To the editor:
Chronic cold agglutinin disease (CAD) is an acquired autoimmune hemolytic anemia (AIHA); usually due to the production of a monoclonal immunoglobulin M (IgM) protein directed against polysaccharide antigens on red blood cells.1,2 An occult lymphoproliferative disorder is often responsible for the production of the IgM paraprotein in patients with symptomatic CAD.3 The discovery of an underlying neoplastic process does guide therapeutic decisions in patients with severe symptomatic CAD. Historically, such patients have been treated with alkylating agents and, more recently, with rituximab or interferon.1,4 Treatments useful in IgG-mediated AIHA, such as corticosteroids, intravenous immunoglobulin and splenectomy, are often ineffective in treatment of CAD.1
Bortezomib is an inhibitor of the 26S proteasome; approved for the treatment of multiple myeloma and mantle cell lymphoma.5 One of the coauthors has successfully used bortezomib to treat some patients with refractory isoagglutinin-mediated pure red-cell aplasia (PRCA) after ABO-mismatched allogeneic hematopoietic stem cell transplantation (J.M., unpublished data, November 2006 [5 patients]). Based upon this observation, we used bortezomib in a patient with transfusion-dependent CAD secondary to IgM κ monoclonal gammopathy. Consent was obtained from the patient in accordance with the Declaration of Helsinki and from the Institutional Review Board regarding bortezomib treatment and publication of associated findings in this case report.
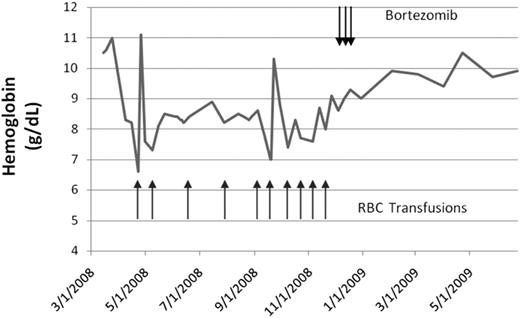
A 78-year-old woman developed transfusion-dependent CAD in April 2008. She was initially diagnosed with CAD in 2001, and was treated with oral cyclophosphamide and later azathioprine with stabilization of hemoglobin in the 9- to 10-g/dL range. She continued to have laboratory evidence of active hemolysis with reticulocytosis, and elevated lactate dehydrogenase (LDH) and total bilirubin. In December 2003, azathioprine was discontinued and weekly epoetin alfa started – and hemoglobin continued to remain stable. In March 2008, epoetin alfa was discontinued and the hemoglobin dropped from 11 g/dL to less than 7 g/dL over a 4-week period. Epoetin alfa was resumed without response, and she became transfusion-dependent (Figure 1). There was no response to 4 doses of rituximab (375 mg/m2) administered at weekly intervals in August and September 2008. Bone marrow biopsy performed in October 2008 showed a small (5%) κ-restricted plasma cell population that was CD20-negative. Serum protein electrophoresis with immunofixation showed a monoclonal IgM κ paraprotein measuring 0.9 g/dL. In December 2008, she received 3 doses of bortezomib (1.3 mg/m2; 4 planned based on the PRCA experience). This resulted in stabilization of hemoglobin and independence from transfusion support. By February 2009, hemoglobin improved from 8.6 to 9.9 g/dL without transfusions, although laboratory evidence of compensated hemolysis persisted. As of August 2009, she has continued to remain transfusion-independent with a hemoglobin level of approximately 10 g/dL. The serum M protein decreased from 0.9 to 0.5 g/dL. The cold agglutinin titer has also declined, but has not disappeared.
Hemoglobin levels. Graph of hemoglobin over time (gray line) with timing of RBC transfusions (black arrows, bottom) and bortezomib administration (black arrows, top).
Hemoglobin levels. Graph of hemoglobin over time (gray line) with timing of RBC transfusions (black arrows, bottom) and bortezomib administration (black arrows, top).
Our report suggests that bortezomib may be an active treatment in patients with AIHA. In contrast to rituximab, which targets CD20+ B lymphocytes, bortezomib targets differentiated plasma cells that typically do not express CD20 but may be responsible for secretion of the abnormal IgM responsible for hemolysis in CAD.
Note added in proof:
Since initial submission, we have treated another patient with refractory cold agglutinin-positive hemolytic anemia with a similar response.
Authorship
Conflict-of-interest disclosure: J.M. served on speakers' bureau for Millennium Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Kenneth R. Carson, MD, Department of Internal Medicine, Division of Medical Oncology, Washington University School of Medicine, 660 S. Euclid Ave, Campus Box 8056, St. Louis, MO 63110; e-mail: kcarson@dom.wustl.edu.