Abstract
During Plasmodium falciparum malaria infections, von Willebrand factor (VWF) levels are elevated, postmortem studies show platelets colocalized with sequestered infected erythrocytes (IEs) at brain microvascular sites, whereas in vitro studies have demonstrated platelet-mediated IE adhesion to tumor necrosis factor-activated brain endothelium via a bridging mechanism. This current study demonstrates how all these observations could be linked through a completely novel mechanism whereby IEs adhere via platelet decorated ultra-large VWF strings on activated endothelium. Using an in vitro laminar flow model, we have demonstrated tethering and firm adhesion of IEs to the endothelium specifically at sites of platelet accumulation. We also show that an IE pro-adhesive state, capable of supporting high levels of binding within minutes of induction, can be removed through the action of the VWF protease ADAMTS-13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13). We propose that this new mechanism contributes to sequestration both independently of and in concert with current adhesion mechanisms.
Introduction
Plasmodium falciparum malaria remains a scourge in many developing tropical regions of the world where it claims the lives of hundreds of thousands of children every year. A defining feature of one of the most severe forms of the disease, cerebral malaria (CM), is the sequestration of infected erythrocytes to endothelium in brain postcapillary venules. Adhesion is mediated by P falciparum erythrocyte membrane protein 1 (PfEMP1) to various host receptors, such as CD36,1 to which almost all pediatric isolates bind.2 With little or no CD36 being expressed on brain endothelium, it had been assumed that CD36 adhesion was not involved in CM. Several observations have challenged this assumption. First, analysis of human postmortem brain sections from fatal CM, severe malarial anemia, and nonmalarial cases showed a strong correlation between platelet accumulation, which generally colocalized with malaria pigment, and disease severity.3 The possible mechanism underlying this association was further developed in vitro, where it was shown that platelets could act as a bridge between activated brain endothelium not expressing CD36 and infected erythrocytes (IEs) that only bind to CD36.4
Endothelial activation is classically achieved using cytokines, such as tumor necrosis factor, which induce the relatively slow process (hours) of de novo protein synthesis. We proposed a mechanism for rapid adhesion via the multimeric protein von Willebrand factor (VWF)5 that mediates platelet adhesion to sites of vascular injury. The mature ultra-large, and physiologically most active, form of VWF is stored in specialized secretory vesicles in endothelial cells (ECs) called Weibel-Palade bodies.6 When released, these large VWF multimers unravel under flow to form platelet-decorated strings that are cleaved and regulated by the endogenous plasma protease ADAMTS-13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13).7 Measurements of clinical plasma samples show that levels of VWF and its propeptide are elevated in malaria and are correlated to disease severity.5 Furthermore, a significant reduction in plasma ADAMTS-13 activity has been recently reported.8 However, given the established limitations of current in vitro ADAMTS-13 assays,8,9 the translational significance of this reduction has not been defined. In addition, it remains unclear whether the high levels of circulating ultra-large VWF multimers present in children with severe P falciparum malaria play a direct role in mediating the underlying pathophysiology.
Here we provide the first evidence that EC activation, and specifically the regulated release of VWF, is capable of mediating the rapid adhesion of IEs via CD36-dependent platelet bridging.
Methods
P falciparum strain ITO4-C24 culturing,10 and isolation of human umbilical vein endothelial cell11 and fresh platelets12 were performed as previously described. Platelet and IE binding flow adhesion assays were performed on confluent-activated human umbilical vein endothelial cell monolayers.
