Abstract
Efficient activation of the fibrinolytic pathway occurs when plasminogen and its activators are sequestered on the cell surface. Identification of receptors responsible for localizing plasminogen to the cell surface has been elusive. Using a proteomics approach, Andronicos and colleagues have identified a novel 17-kDa transmembrane receptor, termed Plg-RKT, that binds plasminogen with high affinity and promotes its activation.1
Plasminogen is a zymogen that, when activated, is the major enzyme responsible for degrading fibrin2 and is therefore critical for maintaining vascular patency. In addition, the plasminogen system facilitates monocyte migration3 by promoting matrix degradation. It also functions in modulating the inflammatory response to bacterial pathogens.4 Plasminogen has long been known to associate with cells via lysine binding sites located within its kringle domains, where it is more readily activated by its physiological activators, tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA), and is protected from inhibition by its primary inhibitor, α2-antiplasmin.5,6
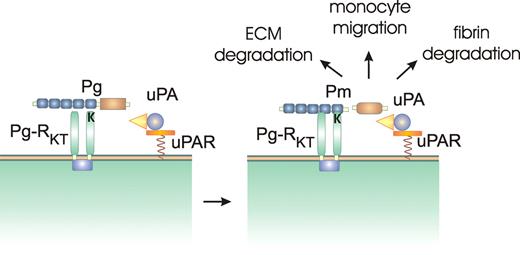
Pg-RKT on the monocyte/macrophage cell surface binds plasminogen (Pg) via interactions involving lysine binding sites on the Pg kringle domains with the C-terminal lysine residue on Pg-RKT. The close proximity to uPA bound to the urokinase receptor (uPAR) greatly facilitates conversion of Pg to plasmin (Pm). Cell-associated Pm is involved in degradation of the extracellular matrix (ECM), cell migration, and in degrading fibrin.
Pg-RKT on the monocyte/macrophage cell surface binds plasminogen (Pg) via interactions involving lysine binding sites on the Pg kringle domains with the C-terminal lysine residue on Pg-RKT. The close proximity to uPA bound to the urokinase receptor (uPAR) greatly facilitates conversion of Pg to plasmin (Pm). Cell-associated Pm is involved in degradation of the extracellular matrix (ECM), cell migration, and in degrading fibrin.
The identification of molecules that sequester plasminogen on the cell surface has proven to be a fruitful yet challenging area of research as a number of cell-associated plasminogen-binding proteins have been identified. Several of these molecules contain carboxy-terminal lysine residues, a requirement for binding to the lysine binding sites on plasminogen. However, most candidate molecules, such as enolase-1,7 have established intracellular functions, and thus it is not apparent how their cell-surface localization might be regulated. An attractive candidate for sequestering plasminogen to the cell surface is annexin II, a member of a family of phospholipid-binding peripheral membrane proteins that binds both plasminogen and tPA, and mediates the plasminogen-dependent invasion of matrix by monocytes.3
To identify a transmembrane cell-surface protein with a carboxy-terminal lysine residue that could function as a plasminogen receptor, Andronicos et al first identified a myloid precursor cell line (Hoxa9-ER4) that was unable to bind plasminogen but could bind plasminogen after differentiation into monocytes.1 To identify the molecule involved, the investigators first tagged the cell-surface proteins with biotin. Then they isolated the cell membranes and subjected the extracted proteins to affinity chromatrography on plasminogen-Sepharose. The proteins eluted from this affinity step were then bound to immobilized avidin to capture the cell-surface proteins tagged with biotin. After elution and digestion of these proteins with trypsin, the digest was subjected to multidimensional chromatography and tandem mass spectrometry.
This procedure identified a single 147–amino acid protein, termed Plg-RKT (plasminogen receptor with a C-terminal lysine) that is predicted to contain 2 transmembrane helices with a C-terminal lysine residue on the ectodomain. Studies demonstrated that the expression Plg-RKT is induced when Hoxa9-ER4 cells are differentiated along the monocytic pathway. Plasminogen as well as tPA, which also contains lysine binding sites in its kringle domains, were shown to specifically bind to a carboxy-terminal peptide representing the C-terminal region of Plg-RKT with a very high affinity. In M-CSF–differentiated Hoxa9-ER4 cells, Plg-RKT colocalizes with the urokinase receptor (uPAR), a cell-surface receptor that binds uPA. Finally, Andronicos et al demonstrated that Plg-RKT promotes the activation of plasminogen.1
Now that a transmembrane-containing receptor has been identified that is capable of binding plasminogen and tPA with high affinity and is able to promote the activation of plasminogen, studies can now be conducted to examine the contribution of this novel receptor to plasminogen biology (see figure). Plg-RKT is widely expressed on cells known to bind plasminogen and likely serves to modulate plasminogen activation and function in a variety of physiological processes.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal