Abstract
In this issue of Blood, Bridges and colleagues report that Plasmodium falciparum–infected erythrocytes are able to attach to the endothelial surface by binding platelet-decorated VWF strands in a CD36-dependent fashion, revealing a new mechanism for erythrocyte cytoadherence in malaria.1
Malarial infection, primarily that caused by P falciparum, kills an estimated 1 million people per year, mainly children. Cerebral malaria is the most lethal form of this disease and is characterized by the sequestration of P falciparum–infected erythrocytes (IE) in the capillaries and postcapillary venules of the brain. Cerebral sequestration is a consequence of adhesion of IE to activated endothelium, with one of the most important adhesive molecules on the IE surface being the parasite-encoded protein, P falciparum erythrocyte membrane protein 1 (PfEMP1). Among the endothelial counterreceptors recognized by PfEMP1 is the scavenger receptor CD36. Although important for IE adhesion, CD36 is not expressed at high levels in the microcirculation of the brain. One potential mechanism by which IE could use this receptor to adhere to cerebral blood vessels was suggested by Wassmer et al,2 who found that platelets could bridge the interaction of IE with endothelial cells by binding the endothelium, thereby providing a rich source of CD36 to which the IE can adhere. This finding was consistent with the electron microscopic observation of platelet adhesion to brain endothelial cells in both human and murine cerebral malaria,3,4 and with the protection against cerebral malaria seen in mice rendered thrombocytopenic.4
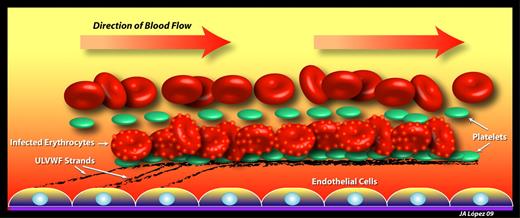
Mechanisms of infected erythrocyte adhesion to endothelium in cerebral malaria. Early in the blood stage of P falciparum infection, endothelial cells become activated to secrete ULVWF strands, which remain attached to the endothelial surface and rapidly bind platelets. Infected erythrocytes, coated with membrane knobs rich in PfEMP1, adhere to the platelet-decorated ULVWF strings through platelet CD36. ULVWF contributes to both the thrombocytopenia and hemolytic anemia of malaria by binding large numbers of platelets and by shearing red cells.
Mechanisms of infected erythrocyte adhesion to endothelium in cerebral malaria. Early in the blood stage of P falciparum infection, endothelial cells become activated to secrete ULVWF strands, which remain attached to the endothelial surface and rapidly bind platelets. Infected erythrocytes, coated with membrane knobs rich in PfEMP1, adhere to the platelet-decorated ULVWF strings through platelet CD36. ULVWF contributes to both the thrombocytopenia and hemolytic anemia of malaria by binding large numbers of platelets and by shearing red cells.
Another important characteristic of both cerebral malaria and severe noncerebral malaria is systemic activation of the endothelium. Activated endothelial cells express on their surfaces a variety of adhesion molecules, including P- and E-selectin, intercellular cell adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1). In addition, acutely activated endothelial cells exocytose storage granules (Weibel-Palade bodies) containing large amounts of von Willebrand factor (VWF), especially ultra-large forms (ULVWF) that bind platelets especially well. In a study of experimental human P falciparum infection, de Mast and coworkers5 noted that platelet counts dropped very early after the onset of blood-stage infection, concomitant with increases in plasma levels of von Willebrand factor and its propeptide, the latter being indicative of acute endothelial activation, as its half-life in the circulation is much shorter than that of VWF. VWF propeptide levels have been shown to be higher in patients with cerebral malaria than in those with noncerebral malaria, and to reflect the acuteness of the syndrome, dropping more rapidly than VWF levels with antimalarial treatment.6 Two recent studies have demonstrated that ULVWF circulates at high levels in cerebral and severe forms of P falciparum malaria.5,7 The hyperreactivity of the VWF was demonstrated both by increased size on multimer gels and enhanced collagen-binding activity7 and by enhanced binding of the unique llama nanobody AU/VWFa-11,5 which recognizes an active conformation of the VWF A1 domain capable of spontaneously binding platelet glycoprotein Ibα.8 This form of VWF is the culprit in the devastating microvascular thrombotic disorder, thrombotic thrombocytopenic purpura (TTP), which is caused by systemic occlusion of small blood vessels by platelet thrombi and is usually fatal unless promptly treated by plasmapheresis. Accumulation of ULVWF in TTP almost always results from failure of its processing by the metalloprotease ADAMTS13. ADAMTS13 deficiency has also been reported in association with malaria.7,9
Bridges and colleagues describe a mechanism by which VWF plays an indispensible role in the worst pathologies caused by P falciparum infection.1 Activated endothelium rapidly releases large strands of ULVWF, many of which remain attached to the endothelium, where they become decorated by platelets to produce a beads-on-a-string pattern.10 Bridges et al show that P falciparum IE are able to bind platelets attached to the ULVWF strands in a CD36-dependent manner (see figure) and that the attachment can be prevented by an antibody against VWF. As with VWF strings decorated with platelets only,10 the erythrocyte-platelet-VWF strings are removed from the endothelium by ADAMTS13.
This involvement of VWF in malarial cytoadherence has several important implications for the understanding and treatment of severe malaria. First, it is very likely that the thrombocytopenia seen in a high percentage of patients with malaria is at least partially due to consumption of platelets as they bind ULVWF. Second, this mechanism explains why IE are able to attach to endothelial cells that are relatively devoid of CD36. Third, this mechanism might also explain why the anemia associated with P falciparum is often out of proportion to the number of IE seen on blood smears, suggesting that a microangiopathic process similar to that seen in TTP is contributing to red cell destruction. Finally, these findings suggest that in cases of severe life-threatening malaria, measures such as plasma exchange should be considered, to eliminate ULVWF and replace ADAMTS13. Thus, as vividly illustrated by Bridges and coworkers in this issue of Blood, it is becoming increasingly apparent that excessive secretion of hyperreactive VWF and failure to inactivate it contributes to many disease processes beyond TTP.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal