolar proteinosis | Died of respiratory failure |
| Wendland et al22 | 15/male | Salmonella enteritis, disseminated MAC, disseminated VZV | Monocytes, B cells, NK cells, T cells | Died of VZV-related multiorgan failure | |
| Khanjari et al23 | 19/male | Disseminated MAC | “idiopathic marrow aplasia,” monocytes, T cells | Pulmonary alveolar proteinosis | Not reported |
| Witzke et al24 | 38/female | Widespread vulvar HPV | Monocytes, B cells, NK cells | Pulmonary alveolar proteinosis, membranous nephropathy | Died of respiratory failure |
| Kobashi et al25 | 14/male | Pulmonary MAC | Monocytes, lymphocytes, platelets | Not reported | |
| Kobashi et al25 | 19/male (brother) | Disseminated MAC (age 22) | MDS (age 17) | Not reported |
Discussion
We describe a novel autosomal dominant syndrome characterized by disseminated mycobacterial, fungal, and viral infections and frequent development of myelodysplasia. In addition, several first-degree relatives have a history of opportunistic infections and myeloid disorders, suggesting an etiologic link to this disorder. The profound circulating monocytopenia with B-cell and NK-cell lymphocytopenias are distinctive features of this syndrome but unusual because of the presence of macrophages and plasma cells at sites of infection. Plasma cells were also seen in bone marrow analyses. Furthermore, serum immunoglobulin levels were essentially normal, and the spectrum of viral infections was limited. Therefore, to some important extent, trafficking of certain cells in this disorder may be abnormal. Infections primarily resulting from intracellular pathogens and PAP clearly indicate macrophage/monocyte dysfunction.
Although circulating monocytopenia and B-cell and NK-cell lymphocytopenia are uniform features of this syndrome, there was significant interpatient variability. Some patients progressed to myelodysplasia or acute leukemia, whereas others developed ongoing infections, PAP, solid cancer, or large granular lymphocytosis. Patient 13.I.2 has remained essentially asymptomatic, except for warts. T cells, granulocytes, erythrocytes, and platelets were inconsistently affected. Hematopoietic involvement ranged from abnormal surface expression of antigens to variable cellular content of granules to frank dysplasia. Unevaluated family members with a similar history of opportunistic infection and/or hematologic derangement probably represent variants of the same syndrome.
If the circulating cytopenias in this syndrome reflect profound diminution of marrow production of monocytes and B cells, tissue macrophages and plasma cells may represent local persistence and proliferation of previously produced cells. Tissue macrophages may arise from local proliferation.26-28 Because lung and alveolar macrophages have proliferative potential, they may maintain a tissue macrophage reservoir somewhat independent of blood reconstitution.29 Murine splenic macrophages and hepatic Kupffer cells have similar ontogeny.30,31 This self-renewing capacity is probably limited and not entirely bone marrow-independent, as tissue macrophages have been shown to be replaced by donor-derived cells several months after human bone marrow transplantation.32,33 One patient (14.II.1) had normal hemograms (including monocyte and lymphocyte numbers) during infancy and early childhood with progressive decline over several years before mycobacterial infection. Although we were not able to retrieve premorbid blood counts on other patients, this finding suggests that, at least in some cases, the underlying defect of this syndrome is not the monocytopenia per se but an alteration in the capacity of the hematopoietic system. Alternatively, these circulating cytopenias may reflect aberrant trafficking out of the circulation because of some exuberantly functioning or abnormally triggered adhesion mechanism that leads to margination and over-rapid depletion of circulating cell numbers.
Neutrophils are variably affected, demonstrating abnormal granule contents, aberrant surface antigen expression, and/or dysplasia (not shown). The involvement of both monocytes and neutrophils points to a lesion of early hematopoeisis because monocytes and neutrophils derive from the same committed myeloid progenitor cell.34,35 Involvement of B cells and NK cells, the thrombocytopenias, and the multiple lineages involved in dysplasia on the bone marrow examinations (Table 3) point to the hematopoietic stem cell or its niche. Infection itself may affect the pace and expression of this disorder.
Monocytopenia and mycobacterial infection are also seen in hairy cell leukemia. The monocytopenia in hairy cell leukemia is profound and persistent, with an incidence of mycobacterial disease of 4% to 9%.36 However, the mean circulating monocyte levels in patients with hairy cell leukemia is 74 cells/μL (442 cells/μL in controls)37 ; the mean level in our cohort was 14 cells/μL. Infections with Aspergillus spp, Cryptococcus sp, P jiroveci (carinii), and Histoplasma sp have also been reported in hairy cell leukemia, similar to our cohort.38,39 Bone marrow findings, lymphocyte immunophenotyping, and B-cell clonality characteristic of hairy cell leukemia40 were absent in our patients. Therefore, monocytopenia and monocyte dysfunction appear to be strongly linked to infection susceptibility in these syndromes, although the mechanism is unclear.