Complete materials and methods are available in the supplemental Methods (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results and discussion
Mature IEs bind to platelets on ultra-large VWF strings via CD36
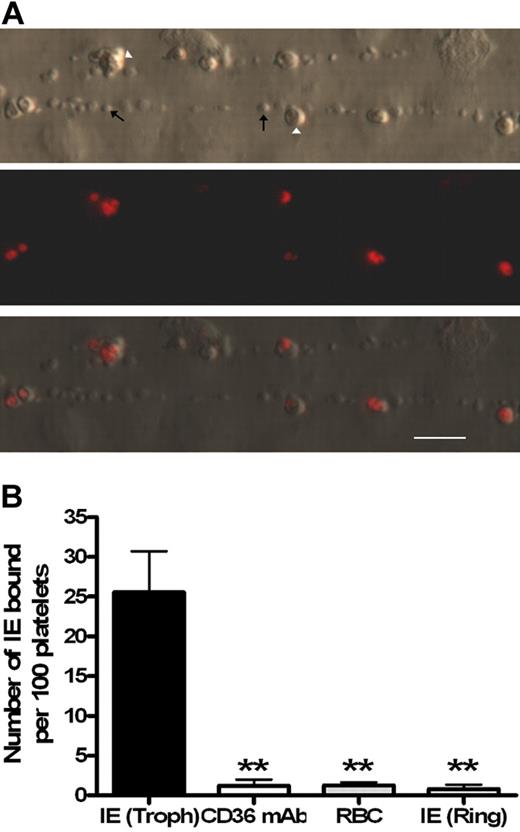
Platelet-decorated strings formed from ultra-large VWF multimers are large macroscopic structures visible under low-power microscopy that only form on activated endothelium.7,13 Using fluorescently labeled trophozoites, the mature adhesive form of the parasite, binding of IEs to platelet-decorated strings was clearly visible (Figure 1A; supplemental Videos 1-2). To confirm that these interactions were parasite mediated, the same experiment was performed replacing the trophozoite culture with uninfected erythrocytes or immature (nonadhesive) ring-stage parasites with only minimal levels of adhesion observed in both cases (Figure 1B). Cytoadherence by the strain ITO-C24 is known to be CD36 dependent; and, as expected, binding was completely inhibited in the presence of an anti-CD36 monoclonal antibody (Figure 1B). From these experiments, binding was confirmed to be CD36 dependent and only possible in the mature trophozoite parasite form, which unlike ring stages express PfEMP-1, the protein mainly responsible for parasite adhesion and known to contain binding sites for CD36.14
Only mature Plasmodium falciparum–infected erthryocytes are able to bind to platelet-decorated VWF strings on endothelial cells stimulated with histamine. (A) Ethidium bromide–labeled parasites (Δ) are the only red blood cell species binding to the platelet–decorated VWF strings ↑. Flow direction is from left to right. (B) Trophozoite (Troph) IE binding to strings (n = 11) was significantly blocked by a CD36 monoclonal antibody (n = 4). Similarly, binding was not observed in the presence of uninfected red blood cells (n = 4) or with ring-stage IE (n = 3). Bar represents 20 μm. **P < .005.
Only mature Plasmodium falciparum–infected erthryocytes are able to bind to platelet-decorated VWF strings on endothelial cells stimulated with histamine. (A) Ethidium bromide–labeled parasites (Δ) are the only red blood cell species binding to the platelet–decorated VWF strings ↑. Flow direction is from left to right. (B) Trophozoite (Troph) IE binding to strings (n = 11) was significantly blocked by a CD36 monoclonal antibody (n = 4). Similarly, binding was not observed in the presence of uninfected red blood cells (n = 4) or with ring-stage IE (n = 3). Bar represents 20 μm. **P < .005.
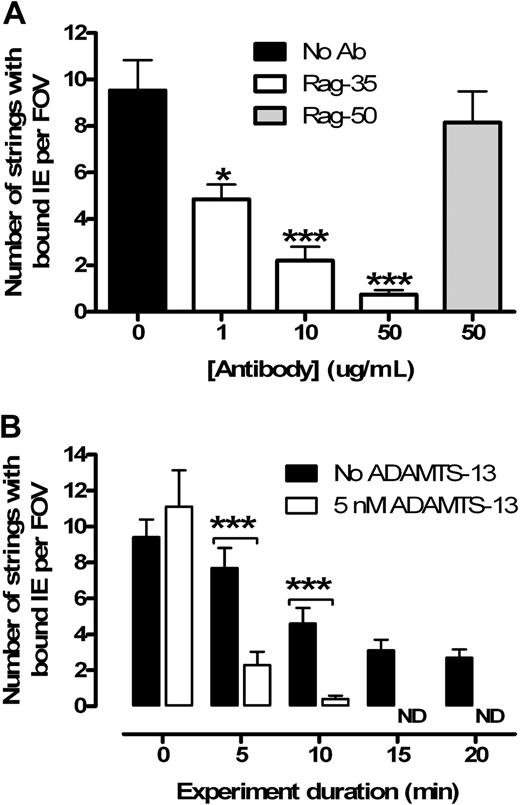
To further characterize binding, we used a monoclonal antibody to selectively block the VWF A1 domain, which is responsible for platelet binding via glycoprotein-Ib-IX-V. As predicted by our original model,5 a dose-dependent reduction in the number of IE-bound strings was observed when blocking the A1 domain, whereas no effect was seen with the control (Figure 2A). In both cases, the binding ratio of IE to platelets was unaffected, confirming that IE binding is proportional to the number of bound platelets. We concluded that IEs are binding to platelets via CD36 and are not interacting directly with the VWF strings.