Monocyte/macrophage dysfunction probably accounts for the PAP observed in this syndrome as well.41-43 Primary autoimmune PAP is the result of autoantibodies to GM-CSF.44 In contrast, the congenital form of PAP is most commonly the result of mutations in surfactant protein B14, but also from defects in the common subunit of the GM-CSF, IL-3, and IL-5 receptors, βc.45 Secondary PAP occurs in association with immunodeficiency, infections, or malignancies. It may be difficult to discern whether PAP is secondary to an aberrant inflammatory response or whether the overaccumulation of alveolar protein provides a hospitable environment for certain infections. Malignancy-associated PAP is most commonly related to MDS or leukemia.14,46,47 Therefore, in both congenital and acquired PAP, alveolar macrophage function is impaired.
There are very few immunodeficiencies in which papillomavirus infection is severe or consistent enough to be a cardinal sign: epidermodysplasia verruciformis, the warts, hypogammaglobulinemia, immunodeficiency, myelokathexis syndrome, and this syndrome. Epidermodysplasia verruciformis differs from this syndrome in spectrum of associated infections and in clinical course. The warts, hypogammaglobulinemia, immunodeficiency, myelokathexis syndrome includes myelokathexis, which was not seen in any patient's bone marrow and sequencing of the gene responsible, CXCR4, was without mutation. Immunity to HPV requires an effective Th1 response as well as NK cells.48 In this syndrome, the combined monocyte and NK-cell deficiency may be permissive for severe HPV disease.19 The NK-cell deficiency in this disease may also account for the susceptibility to some herpesvirus infections49,50 and to recurrent fetal losses in patients 1.II.5 and 4.II.1.51 Viral etiologies have been clearly identified for certain human cancers: HPV with squamous carcinomas52 ; EBV with nasopharyngeal carcinoma, leiomyosarcomas, and some lymphomas53 ; and human herpesvirus 8 with Kaposi sarcoma and Castleman disease.54 Given the common associations of viral infections with leukemias in animals,55 it is possible that some degree of selective viral susceptibility underlies the malignancies in this disorder. Idiopathic CD4+ lymphocytopenia may also present with HPV infection, but those patients have more normal B- and NK-cell numbers and do not have the clinical evolution of this syndrome.56 We included none of the cases previously reported by Zonios et al.56
MDS often terminates in AML.57 These are typically diseases of the elderly (median age at presentation > 65 years),58 whereas our patients were younger (< 40 years) with a strong familial component (more than 1 first-degree relative with MDS/AML). The pattern of autosomal dominant immunodeficiency preceding the development of hematologic malignancy is in keeping with the other familial MDSs or leukemias, collectively referred to as “syndromic MDS/AML” (eg, Shwachman-Diamond syndrome, severe congenital neutropenia).58,59 Monosomy 7 and trisomy 8 are among the most common chromosomal changes found in MDSs or leukemia.60 In sporadic cases, they represent 16% to 17% of identified chromosomal derangements, although they represent the sole change in only 6% to 11%.60 These chromosomal abnormalities are also the most commonly identified in familial MDS or leukemias.58 MDS or leukemia occurred in 11 of our patients, and 3 had monosomy 7 and/or trisomy 8. Familial monosomy 7 pedigrees suggest that leukemogenesis results from mutation in a gene that possesses a mutator effect, which then facilitates the acquisition of chromosomal derangements.59 This would be consistent with the variable expressivity in this syndrome, with one set of presentations for the patients recruited with mycobacterial disease and another set of presentations for first-degree relatives.
This novel syndrome connects susceptibility to bacteria, fungi, viruses, and malignancies through an autosomal dominant gene. It is remarkable for its relatively late onset, its highly selective set of infections, despite their being spread across the entire spectrum of human pathogens, and its unique association with circulating cytopenias. Specific therapy directed at the underlying abnormality must await identification of the mutated gene or genes, which will forge another critical link uniting infection and cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and in part with federal funds from the National Cancer Institute, National Institutes of Health (contracts N01-CO-12400 and HHSN261200800001E). D.C.V. is supported by a Canadian Institutes of Health Research fellowship and by a National Institutes of Health Supplemental Visiting fellowship.
National Institutes of Health
Authorship
Contribution: S.M.H. provided the study concept and design and supervised the study; S.M.H., D.C.V., S.Y.P., and G.U. were responsible for the acquisition of NIH data; S.H. provided data on the referred patient; S.M.H. and D.C.V. provided the analysis and interpretation of the data; S.Y.P., D.C.V., and S.M.H. drafted the manuscript; D.C.V., S.Y.P., G.U., V.L.A., A.F.F., K.N.O., S.H., S.P., M.L.T., E.W.C., and S.M.H. provided critical revision of the manuscript for important intellectual content; S.P. was responsible for reviewing and imaging of histopathology; A.R.W. and F.R.D. performed microarray and analysis; C.S. recruited and coordinated patients and specimens; M.R. and L.R.S. performed and analyzed studies of clonality; and H.Z.E., D.B.K., D.F., D.L.-P., A.P.H., L.D., M.L.P., F.R.D., E.P.S., and D.M.F. provided administrative, technical, or material support.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven M. Holland, Immunopathogenesis Section, Laboratory of Clinical Infectious Diseases, NIAID, NIH, Bldg 10 CRC, Rm B3-4141, MSC 1684, Bethesda, MD 20892-1684; e-mail: smh@nih.gov.
References
Author notes
D.C.V. and S.Y.P. contributed equally to this study.