IE adhesion to endothelium is dependent on binding of platelets to VWF strings, which can be cleaved by recombinant ADAMTS-13. (A) Rag-35 blocks platelets binding to VWF strings in a dose-dependent manner (n = 3). The control antibody (Rag-50) does not inhibit binding (n = 2). (B) Strings can be rapidly cleaved from the endothelium by ADAMTS-13 (added at t = 5 minutes) in the wash media (n = 3). All strings were removed after 15 minutes. ND indicates no detectable strings. *P < .05. ***P < .001.
IE adhesion to endothelium is dependent on binding of platelets to VWF strings, which can be cleaved by recombinant ADAMTS-13. (A) Rag-35 blocks platelets binding to VWF strings in a dose-dependent manner (n = 3). The control antibody (Rag-50) does not inhibit binding (n = 2). (B) Strings can be rapidly cleaved from the endothelium by ADAMTS-13 (added at t = 5 minutes) in the wash media (n = 3). All strings were removed after 15 minutes. ND indicates no detectable strings. *P < .05. ***P < .001.
ADAMTS-13 has previously been shown to remove strings from activated endothelium in vitro.15 In keeping with previous reports,7 the addition of 5nM ADAMTS-13 both cleared tethered IEs from the endothelium (Figure 2B) and ablated string formation if used to pretreat the endothelium. Interestingly, we observed a dose-dependent increase in the number of adherent platelet strings with a progressive reduction in recombinant ADAMTS-13 concentration (from 5nM to 0.1nM; data not shown). At this stage, it is unclear how much string-associated sequestration is necessary in our model to contribute to in vivo malaria pathology.
A dynamic model
Interestingly, although VWF strings are clearly tethered to the endothelium, the majority of the strings appeared to be distanced from, and not in direct contact with, the endothelial monolayer along much of their length. Free rotation of string sections, including bound IEs, was often observed. The dynamic detachment and reattachment of strings under flow led to the concentration of strings into a foci or mesh (supplemental Video 2) as have previously been described in vivo in ADAMTS-13−/− mice.16
Implications in disease pathogenesis
This study establishes a new mechanism by which IEs can sequester in vascular beds, which do not express appropriate IE receptors. In contrast to classic activation pathways involving de novo protein synthesis, we show that a pro-adhesive surface is generated almost instantly through the regulated release of VWF and platelet tethering. Other clinical and experimental correlates suggest that this novel model of adhesion could be of critical importance in malaria pathogenesis. First, EC activation, and specifically the release of Weibel-Palade body contents, occurs very early on in experimental human infections before a person becomes smear positive.17 Although the basis of EC activation remains unknown, inhibition of histamine-mediated signaling protects mice from CM,18,19 whereas P falciparum is known to secrete a functional histamine release factor.20 Finally, ADAMTS-13 levels are reduced while there is an increase in circulating ultra-large VWF multimers in severe malaria.8
The potential role that this new model could play in malaria disease progression (early on, throughout infection, or later on) is striking and suggests that VWF is not just a marker of malaria disease severity but rather is probably a primary player in the pathogenesis of malaria.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Karl Seydel, Prof Terrie Taylor, Dr Happy Phiri, Dr Srabasti Chakravorty, Rachel McGrath, and Dr Sam Wassmer for assistance provided during this project.
This work was supported by the Wellcome Trust (project grant 081345/Z/06/Z). B.d.L. is a research fellow of the Netherlands Heart Foundation (grant 2006T053). J.S.O. was supported by the Science Foundation Ireland, President of Ireland Young Investigator Research Award (06/Y12/0925).
Wellcome Trust
Authorship
Contribution: D.J.B. designed the research, performed experiments, and analyzed the data; J.B., J.A.v.M., G.G., J.S.O., B.d.L., and A.C. designed the research and analyzed the data; R.J.S.P. contributed vital new reagents; and all authors were involved in writing and reviewing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel J. Bridges, Liverpool School of Tropical Medicine, Pembroke Pl, Liverpool, L3 5QA, United Kingdom; e-mail: d.bridges@liverpool.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal